Abstract
Approximately 50% of patients with primary lung cancer have distant metastasis at the time of their first visit, but gastric metastasis is a rare occurrence. Herein, we report a case of progressive dysphagia as the first symptom and the final diagnosis of primary lung squamous cell carcinoma metastasis. A 61‐year‐old man was diagnosed with a solitary left lower lobe tumor and a solitary carcinoma of gastric cardia suspected to be metastatic or malignant stromal tumors. After surgical resection, the final diagnoses were primary differentiated squamous cell lung cancer, metastatic squamous cell carcinoma of the stomach, and secondary lymph node metastasis. Gastrointestinal metastasis should be suspected in lung cancer patients with gastrointestinal symptoms. Gastric cancer metastasis from lung cancer is most likely the result of the aspiration of cancer cells containing sputum into the stomach.
Keywords: Gastric metastasis, primary lung cancer, squamous cell carcinoma
Introduction
Approximately 50% of patients with primary lung cancer have distant metastasis at the time of their first visit.1, 2 Gastric metastasis from lung cancer is a relatively rare development. A case was reported in our hospital, whereby the clinical data showed that the patient experienced progressive dysphagia as the first symptom of primary lung squamous cell carcinoma.
Case reports
A 61‐year‐old male patient was admitted to hospital after suffering progressive dysphagia for more than one month and fever for one week. The patient did not complain of any other obvious symptoms. In the past, this patient had smoked 60 pack‐years of cigarettes and had no drinking history. Both parents had passed away from unknown causes. The patient has four siblings, two of which were diagnosed with lung cancer. Computed tomography (CT) scan results suggested left lower lobe lung cancer with obstructive pneumonia and a left posterior mass shadow in the cardia indicated the possibility of large gastric stromal tumors (Fig 1). Results of positron emission tomography‐CT scans showed left lower lobe lung cancer and a gastric fundal mass located at cardia of the stomach, indicating malignant lesions with the high possibility of stromal tumors (Fig 2). Gastroscopic pathology results revealed a solid cancer nest infiltration (Fig 3). In the official guidelines, no specific conclusion on whether surgery can be performed for NSCLC with single gastric metastases has been made. A review of the literature revealed that patients with no or only a single mediastinal lymph node spread by the malignancy and complete surgical resection of isolated metastases may benefit from surgery. The patient underwent left lower lobe resection, cardia resection with anastomosis of the esophagus and stomach below the aortic arch, and lymphadenectomy.
Figure 1.
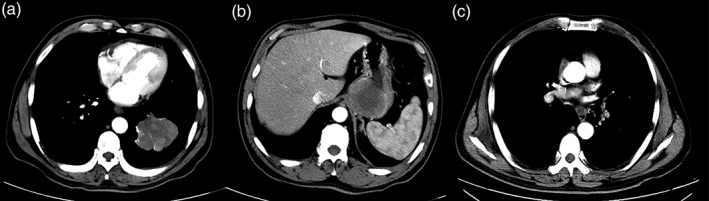
(a) Enhanced computed tomography (CT) scans show a mass shadow (6.9 cm × 6.2 cm) in the left lower lobe (CT value: 28HU plain scan; CT value: 48HU enhanced scan), considered to be lung cancer with obstructive pneumonia. (b) A mass shadow (5.8 cm × 6.3 cm) is shown in the left rear of the cardia with obvious inhomogeneous enhancement, but no abnormal surrounding lymph nodes. (c) An increase to the multiple mediastinal lymph nodes is shown.
Figure 2.
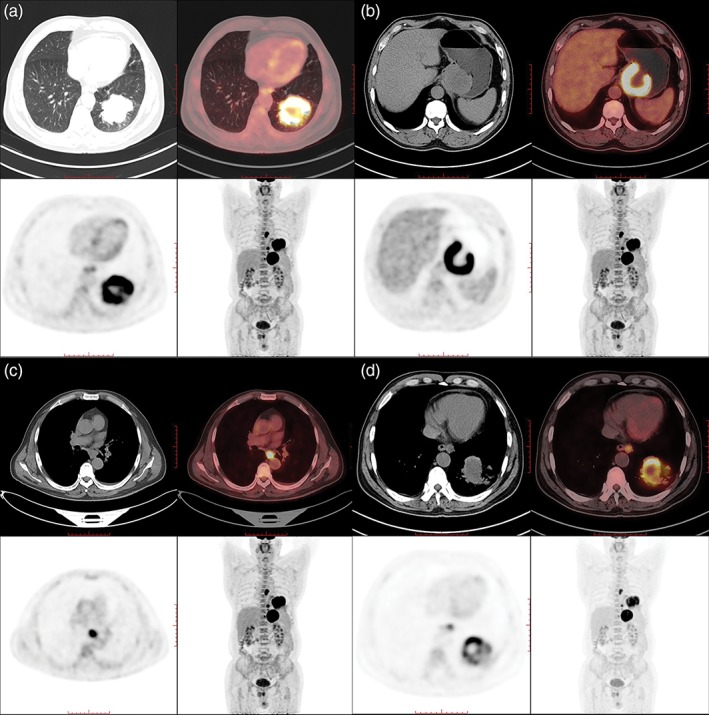
(a) A mass shadow in the left lower lobe with significant 18F‐fluorodeoxyglucose (FDG)‐avidity uptake, considered lung cancer. (b) A mass shadow in the cardia (6.3 cm × 5.4 cm × 6.4 cm) with ring‐like FDG‐avidity uptake and central radioactivity defect, considered malignant lesions. (c) The lymph nodes of the trachea carina and paraesophageal are shown with FDG‐avidity uptake, considered lymph node metastasis. (d) The lymph nodes of the abdominal aorta are shown with FDG‐avidity uptake, considered lymph node metastasis.
Figure 3.
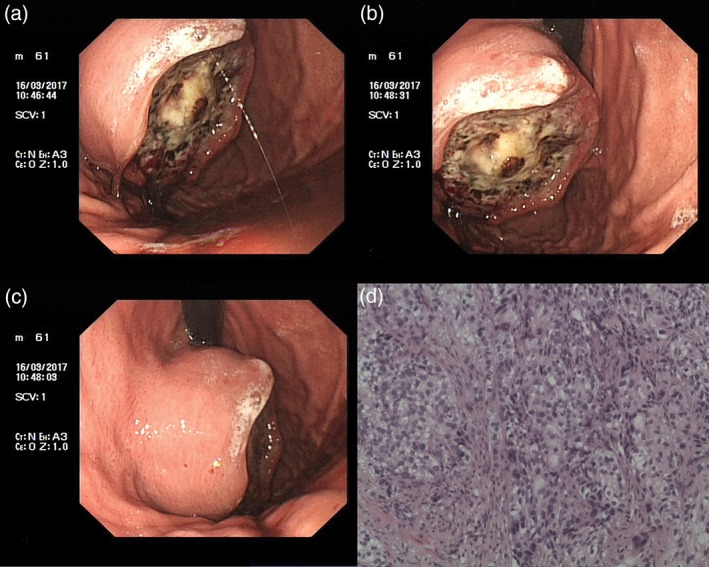
(a,b) Electron gastroscopy: An oval tumor (3 cm × 4 cm) is shown in the fornix gastricus. (c) The surface of the tumor was unclear, covering the fossil moss and tissue brittle. (d) The pathological result: (fornix gastricus) solid cancer nest infiltration can be found in all six pieces of gastric mucosa and fibrous tissue.
Postoperative pathology indicated moderately differentiated squamous cell lung cancer (lung tumor) and moderately differentiated squamous cell carcinoma (gastric mass) (Fig 4). The 7th group lymph nodes are metastatic lymph nodes, while the 8th and 9th group lymph nodes are normal lymph nodes. The results of immunohistochemistry on the lung tumor included cytokeratin (CK) 7‐, TTF‐1‐, Napsin A‐, CK5/6 +, P63 +, P40 +, CD56‐, Syn‐, chromogranin A‐, CK +, and Ki‐67 indexes of approximately 30% +. For the gastric cardia tumor, the immunohistochemical results were CK7‐, TTF‐ 1‐, Napsin A‐, CK5/6 +, P63 +, and Ki‐67 indexes of approximately 30% +. The postoperative diagnosis was moderately differentiated primary squamous cell carcinoma of the lung (pT3N2a1M1a; IVa; 8th edition Tumor Node Metastasis Staging System for Lung Cancer), moderately differentiated squamous cell carcinoma of gastric metastasis, and secondary lymph node metastasis. Since discharge, the patient has been receiving chemotherapy and is regularly followed‐up.
Figure 4.
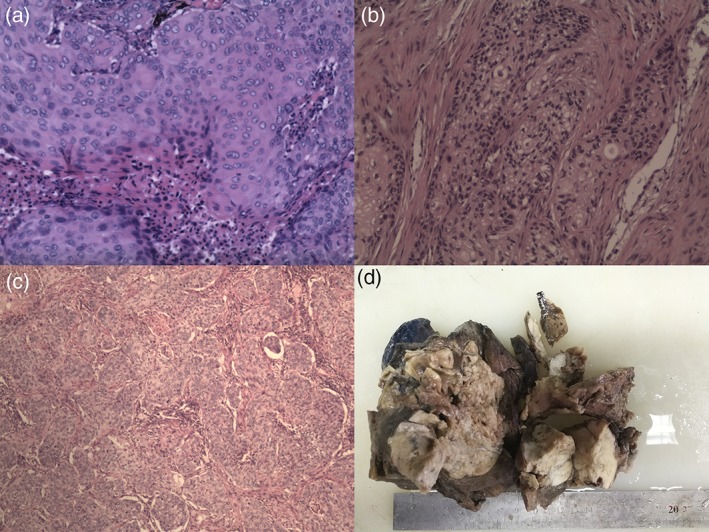
(a) Intraoperative rapid frozen section (lung tumor): moderately differentiated squamous cell carcinoma (diameter 1.4 cm). (b) Intraoperative rapid frozen section (gastric tumor): non‐small cell carcinoma infiltration can be seen in the mucous membranes of the columnar epithelium (2.5 cm × 2 cm × 1 cm). (c) The postoperative pathology report: (i) lung tumor (7 cm × 6 cm × 7 cm), moderately differentiated squamous cell lung cancer; (ii) gastric tumor (diameter 6 cm), moderately differentiated squamous cell carcinoma; (iii) the results of immunohistochemistry: (lung tumor) CK7‐, TTF‐ 1‐, Napsin A‐, CK5 / 6 +, P63 +, P40 +, CD56‐, Syn‐, CgA‐, CK + and Ki‐67 indexes of approximately 30% +; (gastric tumor) CK7‐, TTF‐ 1‐, Napsin A‐, CK5 / 6 +, P63 + and Ki‐67 indexes of approximately 30% +. (d) Left lower lobe specimens (17 cm × 12 cm × 10 cm).
Discussion
Gastrointestinal metastasis of primary lung cancer usually occurs at advanced stages of lung cancer,3 but gastric metastasis is very rare and often manifests as asymptomatic metastatic disease. Gastric metastasis commonly occurs in male smokers aged between 45–90 years.4, 5, 6, 7 The most common type of cells in gastric metastasis are squamous cell carcinoma.8, 9, 10 Although few cases of gastric metastasis have been reported, the autopsy detection rate is higher than that of clinical diagnosis. The detection rate ranges from 0.2% to 1.7%, but has been reported as high as 14%.9, 10 Yang et al. reported that lung cancer patients with stomach metastasis had relatively longer survival compared to other types of intestinal metastasis.8, 11 A final diagnosis of gastric metastasis from primary lung cancer is highly dependent on immunohistochemical results. The results of comprehensive staging show the development of gastrointestinal single organ solitary metastatic lesions, and primary non‐small cell lung cancer can be completely resected among patients with gastrointestinal metastasis. Resection for metastatic gastric cancer can be considered simultaneously with resection of the primary lung tumor.
Lung squamous cell carcinoma is derived from the bronchial epithelium and is easily spread through sputum produced by the respiratory tract. The occurrence of gastric metastasis is greatly increased if the sputum containing cancer cells is swallowed into the digestive tract, especially in smokers who are more susceptible to gastric mucosal damage. In short, a possible explanation for gastric metastasis in primary lung cancer is patients swallowing sputum‐containing cancer cells into their stomach. In this case, smoking was the main causative factor. We speculate that the patient aspirated malignant cells, which caused the gastric mass. One of the main factors affecting the metastasis of malignant cells is the health of the mucous membrane. If damage to the mucous membrane occurs and the patient aspirates multiple malignant cells, more than one gastric mass may develop. However, this explanation should be confirmed by future experiments. Isotope labeling may be an effective research method.
Gastric metastasis from lung cancer is more common in older male smokers. Squamous cell carcinoma is the most common type of lung cancer associated with the occurrence of gastric metastasis. A possible explanation is that patients swallow sputum with cancer cells into their stomach, initiating metastatic gastric cancer. However, our report has only clarified a few points and several problems need to be addressed. Further research is needed to better understand this rare disease.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors are grateful for the grant provided by the High Level Health Technical Personnel Training Project of the Beijing Health System (No. 215R057). We also wish to thank the staff that contributed to the completion of this paper.
References
- 1. Carroll D, Rajesh PB. Colonic metastases from primary squamous cell carcinoma of the lung. Eur J Cardiothorac Surg 2001; 19: 719–20. [DOI] [PubMed] [Google Scholar]
- 2. Del RM, Tsai H. Not all gastric masses are gastric cancer. BMJ Case Rep 2016; 2016: bcr2015213535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossi G, Marchioni A, Romagnani E et al Primary lung cancer presenting with gastrointestinal tract involvement: Clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol 2007; 2: 115–20. [PubMed] [Google Scholar]
- 4. Azar I, Koutroumpakis E, Patel R, Mehdi S. Squamous cell lung carcinoma presenting as melena: A case report and review of the literature. Rare Tumors 2017; 9: 7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casella G, Bella CD, Cambareri AR et al Gastric metastasis by lung small cell carcinoma. World J Gastroenterol 2006; 12: 4096–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzaki N, Hiraki A, Ueoka H et al Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res 2002; 22 (2B): 1209–12. [PubMed] [Google Scholar]
- 7. Fletcher MS. Gastric perforation secondary to metastatic carcinoma of the lung: A case report. Cancer 1980; 46: 1879–82. [DOI] [PubMed] [Google Scholar]
- 8. Yang CJ, Hwang JJ, Kang WY et al Gastro‐intestinal metastasis of primary lung carcinoma: Clinical presentations and outcome. Lung Cancer 2006; 54: 319–23. [DOI] [PubMed] [Google Scholar]
- 9. Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer 1982; 49: 170–2. [DOI] [PubMed] [Google Scholar]
- 10. Mcneill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer 1987; 59: 1486–9. [DOI] [PubMed] [Google Scholar]
- 11. Yong IK, Kang BC, Sun HS. Surgically resected gastric metastasis of pulmonary squamous cell carcinoma. World J Gastrointest Surg 2013; 5: 278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


