Abstract
Pembrolizumab has become the standard first‐line treatment for non‐small cell lung cancer (NSCLC) patients with high PD‐L1expression. MET exon 14 skipping is a rare mutation typically found in older, female, and non‐smoking patients with NSCLC. Herein, we report the case of a 71‐year‐old non‐smoking woman who was diagnosed with NSCLC in the left lung. EGFR mutation and ALK fusion were not detected. Because the biopsy specimen showed high PD‐L1 expression with a tumor proportion score of 95%, pembrolizumab was introduced as first‐line therapy, but resulted in no clinical benefit. The patient was subsequently administered chemotherapy with carboplatin and pemetrexed, leading to remarkable tumor shrinkage. A next‐generation sequencing panel analysis revealed a MET exon 14 skipping mutation. Thus, pembrolizumab might not be effective for NSCLC patients with MET exon 14 skipping mutations, even if PD‐L1 expression is high.
Keywords: MET exon 14 skipping mutation, non‐small cell lung cancer, pembrolizumab, pemetrexed, programmed death‐ligand 1
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide, although new agents targeting oncogenic gene alterations have significantly improved survival.1 More recently, immune checkpoint inhibitors (ICIs) have led to significant changes in treatment strategies, and long‐term survival may be possible in previously treated non‐small cell lung cancer (NSCLC) patients.2, 3, 4 Pembrolizumab monotherapy has become a standard option as the first‐line treatment for advanced NSCLC patients with high PD‐L1 expression and a tumor proportion score (TPS) of ≥ 50%.5
Somatic mutations in the MET gene can cause exon 14 skipping, and the resulting mutant receptor demonstrates increased c‐MET signaling and oncogenic potential. This mutation is only detected in 1% of NSCLC patients, but is often found in sarcomatoid carcinoma, which is characterized by poor prognosis.6, 7 In clinical trials, crizotinib and other MET inhibitors have been shown to be effective for NSCLC with MET amplification or exon 14 skipping mutation, although they have not yet been approved.8, 9, 10, 11 Herein, we present a case of NSCLC with both high PD‐L1 expression and a MET exon 14 skipping mutation.
Case report
A 71‐year‐old woman with no smoking history was referred to our hospital in November 2017 after an abnormal shadow was detected in the left lower lung on chest X‐ray (Fig 1a). A physical examination revealed no significant abnormalities. Computed tomography (CT) of the chest revealed a tumor with a diameter of 30 mm in the left S6 with enlargement of the hilar and peritracheal lymph nodes and small pulmonary metastasis on the bilateral lungs (Fig 1b–d), indicating c‐T3N2M1a (Union for International Cancer Control, 8th edition). Laboratory findings were within the normal ranges, except for a high cytokeratin 19 fragment serum level of 5.1 ng/mL. We conducted a transbronchial biopsy and the pathological diagnosis was non‐small cell lung cancer (NSCLC), which had neither EGFR mutation nor ALK fusion. Hematoxylin and eosin staining of the biopsy specimen revealed medium sized tumor cells of a polygonal shape, with medium cytoplasm and hyperchromatic nuclei (Fig 2). Immunohistochemistry revealed that tumor cells were negative for TTF‐1 and p40. These findings indicated a pathological diagnosis of poorly differentiated lung adenocarcinoma and the pathology indicated histological features of sarcomatoid carcinoma.
Figure 1.
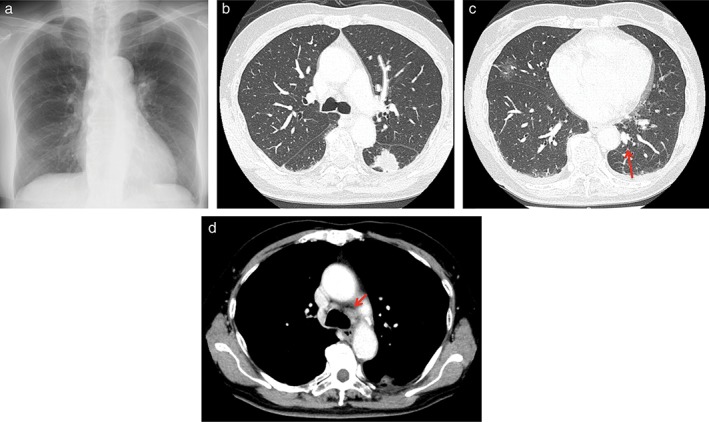
(a) Chest X‐ray on the first visit to our hospital showing a tumor in the left lower lung. (b–d) Computed tomography (CT) of the chest revealed a tumor (30 mm in diameter) in the left S6 with enlargement of the lymph nodes in the hilar and peritracheal regions, as well as a small pulmonary metastasis.
Figure 2.
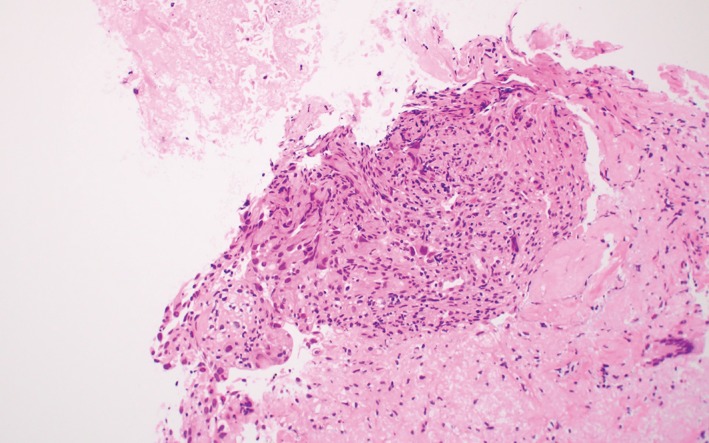
Histopathology of the tumor, showing tumor cells were medium sized and had a polygonal shape with medium cytoplasm and hyperchromatic nuclei (hematoxylin and eosin stain, x20).
Because high PD‐L1 expression with a TPS of 95% was confirmed by immunohistochemistry with a 22C3 antibody (Dako North America, Carpinteria, CA, USA), we commenced first‐line treatment of pembrolizumab (200 mg abs. on day 1). After the first cycle of pembrolizumab, the tumor in the left lung increased in size and atelectasis appeared in the upper lobe as a result of hilar lymph node enlargement (Fig 3a–c). The patient complained of dyspnea and developed hypoxia. We considered obvious disease progression and introduced second‐line chemotherapy of carboplatin (CBDCA, area under the curve = 5) and pemetrexed (PEM, 500 mg/m2 on day 1). These cytotoxic agents were remarkably effective with apparent tumor shrinkage after two cycles of treatment (Fig 3d–f), representing a partial response. Because the patient was a non‐smoker we conducted next‐generation sequencing panel analysis, which revealed a MET exon 14 skipping mutation.
Figure 3.
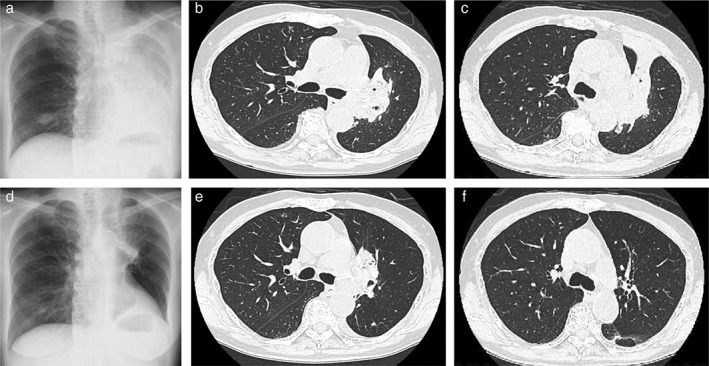
(a) Chest X‐ray performed after 38 days of pembrolizumab treatment showing tumor growth and the development of obstructive atelectasis. (b,c) Computed tomography (CT) scan taken on day 25 after the introduction of pembrolizumab, showing that the tumor had increased in size. The patient was re‐hospitalized that day because of dyspnea and acute respiratory failure. (d) Chest X‐ray taken after 19 days of carboplatin and pemetrexed treatment, showing significant reduction of the tumor in the left lung and recovery of the atelectasis. (e,f) A CT scan performed after 43 days of carboplatin and pemetrexed treatment showing further reduction of the tumor in the left lung.
Discussion
In our case, the patient had both high PD‐L1 expression and a MET exon 14 skipping mutation. Cytotoxic chemotherapy with CBDCA plus PEM was effective, whereas pembrolizumab therapy was not, despite high expression of PD‐L1. In general, ICIs are less effective in NSCLC with EGFR mutation, EML4‐ALK fusion, or other rare mutations.12, 13 Sabari et al. reported that 40% of the NSCLC patients with a MET exon 14 skipping mutation express high PD‐L1 (> 50%) and that the overall response rate of patients treated by ICIs was as low as 33%.14 We considered that this low efficacy of ICIs in those with both high PD‐L1 expression and a MET exon 14 skipping mutation might be explained by a low tumor mutation burden (TMB). Cancer with driver mutations is characterized by low TMB, even with high PD‐L1 expression.15, 16 In fact, most patients with a MET exon 14 skipping mutation had low (56%) or low‐intermediate (32%) TMB.17 On the other hand, the responsiveness of pulmonary sarcomatoid carcinomas, which are likely to have a MET exon 14 skipping mutation, to ICIs can be explained by their high TMB.18, 19 Although we did not measure TMB in the present case, the lack of efficacy of pembrolizumab could be a result of low TMB. Mazieres and Gautschi reported that patients with a MET mutation showed intermediate progression‐free survival (3.4 months) after immunotherapy compared to those with other driver mutations, although PD‐L1 and TMB status were not shown.20
A therapeutic strategy for NSCLC with both high PD‐L1 expression and driver mutation has not been established. Chen et al. reported that PEM‐based chemotherapy was effective in NSCLC patients with EGFR mutation, ALK fusion, and ROS 1 rearrangement (overall response rates: 25.5%, 28.1%, and 57.9%, respectively).21 Our case also indicated the effectiveness of pemetrexed‐based chemotherapy for NSCLC with a MET exon 14 skipping mutation.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors are grateful to the patients and their families, and the investigators, nurses, and staff members who participated in this study, especially Dr. Gotoh and Dr. Matsumoto at the National Cancer Center Central Hospital who performed next generation sequences.
References
- 1. Shea M, Costa DB, Rangachari D. Management of advanced non‐small cell lung cancers with known mutations or rearrangements: Latest evidence and treatment approaches. Ther Adv Respir Dis 2016; 10: 113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 6. Reis H, Metzenmacher M, Goetz M et al MET expression in advanced non‐small cell lung cancer: Effect on clinical outcomes of chemotherapy, targeted therapy, and immunotherapy. Clin Lung Cancer 2018; 19: e441–63. [DOI] [PubMed] [Google Scholar]
- 7. Tong JH, Yeung SF, Chan AW et al MET amplification and exon 14 splice site mutation define unique molecular subgroups of non‐small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016; 22: 3048–56. [DOI] [PubMed] [Google Scholar]
- 8. Tan X, Dai L, Wang Y, Liang G, Yang N, Chen M. Responses to crizotinib and disease monitoring with circulating tumor cells in lung adenocarcinoma patient with MET exon 14 skipping mutation, a case report. Medicine 2017; 96: e8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daoud A, Chu QS. Targeting novel but less common driver mutations and chromosomal translocations in advanced non‐small cell lung cancer. Front Oncol 2017; 7: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu SY, Gou LY, Li AN et al The unique characteristics of MET exon 14 mutation in Chinese patients with NSCLC. J Thorac Oncol 2016; 11: 1503–10. [DOI] [PubMed] [Google Scholar]
- 11. Waqar SN, Morgensztern D, Sehn J. MET mutation associated with responsiveness to crizotinib. J Thorac Oncol 2015; 10: e29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gainor JF, Shaw AT, Sequist LV et al EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016; 22: 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarfaty M, Moore A, Neiman V et al RET fusion lung carcinoma: Response to therapy and clinical features in a case series of 14 patients. Clin Lung Cancer 2017; 18: e223–32. [DOI] [PubMed] [Google Scholar]
- 14. Sabari JK, Montecalvo J, Chen R et al PD‐L1 expression and response to immunotherapy in patients with MET exon 14‐altered non‐small‐cell lung cancers (NSCLC). J Clin Oncol 2017; 35: 8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saito M, Shimada Y, Shiraishi K et al Development of lung adenocarcinomas with exclusive dependence on oncogene fusions. Cancer Res 2015; 75: 2264–71. [DOI] [PubMed] [Google Scholar]
- 16. Spigel DR, Schrock AB, Fabrizio D et al Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD‐1/PD‐L1 targeted therapies. J Clin Oncol 2016; 34: 9017. [Google Scholar]
- 17. Schrock AB, Frampton GM, Suh J et al Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016; 11: 1493–502. [DOI] [PubMed] [Google Scholar]
- 18. Schrock AB, Li SD, Frampton GM et al Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol 2017; 12: 932–42. [DOI] [PubMed] [Google Scholar]
- 19. Zheng D, Wang R, Ye T et al MET exon 14 skipping defines a unique molecular class of non‐small cell lung cancer. Oncotarget 2016; 7: 41691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazieres J, Drilon AE, Mhanna L et al Efficacy of immune‐checkpoint inhibitors (ICI) in non‐small cell lung cancer (NSCLC) patients harboring activating molecular alterations. J Clin Oncol 2018; 36: (15_Suppl): Abstract: 9010. [Google Scholar]
- 21. Chen YF, Hsieh MS, Wu SG et al Efficacy of pemetrexed‐based chemotherapy in patients with ROS1 fusion‐positive lung adenocarcinoma compared with in patients harboring other driver mutations in East Asian populations. J Thorac Oncol 2016; 11: 1140–52. [DOI] [PubMed] [Google Scholar]


