Abstract
Background
PD‐L1 expression in tumor cells has been associated with the efficacy of immune checkpoint inhibitors in non‐small cell lung cancer (NSCLC). The aim of this study was to explore correlations between smoking, genetic profiles, patient outcomes, and PD‐L1 expression in NSCLC.
Methods
PD‐L1 expression was evaluated in 241 surgically resected specimens by immunostaining and 50% was set as the cutoff value.
Results
Of the 241 tumors analyzed, a PD‐L1 tumor proportion score (TPS) of ≥ 50% was detected in 35 cases (14.5%) and a TPS of < 50% in 206 cases (85.5%). A PD‐L1 TPS ≥ 50% was significantly associated with smoking and EGFR wild‐type status (P < 0.001 and P = 0.039, respectively). Detailed assessment of smoking variables showed that total smoking duration was a predictor of a PD‐L1 TPS ≥ 50% (P = 0.001). Univariate and multivariate survival analyses revealed that patients with a PD‐L1 TPS ≥ 50% had poorer disease‐free and overall survival than those with a PD‐L1 TPS < 50% (P = 0.001 and P < 0.001, respectively).
Conclusion
The incidence of a PD‐L1 TPS ≥ 50% was significantly higher in smoking and EGFR wild‐type NSCLC patients, particularly in long‐term smokers. A PD‐L1 TPS of ≥ 50% was an independent adverse prognostic factor for survival in patients with NSCLC.
Keywords: Driver mutation, non‐small cell lung cancer, programmed death ligand 1, smoking
Introduction
Remarkable progress has been made in the treatment of lung cancer in recent years. Blockade of immune checkpoints with monoclonal antibodies has recently emerged as a new therapeutic tool for lung cancer.1, 2, 3, 4, 5 Immune responses are fine‐tuned and regulated through a combination of stimulatory and inhibitory molecules and signal pathways. PD‐L1 binds PD‐1 as counter receptors to offer signals that control and suppress cytotoxic T lymphocyte responses in both autoimmune responses and evasion of tumor immunity.6 Consequently, clinical trials of blocking monoclonal antibodies (mAbs) against PD‐1 and PD‐L1 in a variety of solid tumors have shown promising results and have validated this pathway as a therapeutic target. The KEYNOTE‐024 clinical trial demonstrated that pembrolizumab, an anti‐PD‐1 immune checkpoint inhibitor, is associated with longer progression‐free and overall survival (OS) than platinum‐based chemotherapy in advanced non‐small cell lung cancer (NSCLC) patients with a PD‐L1 tumor proportion score (TPS) of ≥ 50%.7, 8 Therefore, evaluation of the relationship between clinicopathological characteristics and a PD‐L1 TPS ≥ 50% might provide valuable information to predict benefit for patients receiving first‐line immunotherapy.
Although the association between PD‐L1 expression and clinicopathological characteristics in NSCLC has already been examined, the relationship between oncogenic driver mutations, smoking history, and PD‐L1 expression status remains unclear. Recent studies have demonstrated that high PD‐L1expression is more frequently found in resected NSCLC patients with a smoking history,9, 10, 11 while other studies have found no relationship.12 Furthermore, detailed analysis of the association between PD‐L1 expression and smoking variables was not performed in these studies. Several studies have revealed that the level of PD‐L1 expression is significantly higher in patients with ALK fusion or EGFR mutation, and these driver oncogenic alterations induce PD‐L1 expression by activating downstream signaling pathways in NSCLC.13 However, other studies have shown conflicting results.14, 15 Therefore, precise analysis of PD‐L1 expression and correlations with oncogenic driver mutations and smoking is still worthwhile. In this study, we assessed PD‐L1 expression in surgically resected NSCLC patients by SP263 monoclonal antibody and analyzed the correlations of PD‐L1 expression with cigarette smoking, driver oncogenic alterations, and patient outcomes using a cutoff value of 50% PD‐L1 TPS.
Methods
Patients and samples
We retrospectively screened 241 NSCLC patients who underwent surgery at the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS, Beijing, China) between June 2012 and April 2013. Clinicopathological features, including age, gender, smoking history, histology, pathologic tumor node metastasis (TNM) stage (the American Joint Committee on Cancer 8th edition Lung Cancer Staging system), and EGFR and KRAS mutation status were studied. In addition, detailed assessments of smoking variables were also analyzed, including the average number of cigarettes smoked per day, total smoking duration, and cumulative pack‐years. After surgery, routine examinations, including chest computed tomography and blood tests (including serum tumor markers), were performed at three‐month intervals for the first three years and at six‐month intervals thereafter. The Ethics Committee of the Cancer Hospital, CAMS, approved this study protocol and all patients provided written informed consent prior to study commencement.
Immunohistochemical analysis of PD‐L1
Immunohistochemistry (IHC) was conducted using a fully automated Ventana Benchmark XT stainer with the pre‐diluted Ventana PD‐L1 Rabbit monoclonal primary antibody (SP263, CAT No. 740‐4907; Ventana Medical Systems, Roche Group, Tuscon, AZ, USA). Tumor cells showing membranous staining for PD‐L1 were evaluated as positive cells. The TPS was used to evaluate PD‐L1 expression, which was the percentage of PD‐L1 positive tumor cells showing partial or complete membrane staining in the overall tumor sections. We classified PD‐L1 expression into three levels: PD‐L1 TPS ≥ 50%, PD‐L1 TPS 1–49% and PD‐L1 TPS < 1%. Two experienced observers assessed all immunohistochemical images; if the independent judgments did not agree, the observers reviewed the slides together to achieve consensus.
Detection of EGFR and KRAS mutations
Mutation detection was carried out as previously described.16 Briefly, to determine mutation status, four exons that code for the tyrosine kinase domain of the EGFR gene (exons 18–21) and two exons of the KRAS gene (codons 12, 13) were examined.
Statistical analysis
The relationship between PD‐L1 expression and clinicopathologic variables was evaluated statistically by Pearson's χ2 or Fisher's exact test as appropriate. Multivariable analysis was performed using a logistic regression model to investigate the association between PD‐L1 expression and patient characteristics. Disease‐free survival (DFS) was considered as the period between surgery and the date of the recurrence, and OS as the period between surgery and the date of the last follow‐up or death. These rates were estimated using the Kaplan–Meier method with the log rank test. Cox proportional hazards regression analysis was performed to assess the hazard ratios for positive risk factors. Statistical tests were two‐sided, and the significance level for all analyses was set at P < 0.05. Statistics were calculated using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The clinicopathologic characteristics of patients, driver mutation status, and PD‐L1 TPS are reported in Table 1. The majority of patients were female (56.0%) and never smokers (60.2%). Almost all patients had a diagnosis of adenocarcinoma (95.0%). KRAS mutations were detected in 23 out of 241 patients (9.5%) and EGFR mutations in 79: 36 patients with a deletion in exon 19, 37 with an L858R mutation in exon 21, and 6 patients with other mutations. A PD‐L1 TPS ≥ 50% was observed in 35 cases (14.5%), a PD‐L1 TPS 1–49% in 53 cases (22.0%), and a PD‐L1 TPS < 1% in 153 cases (63.5%) (Fig 1).
Table 1.
Patient characteristics, driver mutation status, and PD‐L1 TPS
| Characteristics | No. of patients |
|---|---|
| Gender | |
| Male | 106 (44.0%) |
| Female | 135 (56.0%) |
| Age (years) | |
| Median | 56 |
| Range | 24–77 |
| Smoking history | |
| Never | 145 (60.2%) |
| Current/former | 96 (39.8%) |
| Histology | |
| Adenocarcinoma | 229 (95.0%) |
| Squamous cell carcinoma | 8 (3.3%) |
| Adenosquamous carcinoma | 4 (1.7%) |
| p stage | |
| IA | 40 (16.6%) |
| IB | 46 (19.1%) |
| IIA | 3 (1.2%) |
| IIB | 39 (16.2%) |
| IIIA | 91 (37.8%) |
| IIIB | 22 (9.1%) |
| EGFR status | |
| Wild‐type | 162 (67.2%) |
| Exon 19 deletion | 36 (14.9%) |
| Exon 21 L858R | 37 (15.4%) |
| Others† | 6 (2.5%) |
| KRAS status | |
| Wild‐type | 218 (90.5%) |
| Mutated | 23 (9.5%) |
| PD‐L1 TPS | |
| < 1% | 153 (63.5%) |
| 1–49% | 53 (22.0%) |
| ≥ 50% | 35 (14.5%) |
Others: exon 18, S768I and insertion, exon 21 L861Q, complex mutation with exon 19 deletion + T790M.
TPS, tumor proportion score.
Figure 1.
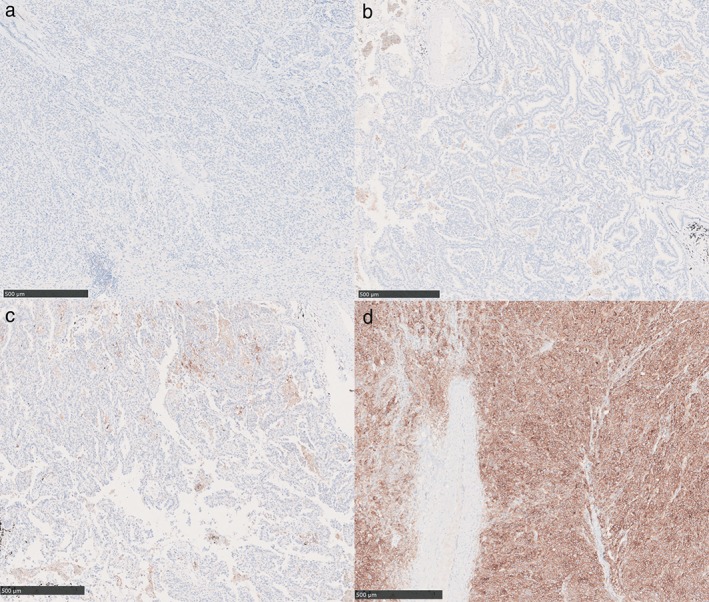
PD‐L1 tumor proportion score (TPS) immunohistochemistry (IHC) results in non‐small cell lung cancer patients using a SP263 antibody on a fully automated Ventana Benchmark XT stainer. (a) Negative staining for PD‐L1; (b) PD‐L1 TPS of 1–10%; (c) PD‐L1 TPS of 11–49%; (d) PD‐L1 TPS of ≥ 50%.
Association between PD‐L1 expression and clinicopathological characteristics
The association between a PD‐L1 TPS ≥ 50% and clinicopathological characteristics was examined by Pearson's χ2 or Fisher's exact test (Table S1). A PD‐L1 TPS ≥ 50% was significantly associated with smoking (P < 0.001) and wild type EGFR (P = 0.012). No significant association was found between a PD‐L1 ≥ 50% and gender (male vs. female, P = 0.885), age (> 56 vs. ≤ 56 years, P = 0.060), histology (adenocarcinoma vs. non‐adenocarcinoma, P = 1.000), pathologic tumor stage (I or II vs. III, P = 0.880), or KRAS status (wild‐type vs. mutated, P = 0.054). Multivariate analysis by logistic regression also revealed that a PD‐L1 TPS ≥ 50% was significantly associated with smoking (P < 0.001) and wild type EGFR status (P = 0.032) (Table S2). The relationship between a PD‐L1 TPS ≥ 50% and smoking variables, including average number of cigarettes smoked per day, total smoking duration, and cumulative pack‐years was assessed in smokers. Positive associations were observed between a PD‐L1 TPS ≥ 50% and total smoking duration (P = 0.001) (Table S3).
Univariate and multivariate survival analyses in non‐small cell lung cancer patients
The median follow‐up time for all 241 patients was 30 months (range: 3–66 months). NSCLC patients with a PD‐L1 TPS ≥ 50% had significantly shorter DFS and OS compared to those with a PD‐L1 TPS < 50% (P = 0.001 and P < 0.001, respectively) (Figs 2, 3).
Figure 2.
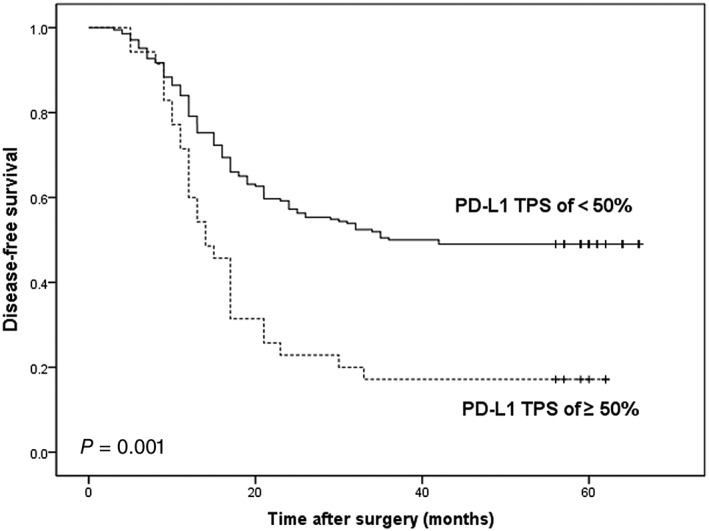
Kaplan–Meier analysis of disease‐free survival according to a PD‐L1 tumor proportion score (TPS) of < 50% or ≥ 50% in non‐small cell lung cancer patients.
Figure 3.
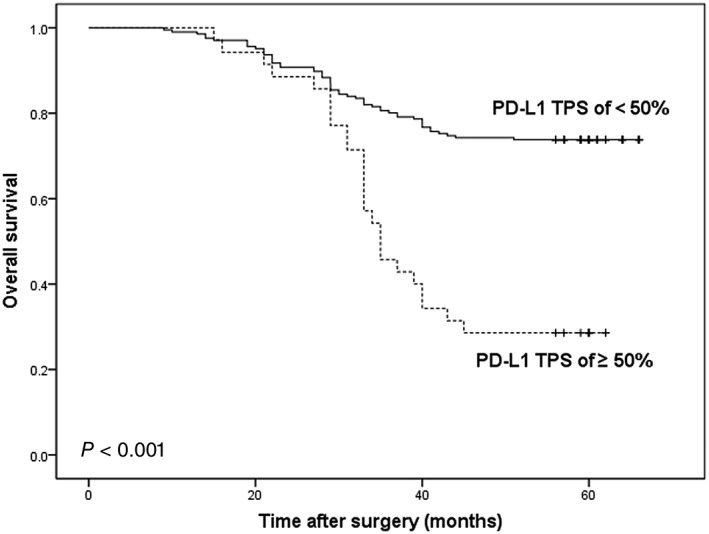
Kaplan–Meier analysis of overall survival according to a PD‐L1 tumor proportion score (TPS) of < 50% or ≥ 50% in non‐small cell lung cancer patients.
Cox proportional hazards regression models showed that male gender, smoking, advanced stage, wild‐type EGFR, KRAS mutation, and a PD‐L1 TPS ≥ 50% were associated with significantly shorter OS (P = 0.036, P = 0.009, P < 0 0.001, P < 0 0.001, P < 0 0.001, and P < 0.001, respectively). In multivariate analysis, advanced stage, wild‐type EGFR, KRAS mutation, and a PD‐L1 TPS ≥ 50% remained predictors of OS (P < 0.001, P = 0.026, P = 0.040, and P < 0.001, respectively). Cox proportional hazards regression models showed that male gender, smoking, advanced stage, and a PD‐L1 TPS ≥ 50% were associated with significantly shorter DFS (P = 0.011, P = 0.010, P < 0.001, and P = 0.001, respectively), and advanced stage and a PD‐L1 TPS ≥ 50% remained predictors of DFS in multivariate analysis (P < 0.001 and P < 0.001, respectively) (Table 2).
Table 2.
Univariate and multivariate analyses of DFS and OS in all patients
| DFS | OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | N (%) | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR 95% CI P | HR 95% CI P | HR 95% CI P | HR 95% CI P | ||||||||||
| Gender | |||||||||||||
| Male | 106 (44.0%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||||
| Female | 135 (56.0%) | 0.64 | 0.46–0.90 | 0.011 | 0.79 | 0.51–1.23 | 0.300 | 0.62 | 0.40–0.97 | 0.036 | 0.77 | 0.45–1.34 | 0.361 |
| Age (years) | |||||||||||||
| ≤ 56 | 118 (49.0%) | 1.0 (ref) | 1.0 (ref) | ||||||||||
| > 56 | 123 (51.0%) | 0.97 | 0.69–1.37 | 0.871 | 1.02 | 0.65–1.58 | 0.946 | ||||||
| Smoking history | |||||||||||||
| Never | 145 (60.2%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||||
| Current/former | 96 (39.8%) | 1.57 | 1.12–2.22 | 0.010 | 1.00 | 0.62–1.62 | 0.994 | 1.81 | 1.16–2.81 | 0.009 | 0.87 | 0.48–1.57 | 0.635 |
| Histology | |||||||||||||
| Adenocarcinoma | 229 (95.0%) | 1.0 (ref) | 1.0 (ref) | ||||||||||
| Non‐Adenocarcinoma | 12 (5.0%) | 1.41 | 0.66–3.03 | 0.373 | 1.84 | 0.80–4.23 | 0.152 | ||||||
| p stage | |||||||||||||
| I/II | 128 (35.7%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||||
| III | 113 (64.3%) | 3.87 | 2.67–5.61 | < 0.001 | 3.87 | 2.66–5.64 | < 0.001 | 2.87 | 1.79–4.62 | < 0.001 | 2.72 | 1.68–4.42 | < 0.001 |
| EGFR status | |||||||||||||
| Wild‐type | 162 (67.2%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||||||||
| Mutated | 79 (32.8%) | 0.70 | 0.48–1.02 | 0.065 | 0.53 | 0.31–0.92 | < 0.001 | 0.48 | 0.25–0.91 | 0.026 | |||
| KRAS status | |||||||||||||
| Wild‐type | 218 (90.5%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||||||||
| Mutated | 23 (9.5%) | 1.54 | 0.90–2.65 | 0.114 | 2.66 | 1.35–5.21 | < 0.001 | 1.88 | 1.03–3.43 | 0.040 | |||
| PD‐L1 status | |||||||||||||
| < 50% | 206 (85.5%) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||||
| ≥ 50% | 35 (14.5%) | 2.13 | 1.39–3.26 | 0.001 | 2.55 | 1.56–4.18 | < 0.001 | 2.37 | 1.24–4.54 | < 0.001 | 3.07 | 1.73–5.45 | < 0.001 |
DFS, disease‐free survival; OS, overall survival; HR, hazard ratio; ref., reference category; TPS, tumor proportion score.
Discussion
During the past few years, immune checkpoint therapies have established a new era for the treatment of patients beyond tumor types, yet the predictors of response remain largely undetermined. Although several studies have evaluated PD‐L1 expression in NSCLC, the clinicopathologic characteristics and molecular features associated with a PD‐L1 TPS ≥ 50% remain controversial. Herein, we evaluated PD‐L1 expression by IHC in 241 surgically resected NSCLC specimens and examined correlations between PD‐L1 expression and smoking history and oncogenic driver mutations. Our data revealed that 14.5% of NSCLC patients had a PD‐L1 TPS ≥ 50%, as measured via SP263 assay. A PD‐L1 TPS of ≥ 50% was significantly higher in smokers and EGFR wild‐type patients. Univariate and multivariate survival analysis revealed that patients with a PD‐L1 TPS ≥ 50% had poorer DFS and OS than those with a PD‐L1 TPS < 50%.
Carcinogens in tobacco are known to be responsible for direct DNA damage and mutagenesis in NSCLC.17 Smoking‐related lung cancers are characterized by a greater mutation burden than lung cancers occurring in never smokers.18, 19 Tumors with a greater number of somatic mutations generate more immunogenic neoantigens, which can drive immune responses, and the levels of neoantigens may correlate with the degree of immune response.20, 21 Studies based on The Cancer Genome Atlas project showed that NSCLC patients with a larger number of somatic mutations are more sensitive to immunotherapy with PD‐1/PD‐L1 inhibitors.22 Moreover, patients with high PD‐L1 expression show greater sensitivity to anti‐PD‐1/PD‐L1 inhibitors.3, 5 Therefore, tumors with excessive somatic mutation burdens tend to be associated with high PD‐L1 expression. Consistent with this data, our results revealed that smokers more frequently had a PD‐L1 TPS ≥ 50% compared to non‐smokers. In addition, persistent exposure to smoking increases the risk of chronic inflammation, which plays a vital role in the regulation of PD‐L1 expression by the interferon‐γ (IFN‐γ) driven inflammatory signaling pathway.23 IFN‐γ is a proinflammatory cytokine that is abundantly produced by T cells upon activation; binding of IFN‐γ to its receptor on tumor cells results in activation of the classic JAK‐STAT signaling pathway, inducing increased PD‐L1 expression.24 Furthermore, we performed a detailed analysis of the association between smoking variables and a PD‐L1 TPS ≥ 50%. Our data showed that tumors with a PD‐L1 TPS of ≥ 50% were more common in patients with a total smoking duration > 20 years than in those who smoked an average of > 20 cigarettes per day or cumulative pack‐years > 20. Our results suggest that total smoking duration is more predictive of a PD‐L1 TPS ≥ 50% than the average number of cigarettes smoked per day or cumulative pack‐years.
PD‐L1 expression was recently found to be elevated in NSCLC patients harboring EGFR mutations.12, 25 Several in vitro studies have shown that EGFR mutation induces PD‐L1 expression via downstream pathways mediated by MEK‐ERK, PI3K‐AKT, or STAT3 signaling pathways.26 However, in the present study, a PD‐L1 TPS of ≥ 50% was more frequently observed in patients with negative driver oncogenic alterations in EGFR, while most patients harboring EGFR mutations had a PD‐L1 TPS of < 50%. In patients with a PD‐L1 TPS ≥ 50%, subgroup analyses revealed that the majority of patients with wild‐type EGFR were smokers (24/30, 80%). Existing evidence shows that EGFR mutations are common in non‐smokers, who are likely to have low mutation burdens and inactive inflammatory signaling. Consequently, high mutation burdens and activation of the inflammatory signaling pathway as a result of smoking tend to have a greater influence on PD‐L1 expression compared to activation of the downstream signaling pathway induced by EGFR mutation. Thus, smokers are more likely to have high PD‐L1 expression than non‐smokers with EGFR mutation.
Recent studies have evaluated the prognostic effect of PD‐L1 expression in NSCLC.27, 28, 29 However, the prognostic relevance of PD‐L1 expression in NSCLC remains controversial. In our study, univariate and multivariate survival analysis showed that patients with a PD‐L1 TPS ≥ 50% showed significantly poorer DFS and OS compared to those with a PD‐L1 TPS < 50%. Immune evasion induced by the PD‐1/PD‐L1 pathway plays a significant role in NSCLC. Cancer cells can evade host immune systems by expressing PD‐L1 to downregulate cytotoxic T lymphocytes through inhibitory pathways, which are usually initiated by PD‐1/PD‐L1 interaction. Cancer cells then become uncontrollable in the host immune system, allowing cancer cells to survive and progress.30 Additionally, our data indicated that patients with EGFR mutation were significantly associated with better OS, possibly because these patients have the opportunity to receive EGFR‐tyrosine kinase inhibitors (TKIs). Ota et al. demonstrated that EGFR‐TKIs downregulate PD‐L1 expression in EGFR‐mutant NSCLC cells but not in those with wild‐type EGFR,31 which indicates that TKIs could not only could induce apoptosis of tumor cells, but also inhibit immune evasion of tumor cells by downregulating PD‐L1 expression. The benefit of TKIs for EGFR mutant patients may be partly attributed to the inhibition of tumor cell immune evasion by downregulating the expression of PD‐L1.
There were several limitations to our study. First, it was retrospective and from a single institution; thus, the possibility of bias cannot be excluded. Second, PD‐L1 expression in tumor cells was only evaluated with an SP263 antibody. A large prospective study of clinicopathological characteristics, molecular features, and prognosis of tumors with evaluated PD‐L1 expression with various antibodies is needed to validate this exploratory result.
In conclusion, our results demonstrate that a PD‐L1 TPS of ≥ 50% is associated with poor DFS and OS in NSCLC patients. Furthermore, current smokers without EGFR mutations, particularly long‐term smokers, were associated with a PD‐L1 TPS ≥ 50%, which indicates that these NSCLC patients may benefit from immunotherapy.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Patient characteristics and PD‐L1 expression status.
Table S2. Multivariate analysis of the relationship between a PD‐L1 tumor proportion score (TPS) of ≥ 50% and patient characteristics.
Table S3. Associations between cigarette smoking and PD‐L1 expression.
Acknowledgments
This work was supported by the National Key R&D Program of China (2016 YFC0905400), the CAMS Initiative for Innovative Medicine (CAMS‐2017‐I2M‐2‐003), and the Beijing Municipal Science & Technology Commission (LC2015A13).
Contributor Information
Jianming Ying, Email: jmying@hotmail.com.
Shugeng Gao, Email: shugenggao@126.com.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 4. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xia B, Herbst RS. Immune checkpoint therapy for non‐small‐cell lung cancer: An update. Immunotherapy 2016; 8: 279–98. [DOI] [PubMed] [Google Scholar]
- 7. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 8. Brahmer JR, Rodriguez‐Abreu D, Robinson AG et al Health‐related quality‐of‐life results for pembrolizumab versus chemotherapy in advanced, PD‐L1‐positive NSCLC (KEYNOTE‐024): A multicentre, international, randomised, open‐label phase 3 trial. Lancet Oncol 2017; 18: 1600–9. [DOI] [PubMed] [Google Scholar]
- 9. Takada K, Toyokawa G, Okamoto T et al A comprehensive analysis of programmed cell death ligand‐1 expression with the clone SP142 antibody in non‐small‐cell lung cancer patients. Clin Lung Cancer 2017; 18: 572–82.e571. [DOI] [PubMed] [Google Scholar]
- 10. Cao L, Wang X, Li S et al PD‐L1 is a prognostic biomarker in resected NSCLC patients with moderate/high smoking history and elevated serum SCCA level. J Cancer 2017; 8: 3251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Igawa S, Sato Y, Ryuge S, Ichinoe M et al Impact of PD‐L1 expression in patients with surgically resected non‐small‐cell lung cancer. Oncology 2017; 92: 283–90. [DOI] [PubMed] [Google Scholar]
- 12. Azuma K, Ota K, Kawahara A et al Association of PD‐L1 overexpression with activating EGFR mutations in surgically resected nonsmall‐cell lung cancer. Ann Oncol 2014; 25: 1935–40. [DOI] [PubMed] [Google Scholar]
- 13. Akbay EA, Koyama S, Carretero J et al Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov 2013; 3: 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoneshima Y, Ijichi K, Anai S et al PD‐L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer 2018; 118: 36–40. [DOI] [PubMed] [Google Scholar]
- 15. Inamura K, Yokouchi Y, Sakakibara R et al Relationship of tumor PD‐L1 expression with EGFR wild‐type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol 2016; 46: 935–41. [DOI] [PubMed] [Google Scholar]
- 16. Li W, Zhang J, Guo L, Chuai S, Shan L, Ying J. Combinational analysis of FISH and immunohistochemistry reveals rare genomic events in ALK fusion patterns in NSCLC that responds to crizotinib treatment. J Thorac Oncol 2017; 12: 94–101. [DOI] [PubMed] [Google Scholar]
- 17. Govindan R, Ding L, Griffith M et al Genomic landscape of non‐small cell lung cancer in smokers and never‐smokers. Cell 2012; 150: 1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexandrov LB, Nik‐Zainal S, Wedge DC et al Signatures of mutational processes in human cancer. Nature 2013; 500: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellmann M, Rizvi N, Wolchok JD, Chan TA. Genomic profile, smoking, and response to anti‐PD‐1 therapy in non‐small cell lung carcinoma. Mol Cell Oncol 2016; 3: e1048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 21. Efremova M, Finotello F, Rieder D, Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol 2017; 8: 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taube JM, Anders RA, Young GD et al Colocalization of inflammatory response with B7‐h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD‐L1 checkpoint. Immunity 2018; 48: 434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Incecco A, Andreozzi M, Ludovini V et al PD‐1 and PD‐L1 expression in molecularly selected non‐small‐cell lung cancer patients. Br J Cancer 2015; 112: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong S, Chen N, Fang W et al Upregulation of PD‐L1 by EML4‐ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti‐PD‐1/PD‐L1 immune therapy for ALK‐TKIs sensitive and resistant NSCLC patients. Oncoimmunology 2016; 5: e1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma G, Deng Y, Jiang H, Li W, Wu Q, Zhou Q. The prognostic role of programmed cell death‐ligand 1 expression in non‐small cell lung cancer patients: An updated meta‐analysis. Clin Chim Acta 2018; 482: 101–7. [DOI] [PubMed] [Google Scholar]
- 28. Takada K, Okamoto T, Toyokawa G et al The expression of PD‐L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer 2017; 104: 7–15. [DOI] [PubMed] [Google Scholar]
- 29. Sun JM, Zhou W, Choi YL et al Prognostic significance of PD‐L1 in patients with non‐small cell lung cancer: A large cohort study of surgically resected cases. J Thorac Oncol 2016; 11: 1003–11. [DOI] [PubMed] [Google Scholar]
- 30. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ota K, Azuma K, Kawahara A et al Induction of PD‐L1 expression by the EML4‐ALK oncoprotein and downstream signaling pathways in non‐small cell lung cancer. Clin Cancer Res 2015; 21: 4014–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics and PD‐L1 expression status.
Table S2. Multivariate analysis of the relationship between a PD‐L1 tumor proportion score (TPS) of ≥ 50% and patient characteristics.
Table S3. Associations between cigarette smoking and PD‐L1 expression.


