Abstract
Background
The purpose of the present study was to retrospectively evaluate the safety and efficacy of proton beam therapy (PBT) in patients with second primary lung cancer after lung resection.
Methods
Patients who were diagnosed with second primary lung cancer after lung resection and underwent PBT between January 2009 and February 2015 were retrospectively recruited. Toxicities were evaluated using Common Terminology Criteria for Adverse Events version 4.0.
Results
Nineteen patients were eligible for inclusion in this study. All of the patients completed the treatment. The median age was 75 (range: 63–82) years, and the median follow‐up time of living patients was 60 months. The median dose of PBT was 76.8 Gy relative biological effectiveness (range: 66.0–80.0 Gy). The three‐year overall survival rate was 63.2% and the three‐year local control rate was 84.2%. No grade 4 or 5 toxicities were observed after PBT.
Conclusions
Our results suggest that PBT is a safe and feasible treatment for second primary lung cancer compared to surgery or X‐ray radiotherapy. PBT may become a treatment choice for patients with second primary lung cancer after lung resection.
Keywords: Lung neoplasm, lung resection, proton beam therapy
Introduction
An estimated 1.8 million new lung cancer cases occurred worldwide in 2012, accounting for approximately 13% of the total cancer diagnoses. Lung cancer was the leading cause of cancer death among men in 2012.1 In addition to primary lung cancer, local recurrence and second primary lung cancer after lung resection for stage I lung cancer are major issues. The risk of second primary lung cancers in patients surviving resection of non‐small cell lung cancer (NSCLC) is 1–2%.2, 3, 4
Pulmonary resection is reported to be a feasible treatment choice for second primary lung cancer after careful resection and is associated with a low rate of operative mortality after pneumonectomy.5, 6, 7 However, physical function is reported to decrease in all patients after lung resection, although most had stable or improved mental health‐related quality of life.8, 9
Radiotherapy is a treatment choice for lung cancer and lung oligometastasis. Recently, an increasing number of patients have been administered stereotactic body radiotherapy (SBRT) for lung tumors, including lung metastasis with high local control.10, 11, 12 SBRT and conventional radiotherapy are also used to treat lung tumors after lung resection.13, 14, 15, 16 These results indicate that the treatments are feasible and safe, even though patients with second lung cancer undergo lung resection before this treatment.
Proton beam therapy (PBT) is a new radiotherapy technique and can deliver a more concentrated dose of radiation while sparing normal tissue than conventional radiotherapy or SBRT using X‐ray irradiation. As a result of this advantage, an increasing number of lung cancer patients choose this treatment.17, 18, 19 Therefore, PBT is also a treatment choice for second lung cancer after lung resection. However there have been no reports on the safety or efficacy of PBT after lung resection.
The purpose of the present study was to retrospectively evaluate the safety and efficacy of PBT in patients with secondary primary lung cancer after lung resection, including segmentectomy, lobectomy, and pneumonectomy.
Methods
Ethics statement
The ethics committees of our institution approved this retrospective study (approval no.: D17‐30). The study was conducted in accordance with the Declaration of Helsinki.
Patients
Patients who were diagnosed with secondary primary lung cancer and were treated with PBT after lung resection between January 2009 and February 2015 at the Southern Tohoku Proton Therapy Center were included in the study. Second primary lung cancer was defined as a lung tumor that occurred at least two years after surgery for primary lung cancer or cancer of a different histology.20 Patients were retrospectively recruited from our database. Histological confirmation of lung tumor pathology was not required as patients who did not receive bronchoscopy or biopsy because of their poor general condition or refusal were also included. If the pathology was not confirmed, an increase in the size of the lung tumor was regarded as a sign of clinical malignancy. The clinical stage of the patients’ second primary lung cancer was determined using computed tomography (CT) and positron emission tomography (PET)‐CT. Written informed consent was obtained from all patients. The inclusion criteria were: a solitary or double lung tumor after lung resection, World Health Organization performance status of 0–2, no lymph node metastasis, and no distant other organ metastasis or other sites of uncontrolled cancer. Patients who received concurrent chemotherapy were excluded.
Proton beam therapy
Treatment planning for PBT was based on three‐dimensional CT images that were taken at 2 mm intervals in the exhalation phase while using a respiratory gating system (Anzai Medical, Tokyo, Japan). A custom‐indexed vacuum‐lock bag was used to immobilize the patients. An Xio‐M treatment planning system (CMS Japan, Tokyo, Japan; Mitsubishi Electric, Tokyo, Japan) was used to calculate the dose distributions for PBT. The gross tumor volume (GTV) included the lung tumor. The clinical target volume was defined as the GTV plus 0.5 cm. The planning target volume was the clinical target volume plus a 0.5 cm margin. The proton energy levels of 150 MeV and 210 MeV for 2–3 portals, and a spread‐out Bragg peak were tuned to the extent that was possible until the planning target volume was exposed to a 90% isodose of the prescribed dose (Fig 1). The PBT system at our institute (Proton Beam System, Mitsubishi) used a synchrotron and a passive scattering method in which a proton beam passes a bar ridge filter, a range shifter, and a customized compensator before entering the patient. Treatment was administered during the exhalation phase using a respiratory gating system. A multileaf collimator, which consisted of 40 iron plates with a width of 3.75 mm that could be formed into an irregular shape, was used. Daily front and lateral X‐ray imaging was used for positioning. The PBT schedule was 66 Gy relative biological effectiveness (RBE) in 10 fractions over two weeks for peripheral lung tumors, and 80 Gy (RBE) in 25 fractions over five weeks for central or centrally located lung tumors. Patients with lung tumors that were located near the digestive tract received 66.0–76.8 Gy (RBE) in 30–33 fractions over seven weeks.
Figure 1.
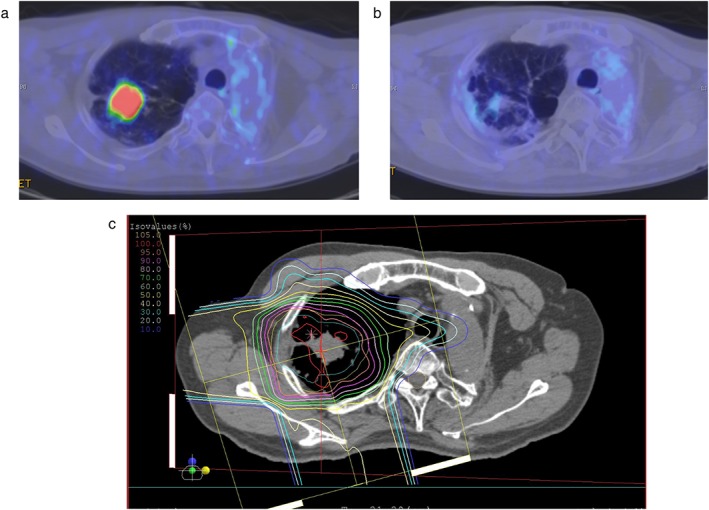
The results of proton beam therapy after pneumonectomy in a 67‐year‐old squamous cell carcinoma patient. Positron emission tomography (a) before proton beam therapy and (b) nine months after treatment. (c) The dose distribution map of proton beam therapy for a lung cancer patient with idiopathic fibrosis. The region inside the innermost orange line received a 95% radiation dose, the pink line received a 90% radiation dose, and outer line is indicated by a 10% step.
Evaluation and follow‐up
All patients underwent either CT or PET‐CT to evaluate the initial tumor response within three months after the completion of treatment. The follow‐up interval was every one to three months for the first year and every three to six months thereafter. Pneumonitis excluding infection, esophagitis, rib fracture, and dermatitis radiation were evaluated using Common Terminology Criteria for Adverse Events version 4.0.21 The following dosimetric factors were examined with the use of a dose volume histogram of the lung minus the GTV: mean lung dose, lung V5, lung V10, lung V15, lung V20, lung V25, and lung V30.
Statistical analysis
All statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA). Overall survival (OS) was defined as the interval between the start of PBT and the date of the last follow‐up examination or death. The Kaplan–Meier method was used to estimate survival probability. The relationships between the occurrence of pneumonitis and the dose volume histogram factors were examined using the Mann–Whitney U test. All P values were two sided, and P < 0.05 was considered to indicate statistical significance.
Results
Patients
The initial study population included 38 patients who had previously undergone lung resection and received PBT for lung cancer. Patients were excluded from the analysis for the following reasons: lymph node metastasis (n = 1), distant other organ metastasis (n = 6), concurrent chemotherapy (n = 2), and failure to satisfy the criteria of second primary lung cancer (n = 10). Thus, the characteristics of 19 patients, including 12 patients (63.2%) with clinically inoperable cancer, were analyzed (Table 1). The following treatments were applied for the patients’ first primary lung cancer: pneumonectomy (n = 3), bilobectomy (n = 2) lobectomy (n = 11), and segmentectomy (n = 3: one and two lesions). Two patients received postoperative radiotherapy outside of the irradiation field of present PBT (one received concurrent chemotherapy). The cohort was composed of 13 men and 6 women, with a median age of 75 (range: 63–82) years. The median follow‐up time was 42 (range: 12–102) months. The median dose of PBT was 76.8 Gy (RBE) (range: 66.0–80.0 Gy [RBE]).
Table 1.
Patient characteristics (n = 19)
| Characteristics | Patients |
|---|---|
| Age (years) | |
| Median (range) | 75 (63–82) |
| Gender | |
| Male | 13 (68%) |
| Female | 6 (32%) |
| Performance status | |
| 0 | 8 (42%) |
| 1 | 9 (47%) |
| 2 | 2 (11%) |
| Fletcher‐Hugh‐Jones classification before treatment | |
| 1 | 12 (63%) |
| 2 | 5 (26%) |
| 3 | 2 (11%) |
| Follow‐up duration (months) | |
| Median (range) | 42 (12–102) |
| Prior surgical methods | |
| Pneumonectomy | 3 (16%) |
| Bilobectomy | 2 (11%) |
| Lobectomy | 11 (57%) |
| Segmentectomy (one or two lesions) | 3 (16%) |
| Postoperative treatment | |
| Chemoradiotherapy | 1 (5%) |
| Radiotherapy | 1 (5%) |
| Chemotherapy | 2 (11%) |
| None | 13 (68%) |
| Unknown | 2 (11%) |
| Prior chemotherapy | |
| None | 15 (79%) |
| Yes | 4 (21%) |
| T category† | |
| T1 | 13 (68%) |
| T2 | 4 (21%) |
| T3 | 2 (11%) |
| Stage† | |
| I | 17 (89%) |
| II | 2 (11%) |
| Tumor location | |
| Right upper lobe | 5 (26%) |
| Right middle lobe | 1 (5%) |
| Right lower lobe | 6 (32%) |
| Left upper lobe | 3 (16%) |
| Left lower lobe | 4 (21%) |
| Time from lung resection (months) | |
| median (range) | 96 (40–324) |
| Peripheral or central | |
| Peripheral | 7 (37%) |
| Central | 12 (63%) |
| Histopathology | |
| Squamous cell carcinoma | 5 (26%) |
| Adenocarcinoma | 3 (16%) |
| Clinical malignancy | 11 (57%) |
| Diameter of lung tumor (mm) | |
| Median (range) | 22.3 (11.0–43.0) |
| Dose per fraction | |
| 6.6 Gy (RBE) per fraction | 7 (37%) |
| 3.2 Gy (RBE) per fraction | 8 (42%) |
| 2.4 Gy (RBE) per fraction | 3 (16%) |
| 2.2 Gy (RBE) per fraction | 1 (5%) |
| Total dose (Gy [RBE]) | |
| Median (range) | 76.8 (66.0–80.0) |
Numbers correspond to the 8th edition International Union Against Cancer Tumor Node Metastasis Classification.
RBE, relative biological dose effectiveness.
Survival
The median follow‐up duration of living patients was 60 (35–102) months. The one, two, and three‐year OS rates were 89.5% (95% confidence interval [CI] 75.8–100%), 73.7% (95% CI 53.9–93.5%), and 63.2% (95% CI 41.4–85.0%), respectively (Fig 2). The median survival time was 57 (95% CI 21.6–92.4) months. Five patients died as a result of lung cancer (metastasis n = 3; local recurrence n = 2), and 6 patients died of other causes (another newly diagnosed cancer after PBT n = 2; other diseases n = 4).
Figure 2.
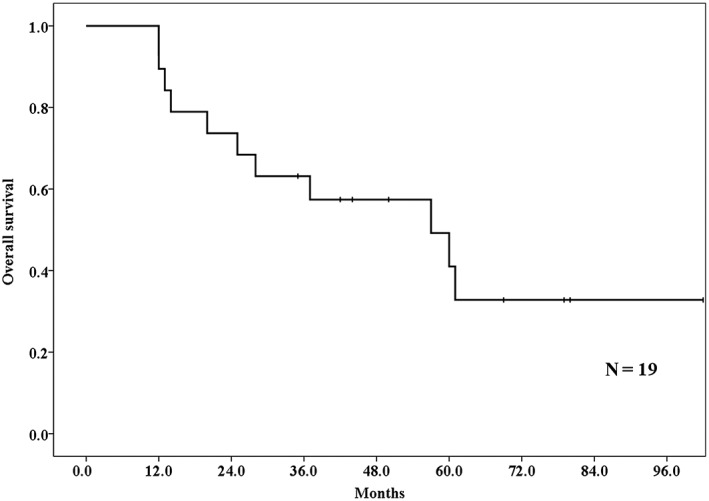
Overall survival rates of lung tumor patients who received proton beam therapy after lung resection. The one, two, and three‐year overall survival rates were 89.5%, 73.7%, and 63.2%, respectively.
Failure patterns
Three patients had local recurrence at 9–12 months. The three‐year local control rate was 84.2% (95% CI 67.7–100%) (Fig 3). Two of the patients did not receive additional treatment because of their poor general condition, and one received chemotherapy after local recurrence. Three had lymph node metastasis and two had lung metastasis outside of the PBT field. Regarding other organ metastasis, one patient had liver metastasis.
Figure 3.
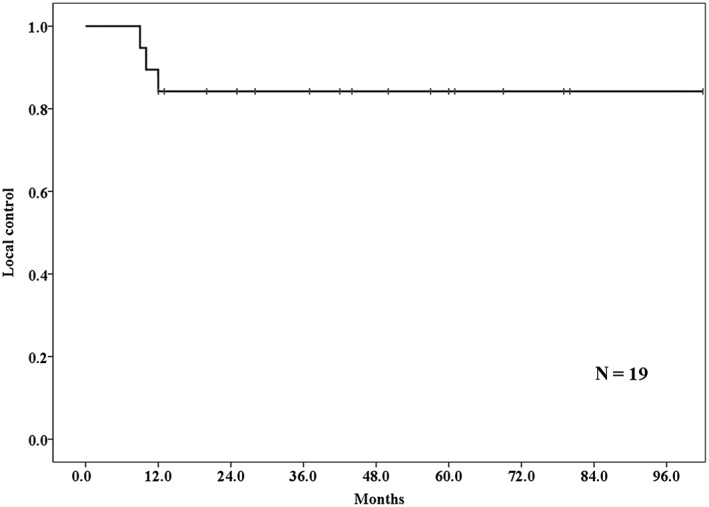
The local control rate among lung tumor patients who received proton beam therapy after lung resection. The three‐year local control rate was 84.2%.
Toxicities
No incidence of grade 4 or 5 toxicities occurred after treatment (Table 2). The toxicities of the 19 patients included: 3 (16%) cases of grade 2 pneumonitis (after lobectomy n = 2, after segmentectomy n = 1), 1 (5%) case of grade 2 esophageal ulcers; and 5 (26%) cases of grade 2 dermatitis radiation. One patient (5%) who had undergone right upper and middle lobectomy seven years before PBT developed grade 3 pneumonitis three months after receiving PBT. The patient had dyspnea, and was treated with steroid therapy and oxygenation. His dyspnea was relieved within one week, and the steroid dosage was gradually tapered. There were no statistically significant differences with regard to the dosimetric factors in relation to the lung and the occurrence of grade 2–5 pneumonitis, although the dosimetric factors for the patients with grade 2–5 pneumonitis tended to be higher than those of other patients (Table 3).
Table 2.
Toxicities
| Toxicities | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4/5 |
|---|---|---|---|---|---|
| Pneumonitis | 3 (16%) | 12 (63%) | 3 (16%) | 1 (5%) | 0 |
| Esophagitis | 18 (95%) | 0 | 1 (5%) | 0 | 0 |
| Rib fracture | 16 (84%) | 3 (16%) | 0 | 0 | 0 |
| Dermatitis radiation | 3 (16%) | 11 (58%) | 5 (26%) | 0 | 0 |
Table 3.
Relationship between dose volume histogram parameters and incidence of grade 2 pneumonitis
| Grade ≥ 2 pneumonitis (n = 4) mean ± SD | Grade < 2 pneumonitis (n = 15) mean ± SD | P | |
|---|---|---|---|
| MLD | 9.6 ± 4.3 | 5.4 ± 1.7 | 0.141 |
| Lung V5 | 24.7 ± 9.5 | 14.4 ± 3.3 | 0.118 |
| Lung V10 | 21.9 ± 8.4 | 12.3 ± 3.2 | 0.104 |
| Lung V15 | 19.8 ± 7.6 | 10.9 ± 3.2 | 0.097 |
| Lung V20 | 18.0 ± 6.9 | 9.7 ± 3.1 | 0.093 |
| Lung V25 | 16.0 ± 5.9 | 8.7 ± 3.0 | 0.085 |
| Lung V30 | 14.2 ± 5.3 | 7.8 ± 2.9 | 0.092 |
MLD, mean lung dose; SD, standard deviation.
Discussion
To the best of our knowledge, this is the first report on the use of PBT for patients with second primary lung cancer after lung resection. Our results show that PBT is a safe and feasible treatment for patients with second primary lung cancer after lung resection.
Pulmonary resection is widely performed for patients with second primary lung cancer or metastases. Pulmonary resection is also performed after pneumonectomy, and Grodzki et al. reported that the two‐year OS rate of patients who underwent this procedure was 61%.5 Our results revealed superior two‐year OS, even though more than half of the patients had a clinically inoperable status. This may be because PBT causes less lung and physical damage than surgery for the treatment of lung cancer. With regard to operative mortality, Voltolini et al. reported an overall hospital mortality rate of 7.4%.22 Pastorino et al. reported that the mortality rates among patients who underwent sublobar resection, lobectomy, and pneumonectomy were 0.6%, 1.2%, and 3.6%, respectively.23 These results suggest that a larger resection volume causes a higher mortality rate; however, no treatment related death occurred in the present study. Thus, PBT for patients after lung resection achieves a comparable OS rate and is safer than surgery.
With regard to radiotherapy, SBRT has been administered to lung tumor patients, even after pneumonectomy.13, 14 Haasabeek et al. reported that the one and two‐year OS rates were both 91%13 which is superior to our results. This may have been a result of the shorter median follow‐up time (the follow‐up time of 40% of the patients was < 12 months) in the Haasabeek et al. study. Senti et al. reported the results of SBRT or conventional radiotherapy for patients with second primary lung cancer after pneumonectomy, and reported a three‐year OS rate of 63%,14 the same as our three‐year OS rate. These findings suggest that PBT after lung resection is not superior to SBRT or conventional radiotherapy in terms of survival. Regarding local control, these reports showed 92–100% local control; our local control rate was not superior comparing X‐ray radiotherapy.13, 14 These distinctions may result from the fact that the RBE of protons and X‐rays are similar.
Pneumonitis after radiotherapy, including PBT, is a major issue. Table 4 summarizes the rates of grade ≥ 3 pneumonitis after radiotherapy in patients who received lung resection before radiotherapy in previous studies and the present study.13, 14, 15 Haasabeek et al. reported that grade 3 pneumonitis occurred in 6.3% of their patients (all patients received SBRT),13 while Senti et al. reported that grade ≥ 3 pneumonitis occurred in 11% of their patients (59% received SBRT and 41% received conventional radiotherapy). These cases included one patient with grade 5 pneumonitis.14 Xiong et al. reported that grade ≥ 3 pneumonitis occurred in 13% of their patients (all patients received SBRT).15 This occurrence rate was higher than the rate among lung cancer patients without lung resection history who received SBRT (1.4–2.4%).12, 24, 25 These results suggest that radiotherapy after lung resection can cause higher rates of pneumonitis than radiotherapy in patients with full lung function. This may be because the radiation volume to the lung is increased because of the loss of part of the lung. In fact, a high radiation volume in the lung has been reported as a cause of pneumonitis.12, 24 On the other hand, only one patient had grade 3 pneumonitis (5.3%) in the present study. This may be because PBT can deliver a more concentrated dose in smaller treatment fields than SBRT. Kadoya et al. reported that PBT for stage I lung cancer can reduce the lung dose compared to SBRT.17 With regard to dosimetric factors, Barriger et al. reported that lung mean lung dose and V20 are associated the incidence of symptomatic pneumonitis, and Matsuo et al. reported that the lung V20 and V25 were associated with the development of radiation pneumonitis.24, 25 These findings suggest that a radiotherapy to a smaller lung volume results in a lower incidence of pneumonitis; however, we found no significant correlation between dosimetric factors and the presence of pneumonitis. Thus, the use of PBT in the treatment of patients with lung tumors after lung resection may be performed more safely than SBRT or conventional X‐ray irradiation.
Table 4.
Summary of incidence of grade ≥ 3 pneumonitis after radiation therapy in lung resected patients
| Study | Number of patients | Treatment | Pneumonitis |
|---|---|---|---|
| Haasabeek et al.13 | 15 | SBRT | 1 (6.3%) |
| Senti et al.14 | 27 | SBRT or conventional RT | 3 (11%) |
| Xiong et al.15 | 23 | SBRT | 4 (13%) |
| Present study | 19 | PBT | 1 (5.3%) |
PBT, proton beam therapy; RBE, relative biological dose effectiveness; RT, radiotherapy; SBRT, stereotactic body radiotherapy.
The present study is associated with three limitations. First, the patient sample was small because it involved the treatment results of single institution. However, other reports concerning the treatment of patients after lung resection also had small study populations. Second, this was a retrospective rather than a prospective study. Third, we included various lung resection procedures. However, there are few reports on the application of PBT for the treatment of lung tumors after lung resection, and not all patients are eligible for further surgery after lung resection because of decreased physical function.8, 9 Thus, evidence of PBT for second lung cancer after lung resection is meaningful.
Our results suggest that PBT is a safe and feasible treatment for second primary lung cancer in patients after lung resection compared to surgery, SBRT, and conventional radiotherapy. PBT may become a treatment choice for second primary lung cancer patients after lung resection.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank the staff of the Radiation Oncology section.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Martini N, Bains MS, Burt ME et al Incidence of local recurrence and second primary tumors in resected stage I. J Thorac Cardiovasc Surg 1995; 109: 120–9. [DOI] [PubMed] [Google Scholar]
- 3. Surapaneni R, Singh P, Rajagopalan K, Hageboutros A. Stage I lung cancer survivorship: Risk of second malignancies and need for individualized care plan. J Thorac Oncol 2012; 7: 1252–6. [DOI] [PubMed] [Google Scholar]
- 4. Johnson BE, Cortazar P, Chute JP. Second lung cancers in patients successfully treated for lung cancer. Semin Oncol 1997; 24: 492–9. [PubMed] [Google Scholar]
- 5. Kittle CF, Faber LP, Jensik RJ, Warren WH. Pulmonary resection in patients after pneumonectomy. Ann Thorac Surg 1985; 40: 294–9. [DOI] [PubMed] [Google Scholar]
- 6. Grodzki T, Alchimowicz J, Kozak A et al Additional pulmonary resections after pneumonectomy: Actual long‐term survival and functional results. Eur J Cardiothorac Surg 2008; 34: 493–8. [DOI] [PubMed] [Google Scholar]
- 7. Westermann CJ, van Swieten HA, Brutel de la Rivière A, van den Bosch JM, Duurkens VA. Pulmonary resection after pneumonectomy in patients with bronchogenic carcinoma. J Thorac Cardiovasc Surg 1993; 106: 868–74. [PubMed] [Google Scholar]
- 8. Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health‐related quality of life after surgical treatment in patients with non‐small cell lung cancer: A systematic review. Lung Cancer 2013; 81: 11–26. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz RM, Yip R, Olkin I, Sikavi D, Taioli E, Henschke C. Impact of surgery for stage IA non‐small‐cell lung cancer on patient quality of life. J Commun Support Oncol 2016; 14: 37–44. [DOI] [PubMed] [Google Scholar]
- 10. Nuyttens JJ, van der Voort van Zyp NC, Verhoef C. Stereotactic body radiation therapy for oligometastases to the lung: A phase 2 study. Int J Radiat Oncol Biol Phys 2015; 91: 337–43. [DOI] [PubMed] [Google Scholar]
- 11. Rusthoven KE, Kavanagh BD, Burri SH et al Multi‐institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009; 27: 1579–84. [DOI] [PubMed] [Google Scholar]
- 12. Li Q, Swanick CW, Allen PK et al Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non‐small cell lung cancer: Exploration of clinical indications. Radiother Oncol 2014; 112: 256–61. [DOI] [PubMed] [Google Scholar]
- 13. Haasbeek CJ, Lagerwaard FJ, de Jaeger K, Slotman BJ, Senan S. Outcomes of stereotactic radiotherapy for a new clinical stage I lung cancer arising postpneumonectomy. Cancer 2009; 115: 587–94. [DOI] [PubMed] [Google Scholar]
- 14. Senthi S, Haasbeek CJ, Lagerwaard FJ et al Radiotherapy for a second primary lung cancer arising post‐pneumonectomy: Planning considerations and clinical outcomes. J Thorac Dis 2013; 5: 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiong W, Xu Q, Xu Y et al Stereotactic body radiation therapy for post‐pulmonary lobectomy isolated lung metastasis of thoracic tumor: Survival and side effects. BMC Cancer 2014; 14: 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pöttgen C, Abu Jawad J, Gkika E et al Accelerated radiotherapy and concurrent chemotherapy for patients with contralateral central or mediastinal lung cancer relapse after pneumonectomy. J Thorac Dis 2015; 7: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kadoya N, Obata Y, Kato T et al Dose‐volume comparison of proton radiotherapy and stereotactic body radiotherapy for non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79: 1225–31. [DOI] [PubMed] [Google Scholar]
- 18. Lee CH, Tait D, Nahum AE, Webb S. Comparison of proton therapy and conformal X‐ray therapy in non‐small cell lung cancer (NSCLC). Br J Radiol 1999; 72: 1078–84. [DOI] [PubMed] [Google Scholar]
- 19. Suit H, Goldberg S, Niemierko A et al Proton beams to replace photon beams in radical dose treatments. Acta Oncol 2003; 42: 800–8. [DOI] [PubMed] [Google Scholar]
- 20. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70: 606–12. [PubMed] [Google Scholar]
- 21. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published 28 May 2009; Revised Version 4.03 June 14, 2010. [Cited 7 May 2018] Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 22. Voltolini L, Paladini P, Luzzi L, Ghiribelli C, Di Bisceglie M, Gotti G. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg 2000; 18: 529–34. [DOI] [PubMed] [Google Scholar]
- 23. Pastorino U, Buyse M, Friedel G et al Long‐term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37–49. [DOI] [PubMed] [Google Scholar]
- 24. Barriger RB, Forquer JA, Brabham JG et al A dose‐volume analysis of radiation pneumonitis in non‐small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012; 82: 457–62. [DOI] [PubMed] [Google Scholar]
- 25. Matsuo Y, Shibuya K, Nakamura M et al Dose‐‐volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: e545–9. [DOI] [PubMed] [Google Scholar]


