Abstract
Background
Although the lower lobes of the lungs occupy half of the chest on both sides, the prognostic value of tumor location in lung cancer in the lower lobe has not been well demonstrated. This study investigated the prognostic value of tumor location (basal vs. superior) in patients with resected lung adenocarcinoma in the lower lobe.
Methods
A total of 207 patients undergoing lobectomy for lung adenocarcinoma in the lower lobe were included in the study. The association between tumor location and mediastinal lymph node metastasis was analyzed. Prognostic factors of overall survival and probability of freedom from recurrence (FFR) were also investigated.
Results
During follow‐up, 71 (34.3%) patients developed recurrence. Patients with basal segment tumors had a significantly higher possibility of developing N2 lymph node metastasis than those with superior segment tumors (P = 0.025). Univariate analysis showed that location in the basal (vs. superior) segment was a significant prognostic factor for a lower probability of FFR (P = 0.013). Basal (vs. superior) segment remained a significant prognostic factor for a lower probability of FFR (P = 0.010) in multivariate analysis.
Conclusions
Basal segment tumors have a significantly higher possibility of developing N2 lymph node metastasis than superior segment tumors in resected lung adenocarcinoma in the lower lobe. Tumor location at the basal segment was a significant prognostic factor for a lower probability of FFR. This information is useful for patient stratification of risk of postoperative recurrence.
Keywords: Lower lobe, lung adenocarcinoma, recurrence, survival, tumor location
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 Surgical resection is the treatment of choice for early‐stage non‐small cell lung cancer (NSCLC).2, 3 Tumor recurrence is the most common cause of treatment failure after resection.4, 5, 6 Post‐recurrence survival in patients undergoing surgical resection for NSCLC is poor.7, 8, 9 Therefore, the identification of prognostic factors in patients with resected NSCLC is necessary to stratify high‐risk patients for further management.
The lower lobes of the lungs occupy half of the chest on both sides, contain a large volume of lung parenchyma, and extend from above the pulmonary hilum to the diaphragm. Anatomically, the lower lobe can be divided into two parts, the superior and basal segments. The prognostic factors of tumors located in a specific lobe of the lung, such as the lower lobe, have not been well demonstrated in the literature.10, 11, 12 Watanabe et al. reported that superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways to the mediastinum.10 In the present study, we investigated the relationships between tumor location (basal vs. superior segment) and clinicopathological variables, and the prognostic significance of tumor location in patients with completely resected lung adenocarcinoma in the lower lobe.
Methods
Patients
The Institutional Review Board of Taipei Veterans General Hospital approved this study. The records of all patients who underwent surgical resection for lung adenocarcinomas at Taipei Veterans General Hospital from January 2004 to December 2013 were retrospectively reviewed. Patients undergoing neoadjuvant chemotherapy or radiotherapy were excluded. A total of 876 patients who had undergone resection for lung adenocarcinoma were identified. Among them, 236 patients had tumors located at their lower lobes. Twenty‐nine of the 236 patients undergoing sublobar resection were excluded from the analysis. The remaining 207 patients underwent lobectomy for lung adenocarcinoma and were included in the study.
The preoperative staging workup, including chest and upper abdomen computed tomography (CT) scans, brain CT scan or magnetic resonance imaging, and a nuclear medicine survey of the bone, was performed as previously described.13, 14 A positron emission tomography (PET)‐CT scan was available as a staging modality in 90 (43.5%) of the 207 patients. Mediastinoscopy or endobronchial ultrasound was performed when enlarged mediastinal lymph nodes (diameter > 1.0 cm) were shown by CT scan or increased uptake at mediastinal lymph nodes was shown by PET‐CT scan. All patients underwent complete resection of the lung cancer with mediastinal lymph node dissection, as previously described.13, 14
Clinicopathological characteristics
Patients were subdivided according to tumor location into superior and basal segment groups. The tumor location was identified by the involved bronchus using preoperative chest CT scans. For tumors involving both the superior and basal segments, the segment with the main tumor volume was determined as the tumor location. Correlations between tumor location and clinicopathological variables were analyzed. To investigating their prognostic value, clinicopathological factors, including tumor location (basal vs. superior segment), were examined in univariate and multivariate analyses. Tumor size was defined as the size of the invasive components of the tumor. The determination of disease stage was based on the 8th edition American Joint Committee on Cancer Tumor Node Metastasis (TNM) Classification.15
Patients follow‐up
All patients were followed‐up at our outpatient department every three months in the first two years after resection and at six‐month intervals thereafter, as previously described.13, 14 The modalities and protocols used during follow‐up have also been previously described.9, 13, 14 CT scans of chest and upper abdomen were routinely performed at every outpatient department visit for follow‐up. A nuclear medicine survey of the bone was taken every six months in the first two years after resection and annually thereafter during follow‐up. Brain CT scan or magnetic resonance imaging was performed when neurological symptoms occurred or when clinical suspicions were raised. Secondary primary lung cancer was differentiated from recurrent NSCLC according to suggestions made by Detterbeck et al.16, 17 Overall survival (OS) was defined as the interval between the date of surgical resection and the date of either death or the last follow‐up. The period of freedom from recurrence (FFR) was defined as the interval between the date of surgical resection and the date of recurrence or the last follow‐up. An observation was censored at the last follow‐up session when the patient was alive with recurrence‐free status or had died without recurrence.
Statistical analysis
The OS and probability of FFR were calculated using the Kaplan–Meier method.18 The log‐rank test was used to make group comparisons. Univariate and multivariate analyses were performed with the Cox proportional hazards model in SPSS version 20 (IBM Corp., Armonk, NY, USA). All variables with P < 0.1 were entered into multivariable analysis, except for T status, N status, and TNM stage, for which only T and N status were entered. Statistical significance was defined as P < 0.05.
Results
The median follow‐up duration of the 207 patients was 33.9 (range 3.2–110.8) months. Patient characteristics are listed in Table 1. The five‐year OS and probability of FFR of all patients were 76.9% and 56.5%, respectively (Fig 1). Of the 207 patients, 73 (35.3%) presented with superior segment tumors, while 134 (64.7%) presented with basal segment tumors (Table 1). Among all patients, 130 (62.8%) were FFR, 71 (34.3%) developed recurrence, and 6 (2.9%) had unknown recurrence status during follow‐up. The six patients with unknown recurrence status were excluded from the analysis of FFR probability.
Table 1.
Clinicopathological variables in 207 patients with lung adenocarcinoma in the lower lobe
| Variables | All patients |
|---|---|
| Age, years (mean ± SD) | 61.6 ± 10.4 |
| Gender, N (%) | |
| Male | 98 (47.3) |
| Female | 109 (52.7) |
| Tumor location, N (%) | |
| Superior segment | 73 (35.3) |
| Basal segment | 134 (64.7) |
| Laterality, N (%) | |
| Left | 95 (45.9) |
| Right | 112 (54.1) |
| Tumor size, cm (mean ± SD) | 2.7 ± 1.4 |
| T status, no. (%) | |
| T1a | 16 (7.7) |
| T1b | 28 (13.6) |
| T1c | 16 (7.7) |
| T2 | 132 (63.8) |
| T3 | 11 (5.3) |
| T4 | 4 (1.9) |
| N status, N (%) | |
| N0 | 152 (73.4) |
| N1 | 22 (10.7) |
| N2 | 33 (15.9) |
| Stage, N (%) | |
| I | 141 (68.1) |
| II | 25 (12.1) |
| III | 41 (19.8) |
| Visceral pleural invasion, N (%) | |
| Absent | 75 (36.3) |
| Present | 129 (62.3) |
| Unknown | 3 (1.4) |
| Angiolymphatic invasion, N (%) | |
| Absent | 139 (67.2) |
| Present | 63 (30.4) |
| Unknown | 5 (2.4) |
| Histologic grade, N (%) | |
| Well differentiated | 21 (10.2) |
| Moderately differentiated | 118 (57.0) |
| Poorly differentiated | 58 (28.0) |
| Unknown | 10 (4.8) |
| No. of LNs dissected/sampled (mean ± SD) | 20.7 ± 8.8 |
| Predominant pattern, N (%) | |
| Lepidic predominant | 15 (7.2) |
| Acinar predominant | 66 (31.9) |
| Papillary predominant | 62 (30.0) |
| Micropapillary predominant | 42 (20.3) |
| Solid predominant | 22 (10.6) |
| Adjuvant chemotherapy, N (%) | |
| No | 113 (54.6) |
| Yes | 94 (45.4) |
LN, lymph node; SD, standard deviation.
Figure 1.
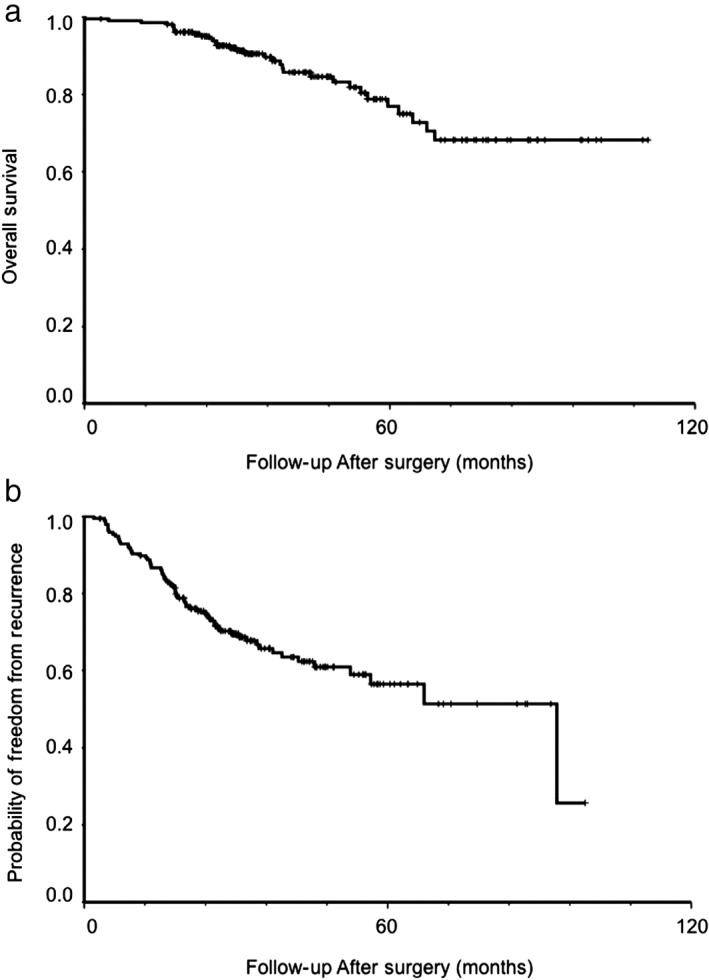
Cumulative probability of (a) overall survival, and (b) freedom from recurrence in 207 patients with resected lung adenocarcinoma in the lower lobe.
Association between tumor location (basal vs. superior segment) in the lower lobe and clinicopathological variables
Associations between tumor location (basal vs. superior segment) and clinicopathological variables are presented in Table 2. Patients with basal segment tumors had a significantly higher possibility of developing N2 lymph node metastasis than those with superior segment tumors (P = 0.025). A trend of significantly larger tumor size was observed in patients with basal segment tumors compared to those with superior segment tumors (P = 0.052). No other clinicopathological variables were significantly associated with tumor location.
Table 2.
Association between tumor location (superior vs. basal segment) and clinicopathological variables in 207 patients with lung adenocarcinoma in the lower lobe
| Variables | Superior segment (n = 73) |
Basal segment (n = 134) |
P |
|---|---|---|---|
| Age, years (mean ± SD) | 64.0 ± 10.7 | 60.6 ± 10.1 | 0.069 |
| Gender, N (%) | |||
| Male | 34 (46.6) | 64 (47.8) | 0.870 |
| Female | 39 (53.4) | 70 (52.2) | |
| Laterality, N (%) | |||
| Left | 34 (46.6) | 61 (45.5) | 0.885 |
| Right | 39 (53.4) | 73 (54.5) | |
| Tumor size, cm (mean ± SD) | 2.3 ± 1.3 | 2.7 ± 1.7 | 0.052 |
| T status, N (%) | |||
| T1 or T2 | 66 (90.4) | 126 (94.0) | 0.337 |
| T3 or T4 | 7 (9.6) | 8 (6.0) | |
| N status, N (%) | |||
| N0 or N1 | 67 (91.8) | 107 (79.9) | 0.025 |
| N2 | 6 (8.2) | 27 (20.1) | |
| TNM stage, N (%) | |||
| I | 53 (72.6) | 88 (65.7) | 0.307 |
| II or III | 20 (27.4) | 46 (34.3) | |
| Visceral pleural invasion, N (%)† | |||
| Absent | 24 (33.8) | 51 (38.3) | 0.521 |
| Present | 47 (66.2) | 82 (61.7) | |
| Angiolymphatic invasion, N (%)† | |||
| Absent | 54 (76.1) | 85 (64.9) | 0.102 |
| Present | 17 (23.9) | 46 (35.1) | |
| Histologic grade, N (%)† | |||
| Well differentiated | 9 (13.2) | 12 (9.3) | 0.395 |
| Moderately or poorly differentiated | 59 (86.8) | 117 (90.7) | |
| No. of LNs dissected/sampled (mean ± SD) | 20.8 ± 8.4 (range 4–39) | 20.7 ± 9.0 (range 5–40) | 0.976 |
| Predominant pattern group, N (%) | |||
| Lepidic/acinar/papillary predominant | 50 (68.5) | 93 (69.4) | 0.892 |
| Micropapillary/solid predominant | 23 (31.5) | 41 (30.6) | |
| Adjuvant chemotherapy, N (%) | |||
| No | 43 (58.9) | 70 (52.2) | 0.357 |
| Yes | 30 (41.1) | 64 (47.8) |
Patients with unknown status were excluded from the analysis.
LN, lymph node; SD, standard deviation; TNM, tumor node metastasis.
We further investigated the association between N2 metastasis and clinicopathological variables other than tumor location. The results showed that women (P = 0.032), larger tumors (P = 0.002), visceral pleural invasion (P = 0.043), angiolymphatic invasion (P < 0.001), histologic grade (moderately or poorly differentiated vs. well differentiated) (P = 0.033), and predominant pattern groups (micropapillary/solid vs. lepidic/acinar/papillary predominant) (P < 0.001) were significantly associated with N2 metastasis.
Overall survival in patients with resected lung adenocarcinoma in the lower lobe
Univariate analysis indicated that larger tumor size (P < 0.001), T status (T3 or T4 vs. T1 or T2; P = 0.044), N status (N2 vs. N0 or N1; P < 0.001), TNM stage (II or III vs. I; P < 0.001), angiolymphatic invasion (P = 0.037), and predominant pattern group (micropapillary/solid vs. lepidic/acinar/papillary predominant) (P = 0.007) were significant prognostic factors of poor OS (Table 3). The tumor location (basal vs. superior segment; P = 0.212) was not a significant prognostic factor of OS (Fig 2a,Table 3). In multivariate analysis, larger tumors (hazard ratio [HR] 1.604, 95% confidence interval [CI] 1.253–2.053; P < 0.001), and N status (N2 vs. N0 or N1, HR 4.755, 95% CI, 2.006–11.273; P < 0.001) were significant prognostic factors of poor OS (Table 4).
Table 3.
Univariate analyses of overall survival and probability of freedom from recurrence in 207 patients with lung adenocarcinoma in the lower lobe
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| Age, years† | 1.024 | 0.992–1.057 | 0.149 |
| Gender | |||
| Male | 1 | ||
| Female | 0.752 | 0.373–1.513 | 0.424 |
| Tumor location | |||
| Superior segment | 1 | ||
| Basal segment | 1.606 | 0.748–3.714 | 0.212 |
| Laterality | |||
| Left | 1 | ||
| Right | 0.754 | 0.376–1.511 | 0.427 |
| Tumor size‡ | 1.526 | 1.302–1.789 | < 0.001 |
| T status | |||
| T1 or T2 | 1 | ||
| T3 or T4 | 2.664 | 1.025–6.925 | 0.044 |
| N status | |||
| N0 or N1 | 1 | ||
| N2 | 4.651 | 2.309–9.368 | < 0.001 |
| N status | |||
| N0 | 1 | ||
| N1 or N2 | 3.586 | 1.782–7.217 | < 0.001 |
| TNM stage | |||
| I | 1 | ||
| II or III | 3.730 | 1.822–7.634 | < 0.001 |
| Visceral pleural invasion | |||
| Absent | 1 | ||
| Present | 1.416 | 0.652–3.076 | 0.380 |
| Angiolymphatic invasion | |||
| Absent | 1 | ||
| Present | 2.149 | 1.048–4.407 | 0.037 |
| Histologic grade | |||
| Well differentiated | 1 | ||
| Moderately or poorly differentiated | 2.775 | 0.378–20.382 | 0.316 |
| No. of LNs dissected/sampled§ | 1.025 | 0.986–1.066 | 0.211 |
| Predominant pattern group | |||
| Lepidic/acinar/papillary predominant | 1 | ||
| Micropapillary/solid predominant | 2.611 | 1.294–5.271 | 0.007 |
| Adjuvant chemotherapy | |||
| No | 1 | ||
| Yes | 1.629 | 0.810–3.278 | 0.171 |
| Probability of freedom from recurrence | |||
| Age, years† | 0.990 | 0.967–1.012 | 0.364 |
| Gender | |||
| Male | 1 | ||
| Female | 1.238 | 0.761–2.013 | 0.390 |
| Tumor location | |||
| Superior segment | 1 | ||
| Basal segment | 2.042 | 1.164–3.583 | 0.013 |
| Laterality | |||
| Left | 1 | ||
| Right | 0.930 | 0.577–1.498 | 0.765 |
| Tumor size‡ | 1.699 | 1.495–1.932 | < 0.001 |
| T status | |||
| T1 or T2 | 1 | ||
| T3 or T4 | 2.082 | 0.976–4.439 | 0.058 |
| N status | |||
| N0 or N1 | 1 | ||
| N2 | 5.946 | 3.622–9.762 | < 0.001 |
| N status | |||
| N0 | 1 | ||
| N1 or N2 | 6.557 | 3.985–10.788 | < 0.001 |
| TNM stage | |||
| I | 1 | ||
| II or III | 6.664 | 3.988–11.135 | < 0.001 |
| Visceral pleural invasion | |||
| Absent | 1 | ||
| Present | 2.365 | 1.309–4.274 | 0.004 |
| Angiolymphatic invasion | |||
| Absent | 1 | ||
| Present | 5.227 | 3.122–8.753 | < 0.001 |
| Histologic grade | |||
| Well differentiated | 1 | ||
| Moderately or poorly differentiated | 8.040 | 1.114–58.002 | 0.039 |
| No. of LNs dissected/sampled§ | 1.003 | 0.975–1.032 | 0.844 |
| Predominant pattern group | |||
| Lepidic/acinar/papillary predominant | 1 | ||
| Micropapillary/solid predominant | 3.561 | 2.195–5.776 | < 0.001 |
| Adjuvant chemotherapy | |||
| No | 1 | ||
| Yes | 3.574 | 2.109–6.058 | < 0.001 |
The hazard ratio (HR) associated with age is the increase in hazard associated with a one‐year increase in age.
The HR associated with tumor size is the increase in hazard associated with a 1 cm increase in size.
The HR associated with number of lymph nodes (LNs) dissected/sampled is an increased hazard per LN of additional LN dissection/sampling.
CI, confidence interval; TNM, tumor node metastasis.
Figure 2.
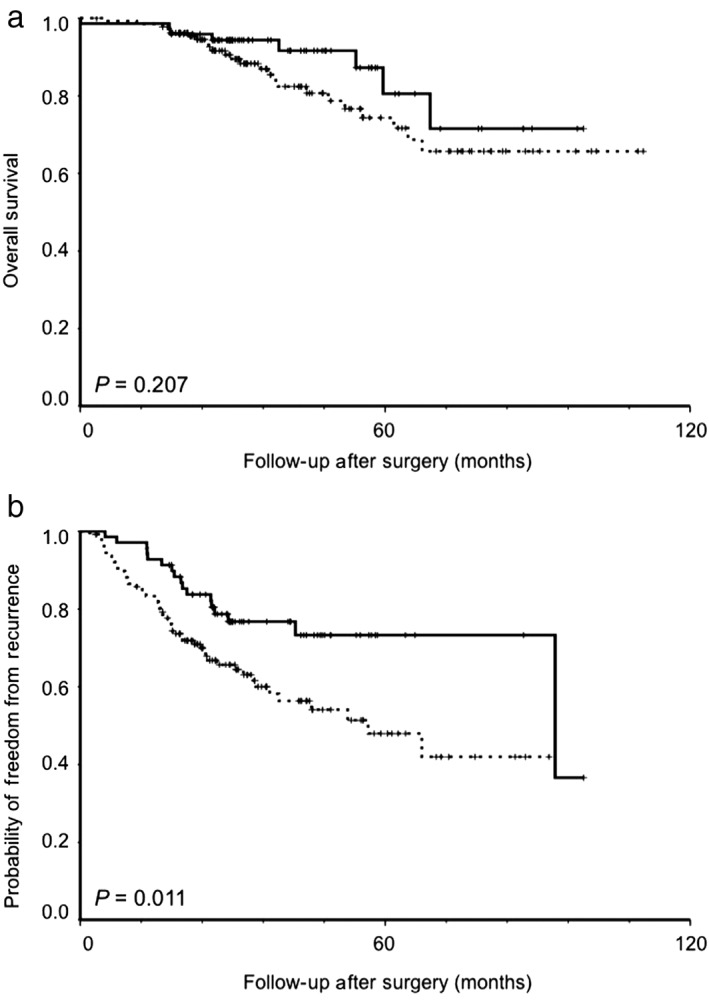
Kaplan–Meier analysis (log‐rank test) for (a) overall survival and (b) probability of freedom from recurrence in 207 patients with resected lung adenocarcinoma in the lower lobe according to tumor location (superior vs. basal segment). ( ) Superior segment, (
) Superior segment, ( ) Basal segment.
) Basal segment.
Table 4.
Multivariate analyses of overall survival and probability of freedom from recurrence in 207 patients with lung adenocarcinoma in the lower lobe
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| Tumor size† | 1.604 | 1.253–2.053 | < 0.001 |
| T status | |||
| T1 or T2 | 1 | ||
| T3 or T4 | 1.220 | 0.344–4.331 | 0.758 |
| N status | |||
| N0 or N1 | 1 | ||
| N2 | 4.755 | 2.006–11.273 | < 0.001 |
| Angiolymphatic invasion | |||
| Absent | 1 | ||
| Present | 0.671 | 0.294–1.532 | 0.343 |
| Predominant pattern group | |||
| Lepidic/acinar/papillary predominant | 1 | ||
| Micropapillary/solid predominant | 1.000 | 0.438–2.281 | 0.999 |
| Probability of freedom from recurrence | |||
| Tumor location | |||
| Superior segment | 1 | ||
| Basal segment | 2.453 | 1.242–4.846 | 0.010 |
| Tumor size† | 1.326 | 1.109–1.585 | 0.002 |
| T status | |||
| T1 or T2 | 1 | ||
| T3 or T4 | 1.927 | 0.745–4.985 | 0.176 |
| N status | |||
| N0 or N1 | 1 | ||
| N2 | 3.337 | 1.699–6.554 | < 0.001 |
| Visceral pleural invasion | |||
| Absent | 1 | ||
| Present | 0.985 | 0.487–1.992 | 0.966 |
| Angiolymphatic invasion | |||
| Absent | 1 | ||
| Present | 2.592 | 1.387–4.845 | 0.003 |
| Histologic grade | |||
| Well differentiated | 1 | ||
| Moderately or poorly differentiated | 2.800 | 0.363–21.582 | 0.323 |
| Predominant pattern group | |||
| Lepidic/acinar/papillary predominant | 1 | ||
| Micropapillary/solid predominant | 2.611 | 1.446–4.712 | 0.001 |
| Adjuvant chemotherapy | |||
| No | 1 | ||
| Yes | 0.677 | 0.314–1.458 | 0.319 |
The hazard ratio (HR) associated with tumor size is the increase in hazard associated with a 1 cm increase in size.
CI, confidence interval.
Probability of freedom from recurrence in patients with resected lung adenocarcinoma in the lower lobe
Univariate analysis indicated that tumor location (basal vs. superior segment; P = 0.013) was a significant prognostic factor for a lower probability of FFR (Fig 2b, Table 3). Larger tumor size (P < 0.001), N status (N2 vs. N0 or N1 and N1 or N2 vs. N0; both P < 0.001), TNM stage (II or III vs. I; P < 0.001), visceral pleural invasion (P = 0.004), angiolymphatic invasion (P < 0.001), histologic grade (moderately or poorly vs. well differentiated; P = 0.039), predominant pattern group (micropapillary/solid predominant vs. lepidic/acinar/papillary predominant; P < 0.001), and adjuvant chemotherapy (P < 0.001) were also significant prognostic factors for a lower probability of FFR (Table 3). In multivariate analysis, tumor location (basal vs. superior segment, HR 2.453, 95% CI, 1.242–4.846; P = 0.010), larger tumor size (HR 1.326, 95% CI 1.109–1.585; P = 0.002), N status (N2 vs. N0 or N1, HR 3.337, 95% CI, 1.699–6.554; P < 0.001), angiolymphatic invasion (HR 2.592, 95% CI 1.387–4.845; P = 0.003), and predominant pattern group (micropapillary/solid vs. lepidic/acinar/papillary predominant, HR 2.611, 95% CI 1.446–4.712; P = 0.001) were still significant prognostic factors for a lower probability of FFR (Table 4).
We further examined the prognostic value of tumor location. For multivariate analysis, N status of N1 or N2 vs. N0 was entered instead of N2 vs. N0 or N1. Tumor location (basal vs. superior segment, HR 1.986, 95% CI, 1.017–3.879; P = 0.045), larger tumor size (HR 1.358, 95% CI 1.124–1.640; P = 0.001), N status (N1 or N2 vs. N0, HR 3.151, 95% CI 1.518–6.542; P = 0.002), angiolymphatic invasion (HR 2.104, 95% CI 1.085–4.081; P = 0.028), and the predominant pattern group (micropapillary/solid vs. lepidic/acinar/papillary predominant, HR 2.084, 95% CI 1.139–3.814; P = 0.017) were still significant prognostic factors for a lower probability of FFR.
Discussion
This study investigated the association between tumor location and clinicopathological variables and the prognostic value of tumor location in patients with completely resected lung adenocarcinoma in the lower lobe. Tumor location (basal vs. superior segment) was not a significant prognostic factor of OS. However, tumor location at the basal (vs. superior) segment was a significant prognostic factor for a lower probability of FFR.
The association between lymphatic drainage pathway and tumor location in the lower lobe has not been well demonstrated. Watanabe et al. reported that superior segment tumors showed a significantly higher incidence of superior mediastinal lymph node metastasis than basal segment tumors.10 They concluded that basal segment tumors metastasize to the superior mediastinum mostly through the subcarinal lymph node, whereas superior segment tumors often metastasize directly to the superior mediastinum without concomitant metastasis to the subcarinal node.10 Although Handa et al. reported that superior segment tumors had a higher incidence of mediastinal lymph node metastasis than basal segment tumors in patients with clinical stage I (clinical N0) lower lobe NSCLC, the difference was not significant.11 Tomizawa et al. also reported that no significant difference existed in subcarinal or superior mediastinal lymph node metastasis between superior and basal segment tumors in the right lower lobe.12 Our study showed that basal segment tumors had a significantly higher possibility of developing N2 lymph node metastasis than superior segment tumors in patients undergoing lobectomy for lung adenocarcinoma in the lower lobe. There was a trend for of significantly larger tumors in patients with basal segment tumors than in those with superior segment tumors.
The prognostic factors of lung cancer in a specific lobe, for example, the lower or upper lobe, have not been well demonstrated. Several reports have examined the prognostic value of tumor location (basal vs. superior segment) in lower lobes.10, 11, 12 In a study of 139 patients with pN2 NSCLC, Watanabe et al. reported that there was no significant difference in OS between the basal and superior segment groups.10 Tomizawa et al. reported that there was no significant difference in disease‐free survival between basal and superior segment groups in 263 patients with NSCLC in the right lower lobe.12 In univariate analysis of patients with pN2 disease, they further showed that disease‐free survival in the superior segment group was significantly lower than in patients in the basal segment group.12 However, they did not perform multivariate analysis. Handa et al. reported that a superior segment tumor was an independent factor of poor OS and recurrence‐free survival in 134 patients with clinical stage I (clinical N0) lower lobe NSCLC.11 However, they mainly examined the preoperative related factors in multivariate analysis instead of pathological variables. Our results show that tumor location at the basal (vs. the superior) segment is a significant prognostic factor for a lower probability of FFR. Our results also show that larger tumor size, N status (N2 vs. N0 or N1), angiolymphatic invasion, and predominant pattern group (micropapillary/solid vs. lepidic/acinar/papillary predominant) are other significant prognostic factors for a lower probability of FFR in patients with lung adenocarcinoma in the lower lobe.
Some limitations of this study should be mentioned. As a retrospective study, patient selection bias and time trend bias are inevitable. The majority of patients in the study had stage I lung adenocarcinoma. The median follow‐up duration (33.9 months) in this study may have been too short to analyze the prognostic factors of OS in these patients. Another limitation is that N status was entered into univariate and multivariate analyses as N2 versus N0 or N1. To solve the problem, we also entered N status as N1 or N2 versus N0 in another multivariate analysis model. Prospective multi‐institutional studies and randomized clinical trials are mandatory to further validate the prognostic value of tumor location (basal vs. superior segment) on survival or recurrence in patients with lung cancer in the lower lobe.
In conclusion, basal segment tumors have a significantly higher possibility of developing N2 lymph node metastasis than superior segment tumors in resected lung adenocarcinoma in the lower lobe. Tumor location at the basal (vs. superior) segment is a significant prognostic factor for a lower probability of FFR in patients with resected lung adenocarcinoma in the lower lobe. This information is useful for patient stratification of risk of recurrence after surgery.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported in part by the Ministry of Science and Technology (MOST 107‐2314‐B‐010‐062); the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (107AC‐D970); Taipei Veterans General Hospital (V107C‐149 and V108C‐199); the National Yang‐Ming University‐Far Eastern Memorial Hospital Joint Research Program (107DN10 and 108DN14); the Taipei Veterans General Hospital‐National Yang‐Ming University‐Excellent Physician Scientists Cultivation Program (107‐V‐B‐079); the Yen Tjing Ling Medical Foundation (CI‐107‐15 and CI‐108‐20); and the Li‐Yang Sheen Medical Education Memorial Foundation. The authors are grateful to Drs Yu‐Chung Wu, Han‐Shui Hsu, Chih‐Cheng Hsieh, Chien‐Sheng Huang, and Teh‐Ying Chou of Taipei Veterans General Hospital, Taipei, Taiwan for contributions to this article. We also thank Dr. Ying‐Shiun Kao for his assistance with data collection.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger DS, Wood DE, Akerley W et al Non‐small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 2015; 13: 515–24. [DOI] [PubMed] [Google Scholar]
- 3. Goldstraw P, Chansky K, Crowley J et al The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 4. Martini N, Bains MS, Burt ME et al Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995; 109: 120–9. [DOI] [PubMed] [Google Scholar]
- 5. Harpole DH Jr, Herndon JE II, Young WG Jr et al Stage I non‐small cell lung cancer. Cancer 1995; 76: 787–96. [DOI] [PubMed] [Google Scholar]
- 6. Hung JJ, Jeng WJ, Hsu WH, Huang BS, Wu YC. Time trends of overall survival and survival after recurrence in completely resected stage I non‐small cell lung cancer. J Thorac Oncol 2012; 7: 397–405. [DOI] [PubMed] [Google Scholar]
- 7. Hung JJ, Hsu WH, Hsieh CC et al Post‐recurrence survival in completely resected stage I non‐small cell lung cancer with local recurrence. Thorax 2009; 64: 192–6. [DOI] [PubMed] [Google Scholar]
- 8. Hung JJ, Jeng WJ, Hsu WH et al Prognostic factors of post‐recurrence survival in completely resected stage I non‐small cell lung cancer with distant metastasis. Thorax 2010; 65: 241–5. [DOI] [PubMed] [Google Scholar]
- 9. Hung JJ, Yeh YC, Jeng WJ et al Prognostic factors of survival after recurrence in patients with resected lung adenocarcinoma. J Thorac Oncol 2015; 10: 1328–36. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe S, Suzuki K, Asamura H. Superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways and prognosis. Ann Thorac Surg 2008; 85: 1026–31. [DOI] [PubMed] [Google Scholar]
- 11. Handa Y, Tsutani Y, Tsubokawa N et al Clinical prognosis of superior versus basal segment stage I non‐small cell lung cancer. Ann Thorac Surg 2017; 104: 1896–901. [DOI] [PubMed] [Google Scholar]
- 12. Tomizawa K, Suda K, Takemoto T et al Prognosis and segment‐specific nodal spread of primary lung cancer in the right lower lobe. Thorac Cancer 2015; 6: 672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hung JJ, Jeng WJ, Chou TY et al Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013; 258: 1079–86. [DOI] [PubMed] [Google Scholar]
- 14. Hung JJ, Yeh YC, Jeng WJ et al Predictive value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol 2014; 32: 2357–64. [DOI] [PubMed] [Google Scholar]
- 15. American Joint Committee on Cancer . AJCC Cancer Staging Manual, 8th edn. Springer, New York, NY: 2016. [Google Scholar]
- 16. Detterbeck FC, Nicholson AG, Franklin WA et al The IASLC Lung Cancer Staging Project: Summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM Classification. J Thorac Oncol 2016; 11: 639–50. [DOI] [PubMed] [Google Scholar]
- 17. Detterbeck FC, Franklin WA, Nicholson AG et al The IASLC Lung Cancer Staging Project: Background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016; 11: 651–65. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]


