Abstract
Background
It has been reported that there are more circulating tumor cells (CTCs) in the pulmonary vein (PV) than in the peripheral blood; however, it is unclear whether the CTC count changes in the PV after resection of a lung lobe.
Methods
Thirty‐three lung cancer patients were recruited for the study, including 17 who underwent lobectomy via video‐assisted thoracoscopic surgery and 16 via open thoracotomy. Sixty‐six blood specimens were sampled from the PV before the PV was interrupted and after lobectomy. The CTCs were quantified using the oHSV1‐hTERT‐GFP method.
Results
Before PV interruption, the CTC (pre‐CTC) detection rate was 79.0% (26/33), the mean number of CTCs was 3.36 (median 2, range: 0–18), and there was no significant relationship between the pre‐CTC count and clinical factors, such as histologic findings and pathological T stage (P > 0.05). After lobectomy, the CTC (post‐CTC) detection rate was 100% (33/33), the average number of CTCs was 14.88 (median 11, range: 1–69), and the post‐CTC count was significantly higher in patients in whom the PV was interrupted prior to the pulmonary artery (PA) than in patients in whom the PA was interrupted before the PV (P = 0.016). Overall, the CTC count was significantly higher following surgery (P < 0.001).
Conclusion
Post‐CTC counts were significantly higher than pre‐CTC counts, suggesting that surgical manipulation may potentially dislodge tumor cells into the PV. Interrupting the PV prior to the PA during lobectomy may prevent partial CTC entry into the circulation.
Keywords: Circulating tumor cell (CTC), lung cancer, oHSV1‐hTERT‐GFP, pulmonary vein, surgical manipulation
Introduction
Circulating tumor cells (CTCs), originating from primary or metastatic tumor sites, mobilize into the bloodstream as a result of intrinsic factors, such as active migration and invasion, or extrinsic factors, such as passive shedding by surgical manipulation, biopsy, and other iatrogenic sources. When CTCs circulate in peripheral blood, only approximately 0.02% can survive the adverse environment,1 which includes mechanical shear forces, immune attack, oxidative stress, and continuous mobility.2, 3 CTCs are very rare in peripheral blood, with an approximate detectable rate of 1 in 106–107 leukocytes.4 Therefore, it is critical to build CTC detection technology with high sensitivity and specificity.
In previous studies, we established a novel technique for CTC capture based on telomerase‐specific, replication‐selective oncolytic herpes‐simplex‐virus‐1 that targets telomerase reverse transcriptase positive cancer cells and expresses green fluorescent protein (oHSV1‐hTERT‐GFP).5 Compared to the CellSearch system (Menarini Silicon Biosystems, Huntington Valley, PA, USA), our method, which does not rely on cell surface markers, detects CTCs with greater sensitivity and specificity in lung cancer patients.6 Furthermore, living tumor cells can be isolated for further analysis of CTC phenotypes and genotypes.
Lobectomy with mediastinal lymph node dissection is the standard treatment for early stage lung cancer. However, 30% of patients suffer recurrence and metastasis after surgical resection.7 This may be the result of tumor cell dissemination and seeding before or during surgical treatment.1 Several studies have demonstrated higher CTC counts in the pulmonary vein (PV) than in peripheral vessels, even in early stage lung cancer.8, 9 However, few studies have explored the effect of surgical manipulation on CTCs in the PV.10 In addition, most CTC detection approaches are epithelial cell adhesion molecule (EpCAM)‐dependent, which may miss EpCAM negative cells (i.e. low‐expressing tumor cells) and capture EpCAM+ epithelial cells, leading to inaccurate results.8, 9, 10, 11, 12
Using our highly effective oHSV1‐hTERT‐GFP method, we conducted this prospective study to assess differences in CTC count in the PV before and after resection of the lobe via video‐assisted thoracoscopic surgery (VATS‐L) or open thoracotomy (OT‐L).
Methods
Patients and surgical procedure
We enrolled 33 patients with peripheral‐type lung cancer from the Department of Thoracic Surgery at Beijing Chest Hospital affiliated with Capital Medical University between January and May 2018. After receiving a clear diagnosis of lung cancer based on preoperative biopsy or identification of a larger tumor by positron emission tomography (PET) scan, the patients underwent lobectomy via VATS or OT. Before surgery, thoracic‐enhanced computed tomography (CT) scanning, brain magnetic resonance imaging, isotope bone scanning, abdomen B‐ultrasound or abdominal enhanced CT were routinely performed to exclude overt distant metastases. The evaluation of tumor stage was based on the 8th edition of the Tumor Node Metastasis (TNM) Classification for Lung Cancer.13 The inclusion criteria were as follows: (i) patients with a pathological diagnosis of lung cancer; (ii) a clean simple lobectomy with systemic mediastinal lymphadenectomy had been performed; (iii) a tumor diameter of ≤ 4 cm, as indicated by preoperative chest CT, without obvious mediastinal lymph node metastasis; (iv) after VATS‐L, the resected pulmonary lobe was gently and smoothly removed from the thoracic cavity; and (v) the resected pulmonary lobe and the residual vessels remained intact intraoperatively. The exclusion criteria were: (i) patients with a pathological diagnosis of benign tumor; (ii) other resection procedures had been performed, including wedge resection, bilobectomy, sleeve lobectomy, pneumonectomy, or first wedge resection followed by lobectomy; (iii) a tumor diameter of > 4 cm, as indicated by preoperative chest CT; (iv) the resected lung lobe could not be smoothly extracted from the thoracic cavity; and (v) excessive bleeding resulting from rupture of the resected pulmonary lobe or residual vessels. Minimally invasive surgery was performed in our center using two‐port VATS, with an operation port of 5–7 cm and an observation‐port of 2–3 cm. The Ethics Committee of Beijing Chest Hospital approved this prospective study (2018‐04). All participants provided informed written consent prior to study commencement.
Sample collection
Four milliliters of blood were obtained from the PV via a 24 or 25‐gauge disposable vein infusion needle attached to a 10 mL syringe before the PV was interrupted (pre‐CTC) (Fig 1). When the resected pulmonary lobe was removed from the thoracic cavity, another 4 mL blood sample was collected from the residual end of the PV through a 10 mL syringe (post‐CTC) (Fig 2). All blood samples were preserved in 4 mL ethylenediaminetetraacetic acid tubes, which were stored and transported at 2–8°C and submitted for analysis within four hours.
Figure 1.
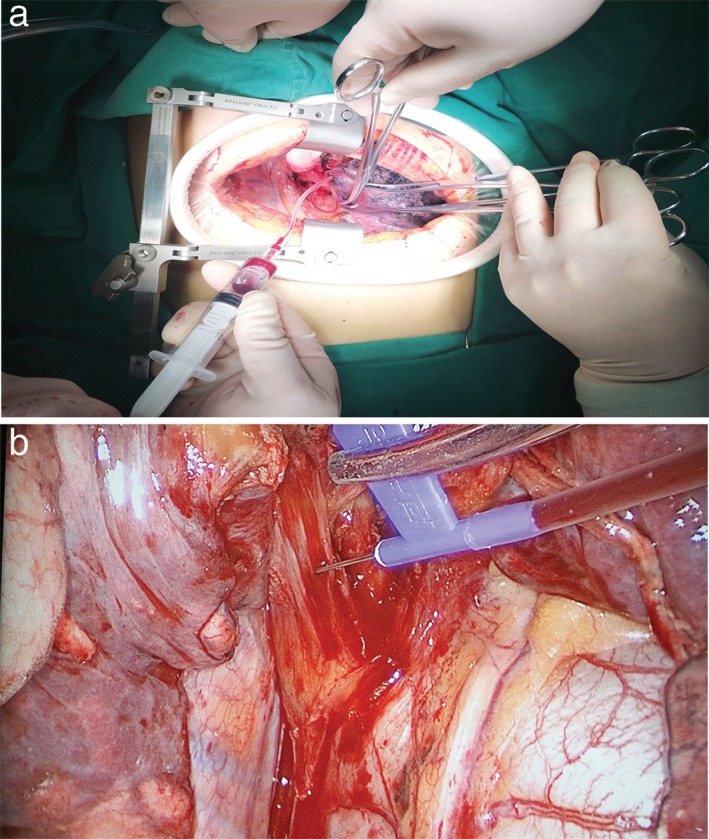
During a right lower lobe resection via (a) open thoracotomy and (b) video‐assisted thoracoscopic surgery, 4 mL pulmonary vein (PV) blood was sampled by a 24 or 25‐gauge disposable vein infusion needle attached to a 10 mL syringe before the PV was interrupted.
Figure 2.
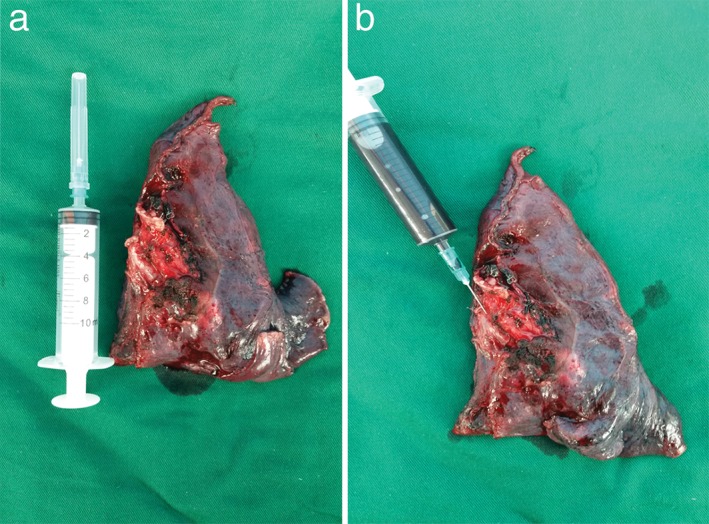
After (a) a left lower lobe was resected via video‐assisted thoracoscopic surgery (VATS), (b) a pulmonary vein (PV) blood sample was collected using a 10 mL syringe.
Detection of circulating tumor cells (CTCs)
The workflow of the oHSV1‐hTERT‐GFP method is briefly described as follows. Blood samples were incubated with erythrocyte lysis buffer and the remaining cells were collected and seeded in a six‐well cell culture plate with transfection reagent (oHSV1‐hTERT‐GFP). In the following 20–24 hours, the transduced cells were incubated in a humidified atmosphere of 5% CO2 at 37°C. The transduced cells were then gathered and stained with CD45. Flow cytometry was used to identify CTCs: negative selection with CD45 labeling and positive selection with GFP expression (CD45‐ GFP+).
In addition, 4 mL postoperative PV blood was collected from one patient who underwent OT‐L because of peripheral lung cancer (3.2 cm) in his right upper lung lobe. The specimen was stained with an additional maker‐EpCAM, and CTCs were detected and validated using a FlowSight Imaging Flow Cytometer (Merck, Kenilworth, NJ, USA).
Statistics
SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used to analyze data and calculate numbers, means, medians, and minimum and maximum values for quantitative data and to determine numbers, rates, and percentages for qualitative data. If the sample distribution was normal, continuous data were compared between groups using a Student's unpaired t‐test. If the sample distribution was asymmetric, continuous data were compared between groups using a non‐parametric Mann–Whitney U test. Comparison of pre‐CTC and post‐CTC counts was conducted using a non‐parametric Wilcoxon's signed rank test. P < 0.05 was considered statistically significant.
Results
Demographic patient information
Among the 33 enrolled patients, 18 were male, and 15 were female, with an average age of 61 years (median 63, range: 40–75 years). Fifteen patients had a smoking history. Twenty‐one patients were at pathological stage I, six at stage II, and six at stage III. Postoperative pathologic diagnoses indicated that 25 patients had adenocarcinomas, 3 had squamous cell carcinomas, 1 had small cell lung cancer, 1 had large cell lung cancer, and 3 had mixed cancers. Fourteen patients had a preoperative CT biopsy, 4 patients had a preoperative bronchoscopic biopsy, and biopsies were not performed in 15 patients. Finally, tumor markers (TMs) with single carcinoembryonic antigen (CEA) positive or single soluble fragment of cytokeratin‐19 (CYFR21‐1) positive were found in 10 patients, while the other 23 patients were negative. All clinical factors are presented in Table 1.
Table 1.
Demographic data of 33 lung cancer patients
| Variable | No. of patients | Percentage | |
|---|---|---|---|
| Gender | Male | 18 | 54.5% |
| Female | 15 | 45.5% | |
| Age | Mean, median, range (years) | 61, 63, 40–75 | |
| Smoking history | Smoker | 15 | 45.5% |
| Non‐smoker | 18 | 54.5% | |
| TMs | Positive | 10 | 30.3% |
| Negative | 23 | 69.7% | |
| LND classification | Solid nodule | 30 | 90.9% |
| Part solid nodule | 3 | 9.1% | |
| Preoperative biopsy | Yes | 18 | 54.5% |
| No | 15 | 45.5% | |
| Sequence of vessel interruption | PV‐PA | 19 | 57.6% |
| PA‐PV | 14 | 42.4% | |
| Histology | Adenocarcinoma | 25 | 75.8% |
| Sq + others | 8 | 24.2% | |
| pT stage | Mean, median, range (cm) | 2.7, 2.5, 1.2–4.5 | |
| p stage | I | 21 | 63.6% |
| II | 6 | 18.2% | |
| III | 6 | 18.2% | |
| pN stage | N0 | 23 | 69.7% |
| N1–2 | 10 | 30.3% | |
| Vessel/lymphatic invasion | Positive | 10 | 30.3% |
| Negative | 23 | 69.7% | |
| Pleural invasion | Positive | 5 | 15.2% |
| Negative | 28 | 84.8% |
LND, lung nodule density; PA, pulmonary artery; pN stage, pathological N stage; pT stage, pathological T stage; PV, pulmonary vein; Sq, squamous cell carcinoma; TMs, tumor markers.
Correlation between the number of CTCs in the pulmonary vein and clinical factors
Before the PV was interrupted, the pre‐CTC detection rate was 79.0% (26/33), the mean pre‐CTC count was 3.36 (median 2, range: 0–18), and there was no significant correlation between the pre‐CTC count and clinical factors (age, gender, smoking history, TMs, classification of lung nodule density, preoperative biopsy findings, histologic findings, sequence of vessel interruption, and pathological T and N stage; P > 0.05) (Table 2).
Table 2.
Associations between clinical variables and CTC count
| Variable | No. of patients | Median pre‐CTC | P | Median post‐CTC | P | Change | P |
|---|---|---|---|---|---|---|---|
| Male | 18 | 3.5 | 10.5 | 9 | |||
| Female | 15 | 2 | 11 | 7 | |||
| Age | 0.052 | 0.486 | 0.929 | ||||
| ≥ 63 | 18 | 2 | 10 | 7.5 | |||
| < 63 | 15 | 4 | 12 | 11 | |||
| Smoking history | 0.762 | 0.986 | 0.901 | ||||
| Smoker | 15 | 2 | 10 | 10 | |||
| Non‐smoker | 18 | 2 | 11.5 | 7.5 | |||
| TMs | 0.451 | 0.923 | 0.862 | ||||
| Positive | 10 | 3 | 10 | 8 | |||
| Negative | 23 | 2 | 11 | 7 | |||
| LND classification | 0.614 | 0.288 | 0.211 | ||||
| Solid nodule | 30 | 2 | 11.5 | 9 | |||
| Part solid nodule | 3 | 4 | 9 | 4 | |||
| Preoperative biopsy | 0.630 | 0.073 | 0.067 | ||||
| Yes | 18 | 2.5 | 14 | 11 | |||
| No | 15 | 2 | 10 | 6 | |||
| Sequence of vessel interruption | 0.957 | 0.016 | 0.014 | ||||
| PV‐PA | 19 | 3 | 15 | 11 | |||
| PA‐PV | 14 | 2 | 7 | 4.5 | |||
| Histology | 0.655 | 0.236 | 0.162 | ||||
| Adenocarcinoma | 25 | 2 | 10 | 7 | |||
| Sq + Others | 8 | 2 | 14 | 12.5 | |||
| pT stage | 0.427 | 0.839 | 0.985 | ||||
| T1b–1c | 21 | 3 | 12 | 8 | |||
| T2a–2b | 12 | 1.5 | 10.5 | 7.5 | |||
| pN stage | 0.475 | 0.221 | 0.207 | ||||
| N0 | 23 | 2 | 12 | 11 | |||
| N1‐2 | 10 | 3 | 8 | 7 | |||
| Vessel/lymphatic invasion | 0.550 | 0.180 | 0.253 | ||||
| Positive | 10 | 3.5 | 13.5 | 12.5 | |||
| Negative | 23 | 2 | 10 | 6 | |||
| Pleural invasion | 0.542 | 0.314 | 0.338 | ||||
| Positive | 5 | 5 | 11 | 11 | |||
| Negative | 28 | 2 | 10.5 | 7 |
CTC, circulating tumor cell; LND, lung nodule density; PA, pulmonary artery; pN stage, pathological N stage; pT stage, pathological T stage; PV, pulmonary vein; Sq, squamous cell carcinoma; TMs, tumor markers.
After resection of the lobe, the post‐CTC detection rate was 100% (33/33), the average number was 14.88 (median 11, range: 1–69), and the post‐CTC count was significantly higher in patients in whom the PV was interrupted prior to the pulmonary artery (PA; V‐first) than in patients in whom the PA was interrupted before the PV (A‐first) (P = 0.016) (Table 2, Fig 3d). Increases in the CTC (post‐CTC count minus pre‐CTC count) were significantly higher in the V‐first than in the A‐first group (P = 0.014).
Figure 3.
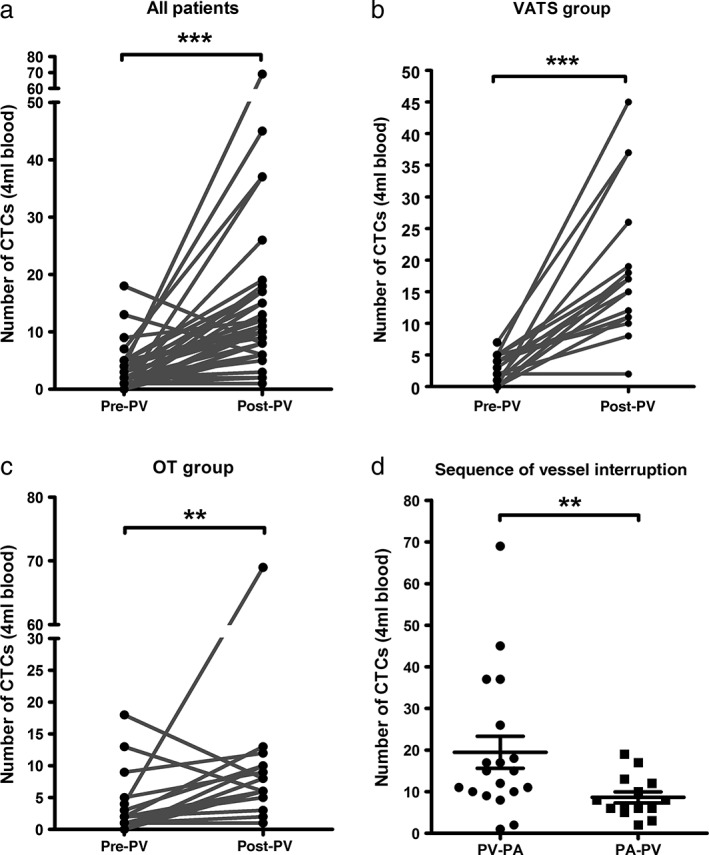
Comparison of the change in circulating tumor cell (CTC) counts among (a) all patients, (b) the video‐assisted thoracoscopic surgery‐lobectomy (VATS‐L) group and (c) the open thoracotomy‐lobectomy (OT‐L) group. (d) Post‐CTC counts were significantly higher when the pulmonary vein (PV) was interrupted prior to the pulmonary artery (PA), compared to when the PV was interrupted prior to the PV. (a–c) Wilcoxon's signed rank and (d) Mann–Whitney U tests were used to analyze significant differences (**P < 0.01; ***P < 0.001).
Comparison of pre‐CTC and post‐CTC counts
Post‐CTC counts were significantly higher than pre‐CTC counts (P < 0.001). Furthermore, compared to pre‐CTC counts, the post‐CTC counts were significantly higher in both the VATS‐L (P < 0.001) and the OT‐L (P = 0.031) groups (Table 3, Fig 3a–c).
Table 3.
Comparison of pre‐CTC and post‐CTC counts
| Pre‐CTC | Post‐CTC | P | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | ||
| VATS‐L | 2.82 | 3 | 0–7 | 18.65 | 17 | 2–45 | < 0.001 |
| OT‐L | 3.94 | 2 | 0–18 | 10.88 | 7 | 1–69 | 0.031 |
| All patients | 3.36 | 2 | 0–18 | 14.88 | 11 | 1–69 | < 0.001 |
CTC, circulating tumor cell; OT‐L, open thoracotomy‐lobectomy; VATS‐L, video‐assisted thoracoscopic surgery‐lobectomy.
Verification of circulating tumor cells by FlowSight image
To identity whether CTCs from PV blood in lung cancer patients were captured by our detection method, we used FlowSight imaging to validate its accuracy. A representative FlowSight image is provided in Figure 4. The cells identified as CTCs by GFP‐expression were also marked with an EpCAM antibody.
Figure 4.
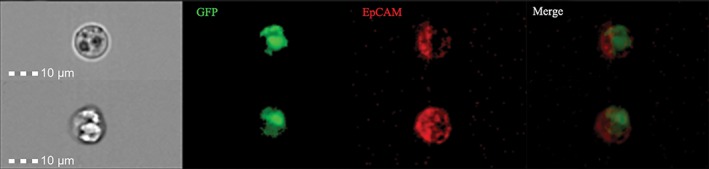
Typical circulating tumor cells (CTCs) in the pulmonary vein (PV) were visualized using green fluorescent protein (GFP) expression. Blood specimens were incubated with oHSV1‐hTERT‐GFP and then stained with allophycocyanin‐labeled anti‐epithelial cell adhesion molecule (EpCAM) antibody. From left to right, pictures were taken sequentially in white light, then using green (GFP) and red (EpCAM) fluorescence. The picture on the far right depicts the overlap of all fluorescence. Scale 10 μm.
Discussion
In the present study, we used a CTC detection method (oHSV1‐hTERT‐GFP) independent of EpCAM to isolate CTCs in the PV. Our primary aim was to evaluate the difference in CTC count in the PV following lobectomy. Data showed that the number of post‐CTCs increased significantly, both after VATS‐L and OT‐L. Analyses of correlation between CTC counts and clinical factors found that the sequence of vessel interruption influenced CTC counts.
Independent of tumor stage, the number of CTCs in the PV after lobectomy was significantly higher than that in preoperative PV and peripheral blood. Okumura et al. showed that there were more CTCs in postoperative PV than in peripheral blood.9 Reddy et al. used microfluidic chip technology based on EpCAM and a series of surface makers and reached the same conclusion.8 In a study of 30 lung cancer patients who underwent open thoracotomy, Hashimoto et al. detected CTCs in PV using the CellSearch method and found that postoperative CTC counts were significantly higher than preoperative counts;10 our findings are consistent with these results. Furthermore, patients in our study all underwent simple lobectomies performed by either OT or VATS, adding weight to our results. However, more evidence is needed to validate whether these captured CTCs are real tumor cells. In addition, follow‐up studies should be conducted to investigate whether post‐CTC levels contribute to long‐term survival. Hashimoto et al. reported that patients with an increased number of CTCs after surgery are more susceptible to distant metastasis.14
Some research suggests that CTC count in the PV is related to clinical and histological factors. Reddy et al. reported that the number of CTCs in the PV was associated with pathological tumor size.8 Furthermore, patients who underwent preoperative bronchoscopic biopsy (9 cases) had more PV CTCs than those who underwent CT‐guided biopsy (12 cases). In a recent study, Chudasama et al. recruited 20 patients awaiting endobronchial cryotherapy treatment and found that 75% had increased CTC counts after the treatment.15 We did not find that preoperative biopsy was related to PV CTC counts, possibly because all of the patients in our study had peripheral‐type lung cancer. Tumor histology may also correlate with CTC count. As Okumura et al. reported, compared to adenocarcinoma patients, squamous carcinoma patients have a higher incidence of CTCs in their peripheral blood after surgery.15 Moreover, Hashimoto et al. reported that lymphatic tumor invasion was associated with higher CTC counts in postoperative PV.10 However, inconsistent with these results, our study found that the sequence of vessel interruption impacted CTC counts: interrupting the PV resulted in higher post‐CTC counts than interrupting the PA first.
The high PV CTC counts observed after surgery show that surgical manipulation can dislodge tumor cells shedding from primary tumors. CTC shedding may also be impacted by the biological characteristics of tumors or clinical factors, such as histologic type, tumor size, preoperative biopsy, and classifications of lung nodule density. These shedding tumor cells from the primary site will drain through the PV into the circulation during lobectomy; however, when the PV is interrupted, the residual end of the PV plays a role as a “reservoir” to intercept the CTCs and prevents them from entering the circulation. Thus, CTCs will accumulate in the residual end of the PV. This may be the reason why the post‐CTC counts were significantly higher than the pre‐CTC counts and why more CTCs were detected when interrupting the PV prior to the PA than when the PA was interrupted prior to the PV. The pre‐CTC counts indicated that shedding tumor cells are running through the PV with the bloodstream and are occasionally captured at the time of sampling. Therefore, we postulate that the sequence of pulmonary vessel interruption16, 17 and the timing of PV interruption determines whether or not the shedding tumor cells will travel through the PV. Thoracic surgeons should be cognizant of the principle of no‐touch tumor18 and preferential PV interruption. PV CTCs are characterized not only by a large quantity but also by robust activity because of the absence of an immune attack and mechanical shear forces in the circulation. Further research involving CTCs is warranted, including drug sensitivity experiments and molecular characterization analyses by single cell sequences.
Because of our small sample size, we did not find evidence of a correlation between postoperative PV CTC counts and tumor histology, classification of lung nodule density, and preoperative biopsy. However, we plan to address this shortcoming in future research.
In conclusion, our results confirm that CTC counts significantly increase following lobectomy. This finding may be explained by surgical manipulation, possibly dislodging tumor cells into the PV during lobectomy. Interrupting the PV prior to the PA during surgery may prevent partial CTC entry into the circulation.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This research was supported by grants from the National Key R&D Program of China (No. 2017YFC1308702, No. 2017YFC1308700), the CAMS Initiative for Innovative Medicine (No. 2017‐I2M‐1‐005), and the National Natural Science Foundation of China (No. 81472013).
References
- 1. Martin OA, Anderson RL, Narayan K, MacManus MP. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol 2017; 14: 32–44. [DOI] [PubMed] [Google Scholar]
- 2. Steinert G, Scholch S, Niemietz T et al Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 2014; 74: 1694–704. [DOI] [PubMed] [Google Scholar]
- 3. Zheng Y, Miyamoto DT, Wittner BS et al Expression of beta‐globin by cancer cells promotes cell survival during blood‐borne dissemination. Nat Commun 2017; 8: 14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manicone M, Poggiana C, Facchinetti A, Zamarchi R. Critical issues in the clinical application of liquid biopsy in non‐small cell lung cancer. J Thorac Dis 2017; 9: S1346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Bao L, Yang S et al Tumor‐selective replication herpes simplex virus‐based technology significantly improves clinical detection and prognostication of viable circulating tumor cells. Oncotarget 2016; 7: 39768–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao H, Liu W, Yang S et al Detection of circulating tumor cells using oHSV1‐hTERT‐GFP in lung cancer. Thorac Cancer 2018; 9: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early‐stage non‐small cell lung cancer: A systematic review of the video‐assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008; 86: 2008–2016–8. [DOI] [PubMed] [Google Scholar]
- 8. Reddy RM, Murlidhar V, Zhao L et al Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early‐stage lung cancer. J Thorac Cardiovasc Surg 2016; 151: 852–8. [DOI] [PubMed] [Google Scholar]
- 9. Okumura Y, Tanaka F, Yoneda K et al Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg 2009; 87: 1669–75. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto M, Tanaka F, Yoneda K et al Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18: 775–83. [DOI] [PubMed] [Google Scholar]
- 11. Lv C, Zhao B, Wang L et al Detection of circulating tumor cells in pulmonary venous blood for resectable non‐small cell lung cancer. Oncol Lett 2018; 15: 1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsutani N, Sawabata N, Yamaguchi M et al Does lung cancer surgery cause circulating tumor cells?‐A multicenter, prospective study. J Thorac Dis. 2017; 9: 2419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 14. Hashimoto M, Tanaka F, Yoneda K et al Positive correlation between postoperative tumor recurrence and changes in circulating tumor cell counts in pulmonary venous blood (pvCTC) during surgical manipulation in non‐small cell lung cancer. J Thorac Dis. 2018; 10: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chudasama D, Rice A, Soppa G, Anikin V. Circulating tumour cells in patients with lung cancer undergoing endobronchial cryotherapy. Cryobiology 2015; 71: 161–3. [DOI] [PubMed] [Google Scholar]
- 16. Kurusu Y, Yamashita J, Hayashi N, Mita S, Fujino N, Ogawa M. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg 1998; 116: 107–13. [DOI] [PubMed] [Google Scholar]
- 17. Sienel W, Seen‐Hibler R, Mutschler W, Pantel K, Passlick B. Tumour cells in the tumour draining vein of patients with non‐small cell lung cancer: Detection rate and clinical significance. Eur J Cardiothorac Surg 2003; 23: 451–6. [DOI] [PubMed] [Google Scholar]
- 18. Wiggers T, Jeekel J, Arends JW et al No‐touch isolation technique in colon cancer: A controlled prospective trial. Br J Surg 1988; 75: 409–15. [DOI] [PubMed] [Google Scholar]


