Abstract
Background
Approximately 15% of lung cancer patients are diagnosed in early stages. Microscopic proof of disease cannot always be obtained because of comorbidity or reluctance to undergo invasive diagnostic procedures. In the current study, survival data of patients with and without pathology are compared.
Methods
One hundred and sixty three patients with NSCLC I–IIb (T3 N0) treated between 2002 and 2016 were eligible: 123 (75%) had pathological confirmation of disease, whereas 40 (25%) did not. In accordance with international guidelines, both groups received radiotherapy. Comorbidity was assessed with the Charlson Comorbidity Index (CCI).
Results
The median follow‐up was 28.6 months (range: 0.3–162): 66 (40%) patients are still alive, while 97 (59%) patients died: 48 (29%) cancer‐related deaths and 49 (30%) from causes other than cancer. Median overall survival (OS) in patients without pathological confirmation was 58.6 months (range: 0.5–162), which did not differ from those with microscopic proof of disease (39.4 months, range: 0.3–147.5; logrank P = 0.481). Median cancer‐specific survival (CSS) also did not differ at 113.4 months (range: 0.5–162) in the non‐confirmation group (logrank P = 0.763) versus 51.5 months (range: 3.7–129.5) in patients with pathology. In Cox regression, a CCI of ≥ 3 was associated with poor OS (hazard ratio 2.0; range 1.2–3.4; P = 0.010) and CSS (hazard ratio 2.0; 1.0–4.0; P = 0.043).
Conclusion
OS and CSS in early lung cancer patients depend on comorbidity rather than on pathological confirmation of disease.
Keywords: Charlson comorbidity index, early stage NSCLC, overdiagnosis, overtreatment, SBRT
Introduction
Lung cancer causes 27% of all cancer deaths.1, 2 For 2017, the predicted age‐standardized lung cancer death rates in Europe are 37 and 14 per 100 000 for men and women, respectively.1 Approximately 15% of patients are diagnosed in Union for International Cancer Control (UICC) stages I–IIb. Without treatment, median overall survival (OS) is 10 months.3 Although surgery is the standard of care, approximately 30% of patients with early stage lung cancer4 are inoperable, which is one of the reasons why treatment mortality rates range from 5.2% to 7.4%.5, 6, 7 In the past decade, stereotactic body radiotherapy (SBRT) has evolved as an effective treatment option with local control rates comparable to surgery combined with low toxicity.3, 8 This has led to a rise in the number of patients clinically diagnosed, which entails the risk of overtreatment. On the other hand, observation alone may result in disease progression and subsequent death.
The complication rate of invasive diagnostic procedures may be acceptable in patients in good general condition, but the risk may outweigh the benefit in patients with significant comorbidity.9, 10 The biopsy rates before SBRT are between 41% in the Netherlands and up to 100% in the United States, which reflects ethnic differences.11 Because pathological confirmation of disease is generally higher in surgical cohorts than in irradiated patients, the issue of artificially raising local control rates by irradiating non‐cancerous lesions has been discussed.3 Surgical literature corroborates that up to 14% of the lung lesions initially regarded as malignant are in truth benign.12 In Dutch patients who represent a rather uniform population, the rate of false positives is < 5%.12, 13, 14 Indeed, overtreatment in the context of survival becomes an increasingly eminent issue with the growing number of elderly patients in frail general condition and the adoption of screening programs for lung cancer.15, 16
If invasive diagnostic procedures are not applicable or do not lead to definitive histological proof, the literature suggests several algorithms to estimate the probability of cancer17, 18 based on age, smoking history, and F18‐fluorodeoxyglucose positron emission tomography‐computed tomography (F18‐FDG PET‐CT), as well as lesion size changes in follow‐up CTs. If the probability of cancer is > 65%, the American Joint Committee on Cancer (AJCC) suggests surgery.19, 20, 21, 22
Because patients in a frail condition may die as a result of many causes other than cancer, an evaluation of general condition plays an important role in the context of overtreatment. One of the most common tools to assess comorbidities is the Charlson Comorbidity Index (CCI).23, 24 It includes 17 comorbidities that are denoted by an individual number which in sum estimates the 10‐year risk of death.23
A Surveillance, Epidemiology, and End Results (SEER) analysis in an ethnically heterogeneous population found significantly better cancer‐specific survival (CSS) in patients with clinically diagnosed tumors compared to controls with histological confirmation, which led the authors to conclude that patients without confirmed disease were overtreated.25 Although microscopic proof should be sought whenever possible, it cannot always be obtained in elderly patients with severe comorbidity. Because of demographic shifts, this patient population is increasing, which leads to the important question of whether these patients should be treated at all or kept under close observation in order to avoid overtreatment. The aim of this single center analysis was to clarify whether patients without confirmed disease are adequately treated by comparing their survival rates to those of patients with pathologically proven lung cancer.
Methods
Patients
Between 2002 and 2016, 211 patients with lymph node negative non‐small cell lung cancer (NSCLC) stages I–IIb (T3 N0) were irradiated at our department (8th edition Tumor Node Metastasis [TNM] Classification). After excluding patients with simultaneous lung tumors or concurrent second malignancies, 163 patients were eligible for the analysis. Patients were divided into two groups according to pathology: 123 (75%) patients had histologically confirmed disease, 40 (25%) did not. Patient data are summarized in Table 1. All patients provided informed consent for treatment. In order to perform this retrospective analysis, approval from the local ethics board was obtained.
Table 1.
Patient and treatment characteristics
| Characteristics and treatment | Pathological confirmation (n = 123) | No pathological confirmation (n = 40) | Mann–Whitney P |
|---|---|---|---|
| Age | |||
| Median | 71.8 | 73.4 | NS |
| Range | 54.7–88.2 | 52.0–90.0 | |
| Gender | |||
| Male | 45 (37%) | 17 (43%) | NS |
| Female | 78 (63%) | 23 (58%) | |
| T‐stage | |||
| 1 | 64 (52%) | 35 (88%) | < 0.001 |
| 2 | 43 (35%) | 5 (12%) | |
| 3 | 16 (13%) | 0 (0%) | |
| UICC stage | |||
| Ia1 | 8 (7%) | 8 (20%) | 0.002 |
| Ia2 | 23 (19%) | 17 (43%) | |
| Ia3 | 34 (28%) | 10 (25%) | |
| Ib | 23 (19%) | 3 (8%) | |
| IIa | 19 (15%) | 2 (5%) | |
| IIb | 16 (13%) | 0 (0%) | |
| COPD grade | |||
| 0 | 14 (11%) | 3 (8%) | NS |
| 1 | 10 (8%) | 4 (10%) | |
| 2 | 35 (28%) | 10 (25%) | |
| 3 | 36 (29%) | 11 (28%) | |
| 4 | 22 (18%) | 10 (25%) | |
| Unknown | 6 (5%) | 2 (5%) | |
| Smoking status | |||
| Never | 3 (2%) | 0 (0%) | NS |
| Former | 63 (51%) | 23 (58%) | |
| Current | 47 (38%) | 13 (33%) | |
| Unknown | 10 (8%) | 4 (10%) | |
| Pack years | |||
| Median | 50 | 50 | NS |
| Range | 15–200 | 7–100 | |
| Unknown cases | 33 | 10 | |
| Charlson score | |||
| Median | 4 | 4 | NS |
| Range | 2–14 | 2–8 | |
| Radiotherapy | |||
| DART | 89 (72%) | 19 (48%) | NS |
| STX | 30 (24%) | 18 (45%) | |
| Conventional (=2 Gy/d) | 4 (3%) | 3 (8%) | |
| Chemotherapy | |||
| Yes | 32 (26%) | 0 (0%) | < 0.001 |
| No | 91 (74%) | 40 (100%) | |
COPD, chronic obstructive pulmonary disease; DART, dose‐differentiated accelerated radiotherapy; NS, not significant; STX, stereotactic radiotherapy; UICC, Union for International Cancer Control.
Diagnostic procedures
An F18‐FDG PET‐CT scan is compulsory in the diagnostic workup of all patients, which marks a difference between our study and previous SEER analysis25 and large screening trials.15, 16 In case of positivity, histological – or at least cytological – confirmation by bronchoscopy is indicated. Treatment decisions are based on the patient's age and smoking history, new or growing nodule(s) on CT, and positive F18‐FDG PET‐CT scan.8
Radiotherapy
Between 2002 and 2011, dose‐differentiated accelerated radiotherapy (DART) was delivered in two daily fractions of 1.8 Gy26, 27, 28, 29 As of 2011, stereotactic body radiotherapy (SBRT) was also implemented at our institution. The study cohort was treated as follows: 108 (66%) patients received DART, 48 (29%) received SBRT, and 7 (4%) were administered conventional RT. The biologically effective doses (BED) calculated with an α/β of 10 ranged from 87 to 106 Gy for DART and 85 Gy (65% isodose, 130 Gy International Commission on Radiation Units) for SBRT.
Induction chemotherapy
Thirty‐two (20%) patients were administered two cycles of induction chemotherapy with platinum‐based doublets. The regimens were either cisplatinum (75 mg/m2) combined with pemetrexed (500 mg/m2) or gemcitabine (1000 mg/m2); in case of renal dysfunction, carboplatin AUC5 (absolute maximum dose 1100 mg) was administered.
Comorbidity
The patient's general condition was assessed using the CCI.23, 24 The 10‐year OS probability was calculated using the CCI + patient age: 0.983x, where x equals e0.9*(comorbidity‐age score).23 The reference population in this calculation had a high 10‐year OS rate of 98.3%.
Probability of cancer
For patients without pathological proof of disease, the probability of cancer was calculated according to the algorithm proposed by Herder: probability of malignancy = 1/(1 + e−x), with x = −4739 + 3691*(percentage probability using the Swenson model) + 2322 (faint standardized uptake value [SUV]) + 4617 (moderate SUV) + 4771 (intense SUV).17 For the purpose of this analysis, we defined SUV < 5 as faint, 5–10 as moderate, and > 5 as intense. The model calculates cancer probability using the following formula: probability of malignancy = 1/(1 + e−x), with x = −6.8272 + 0.0391*(age) + 0.7917*(smoking status) + 1.3388*(cancer) + 0.1274*(diameter) + 1.0407*(spiculation) + 0.7838*(upper).18 Patient age is given in years; the smoking status is 0 if the patient is a never smoker and 1 for current or former smokers; cancer is 1 in case of extrathoracic cancer > 5 years ago; the diameter is measured in millimeters; spiculation is 1 for lesions with spicula; and upper is 1 if the tumor is located in one of the upper lobes.
Statistics
The co‐primary endpoints of this study were OS and CSS. Groups with and without pathology were compared by logrank test. CSS was defined as survival excluding causes of death other than lung cancer. Initially, non‐parametric testing (Mann–Whitney U) was used to compare differences in clinical and treatment parameters. Cox regression (forward stepwise) was used to identify potential predictors of OS and CSS. The following variables were included in the model: gender, pathological confirmation, T‐stage, UICC stage, CCI, and type of radiotherapy. The secondary endpoint was a survival comparison between subgroups of T1 and T2 patients either with or without pathology.
Results
Overall survival (OS) and cancer‐specific survival (CSS) are independent of pathological proof
The patient related variables were equally distributed among groups, except for T‐stage (P < 0.001), UICC stage (P = 0.002), and the administration of chemotherapy (P < 0.001). A probable reason is that pathological confirmation is easier to obtain in patients with larger tumors. Higher T‐stage also triggered the administration of chemotherapy before radiation treatment. The median follow‐up in the whole cohort was 28.6 (range: 0.3–162) months: 66 (40%) patients are still alive; 97 (59%) patients died: 48 (29%) cancer‐related death and 49 (30%) as a result of other reasons, such as cardiac or respiratory failure. One patient was lost to follow‐up. The median OS and CSS rates in all patients were 40.1 (95% confidence interval [CI] 28.4–51.8) and 51.7 months (95% CI 40.2–63.2), respectively. The one, three, and five‐year OS rates were 86%, 56%, and 32%, respectively. In the 114 (71%) patients who died of cancer or are still alive, the corresponding CSS rates amounted to 92%, 80%, and 42%, respectively.
The median OS in the group of patients without pathological confirmation of disease was 58.6 (range: 0.5–162) months, which did not differ significantly from those with pathological confirmation (39.4 months, range 0.3–147.5; logrank P = 0.481) (Fig 1). No statistically significant difference was observed in CSS either. The median OS in the non‐confirmation group was 113.4 (range: 0.5–162) compared to 51.5 (range: 3.7–129.5) months in the patients with histological proof (logrank P = 0.585) (Fig 2). Similarly, in the T1 and T2 subgroups, there was no statistically significant difference in OS and CSS (Figures S1–S4).
Figure 1.
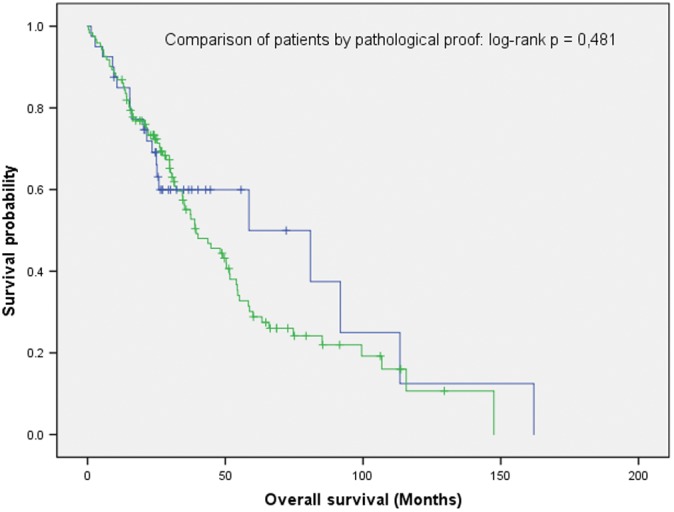
Overall survival in patients with or without pathological proof of disease did not differ significantly (n = 163; logrank P: 0.481).
Figure 2.
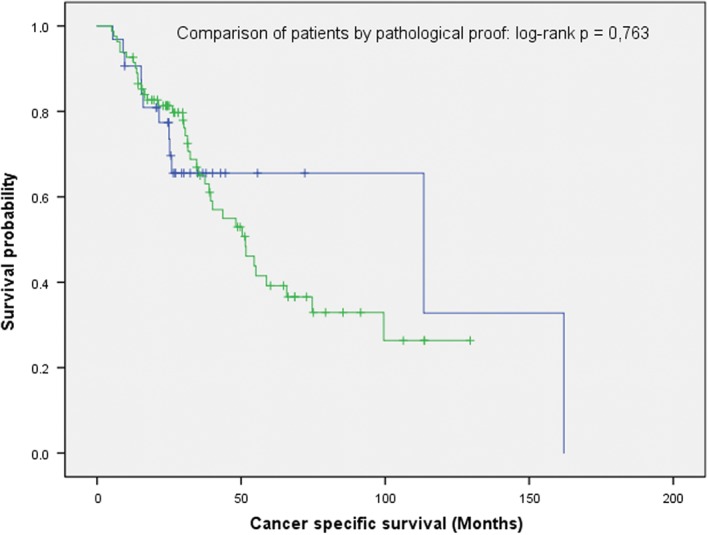
Cancer‐specific survival in patients with or without pathological proof of disease did not differ significantly (n = 114; logrank P: 0.763).
Local (logrank P = 0.819), regional (logrank P = 0.131), and distant (logrank P = 0.093) control did not significantly differ between the groups. Outcome data are summarized in Table 2.
Table 2.
Clinical outcomes
| Clinical outcome | Pathological confirmation (n = 123) | No pathological confirmation (n = 40) | Logrank P |
|---|---|---|---|
| Overall survival | |||
| Deaths (n) | 76 (62%) | 21 (53%) | 0.481 |
| Alive (n) | 47 (38%) | 19 (48%) | |
| Median (months) | 39.4 | 58.6 | |
| Range (months) | 0.3–147.5 | 0.5–162.0 | |
| Cause of death | |||
| Cancer (n) | 35 (28%) | 13 (33%) | 0.24 |
| COPD (n) | 7 (6%) | 3 (8%) | |
| Cardiovascular (n) | 19 (15%) | 2 (5%) | |
| Unknown (n) | 15 (12%) | 3 (8%) | |
| Cancer specific survival | |||
| Median (months) | 51.5 | 113.4 | 0.763 |
| Range (months) | 3.7–129.5 | 0.5–162.0 | |
| Local control | |||
| Median (months) | Not reached | Not reached | 0.819 |
| Range (months) | 0.3–147.5 | 0.5–161.7 | |
| Regional control | |||
| Median (months) | Not reached | Not reached | 0.131 |
| Range (months) | 0.3–147.5 | 0.5–162.0 | |
| Distant control | |||
| Median (months) | Not reached | 38.2 | 0.093 |
| Range (months) | 0.3–115.7 | 0.5–162.0 | |
COPD, chronic obstructive pulmonary disease.
Probability of cancer in patients without pathological confirmation of disease is high
Forty (25%) patients had no pathological proof of disease for one of the following reasons: ineligible for invasive procedures because of comorbidities; refusal to undergo invasive procedures (bronchoscopy or transthoracic biopsy); or because the tumor was located peripherally, no histology or cytology could be obtained during bronchoscopy. The probability of cancer was estimated in 33 (83%) of the patients without pathology. In 7 (17%) patients one of the parameters included in the algorithm suggested by Herder was missing.17 The median probability of cancer was 89% (range: 2–98%): 26 (65%) patients had at least a 65% probability of cancer, while in the remaining 7 (17%) the cancer probability ranged between 33% and 65%.
Charlson Comorbidity Index is the main prognostic factor for OS and CSS
The CCI, as an indicator for comorbidity, did not differ significantly between patients with and without pathological confirmation. In both groups, the median was 4, ranging from 2 to 14 and 2 to 8, respectively (Table 1). However, in multivariate analysis (MVA) of prognostic factors for OS (Table S1), a CCI of ≥ 3 was the only variable that remained significant (hazard ratio [HR] 2.0, range 1.2–3.4; P = 0.010). In the comparison by logrank test, a similar difference was observed (P = 0.009) (Fig 3), which persisted as a trend in the T1 (P = 0.062) and T2 (P = 0.099) subgroups (Figure S5, S6). As for CSS, MVA (HR 2.0, range 1.0–4.0; P = 0.043) (Table S1), as well as logrank comparison (P = 0.039) (Fig 4), revealed a significant difference between patients with and without histology, independent of CCI. This was a trend in the T1 (P = 0.081) (Figure S7) but not in the T2 subgroup (P = 0.349) (Figure S8).
Figure 3.
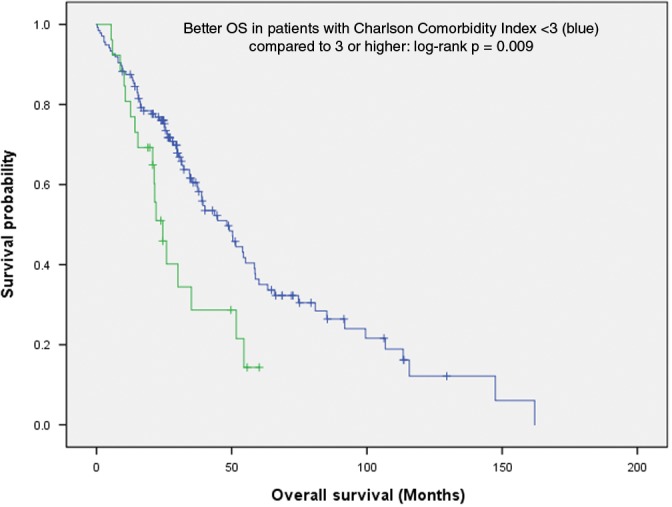
Charlson Comorbidity Index < 3 is a positive prognostic factor for overall survival (n = 163; logrank P: 0.009).
Figure 4.
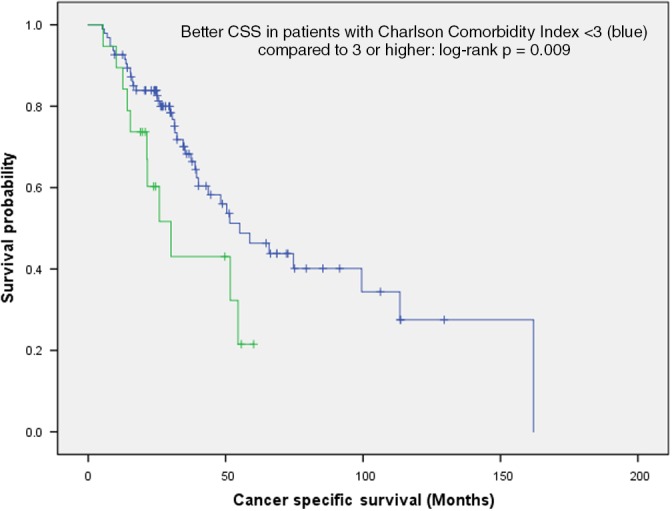
Charlson Comorbidity Index < 3 is a positive prognostic factor for cancer‐specific survival (n = 114; logrank P: 0.039).
Based on CCI, the calculated median 10‐year OS probability was 21% whereas the actual Kaplan–Meier estimate of 11% was slightly lower. No significant differences between patients with or without pathological proof were found (Mann–Whitney U test P = 0.734).
Discussion
This single center analysis of an ethnically homogeneous cohort shows that OS and CSS are independent of pathologic proof of disease, which corroborates the idea that the risk of overtreatment is low and patients are adequately treated by high dose irradiation. The only prognosticator for survival is the CCI.
The question of pathologic confirmation of disease becomes increasingly important as the average age of patients referred for treatment of early lung cancer is on the rise. Simultaneously, the adoption of screening programs for lung cancer15, 16 will lead to the detection of numerous small lesions in high‐risk populations in potentially limited general condition, which may preclude invasive diagnostic procedures. The development of modern radiation technologies ensures that these elderly, frail patients can be treated safely.30, 31, 32
The results of the current study stand in contrast to a SEER analysis by Shaikh et al., which shows that CSS and – on univariate analysis – OS are better in patients without histologically confirmed disease.25 More than 7000 patients were included, 90% of which had pathology. Because patients without microscopic confirmation had better CSS (MVA HR 0.82, 95% CI 0.71–0.96; P = 0.013), the authors concluded that a significant number of non‐cancerous lesions were included, especially in the group of patients with small peripheral tumors. This result is not surprising as larger tumors (= higher T‐stage) are generally associated with a higher proportion of histologically confirmed cancers – a fact that is consistent with our data. The discrepancy between our results and this study may be because of several differences in the patient population. The SEER database includes a very ethnically heterogeneous population, whereas the patients in our study are all from a central European region. SEER does not provide information on comorbidities, diagnostic workup and imaging, or radiation treatment, whereas this information was prospectively collected in our institutional database for each patient. The obligatory inclusion of F18‐FDG PET‐CT in the diagnostic workup adds to the reduction of erroneous cancer diagnosis. As shown in Dutch publications, the reliance on molecular imaging makes sense in surroundings where the risk of false positives as a result of infections is low, as in our cohort.3, 5, 7 Although misdiagnosis cannot entirely be excluded, the chances of false positives are further minimized as 98.2% of the patients were former smokers, 36.8% current smokers, and the median age of the whole cohort was 72.2 years.
In a retrospective analysis by Verstegen et al., 591 patients were treated with SBRT for stage I lung cancer with local control rates > 90% at three years.3 Similar to our study, no significant difference in OS between patients with or without pathology was detected either in the whole cohort or in the T1 and T2 subgroups. While our patient cohort was more homogeneous, Verstegen et al. also included F18‐FDG PET‐CT in the diagnostic workup, meaning our results are comparable. The calculated mean probability of malignancy was 92.5%, which is also in the same range. Not surprisingly, patients without pathology had a smaller mean tumor diameter, which is also corroborated by the present analysis (Table 1). The MVA model included similar parameters as ours and showed no differences, except for lung function and tumor size (i.e. T‐stage and tumor diameter). As opposed to our results, the CCI did not significantly predict OS in this cohort.3
A similar study by Lagerwaard et al. included 177 patients with a median CCI of 2 (range: 0–5). In contrast to the previous report by Verstegen et al., all of these patients were potentially eligible for surgery. About two thirds of the patients had no pathological confirmation of disease.8 The patient population is not exactly comparable to our results as the median CCI of 2 – comparable to a surgical series – is markedly lower than ours (median 4, range: 2–14). Additionally, the number of patients without pathological confirmation of disease is approximately three times as high as in our study. Nevertheless, the 90% mean likelihood of malignancy is close to the 89% in our cohort, including one patient with a very low probability of cancer.8
Data from the Amsterdam Cancer Registry revealed that patients with T2 tumors or a lack of pathology have poor survival.5 This is inconsistent with the notion that patients with benign lesions were included in the no‐pathology population.25 An OS gain was observed only in the radiation group (not in the surgery or no treatment group). SBRT is suitable for frail and elderly patients because of little toxicity and yields higher local control rates than conventional RT. Although – as the authors mention – the study is only observational and therefore no direct relationship between SBRT and changes in OS can be established, the link between SBRT and OS is not counterintuitive. To some extent, our data corroborate this finding as SBRT shows the best OS. Because of the long time course of inclusion (2002–2016), irradiation techniques and treatment schedules have altered. While this reflects the changes in treatment approach during the accrual period, the BEDs do not differ substantially between accelerated mode and SBRT, which may explain why OS in patients treated either with DART or SBRT is alike. During the 15 years encompassed by the current analysis it became clear that a BED >100 Gy should be applied.32, 33 In our cohort, the minimum BED was approximately 15% lower, leading to median OS of 40 months in both groups. The published OS data for early stage lung cancer patients ranges from 21 months for elderly patients with presumably higher Eastern Cooperative Oncology Group performance status5 and 61.5 months for operable patients.8
The use of surrogate markers to estimate the probability of cancer may minimize the risk of overtreatment. We applied the algorithm proposed by Herder, which extends a previously published model by including F18‐FDG PET‐CT results in the calculation.17, 18, 34 According to this formalism, the median probability of cancer in our study was 89% (2–98%), which is in line with the Dutch studies mentioned previously.3, 8 These figures are above the threshold of 65% suggested for surgical treatment by the American College of Clinical Pharmacy, as well as the 85% for SBRT by the International Association for the Study of Lung Cancer.21, 22 High dose irradiation is a feasible approach in patients without pathological confirmation diagnosed by F18‐FDG PET‐CT, preferably in populations with a generally low probability of non‐cancerous PET‐positive lesions.21
However, if pathology is lacking, dose adaptation to the tumor is not possible. In a cohort of 740 patients treated for early lung cancer between 2003 and 2015, Woody et al. indicated that differences in histology should trigger modified dose schedules:35 30% of the patients had no pathology, while 70% were diagnosed histologically, which is consistent with the higher biopsy rate compared to European series.21 Centrally located tumors were treated with 50 Gy in five fractions, whereas peripheral tumors received 60 Gy in three fractions. This treatment approach showed a significantly higher local failure rate in squamous cell carcinoma than in adenocarcinoma.35 The absence of microscopic proof may hamper optimal radiation treatment, thus histological proof should be sought whenever possible.
Because a significant number of non‐cancer deaths occur in long‐term survivors,36 international guidelines recommend the evaluation of comorbidity before treatment planning in patients with lung cancer.37 In the current study, Cox regression revealed that CCI as a measure for patients’ general condition was the only significant prognosticator for OS. This score was validated in 685 breast cancer patients.23 Although aged approximately 30 years old, the fact that the validation cohort in the report by Charlson revealed a low risk of cancer death makes its results – at least to some extent – applicable to our study population. Indeed, the predicted 10‐year OS rate was 21% compared to the actuarial rate of 11%.
Mellemgaard et al. analyzed approximately 20 000 patients from the Danish Cancer Registry and showed that survival in lung cancer patients was mainly influenced by comorbidity.36 Of note, the most significant difference was found in the group of patients with CCI ≥ 3. Although the authors describe CCI as a “modest prognostic factor” and the performance status at the time of diagnosis as more important, they admit that comorbidity may interfere with performance status.36 These results corroborate the usefulness of the CCI for lung cancer patients, despite criticism of the lack of specificity with respect to pulmonary conditions, such as fibrosis.38
Because of the retrospective nature of the current analysis, it is difficult to draw definitive conclusions. However, because of the lack of prospective trials in the field, our data may obtain significance. The variety in the patient population with respect to tumor stage and the administration of chemotherapy results from the clinical fact that pathological confirmation is easier to obtain in patients with larger tumors. Similarly, in accordance with international guidelines, higher T and UICC stages trigger the administration of chemotherapy before radiation treatment. While this reflects the diversity of clinical practice in a tertiary referral center, the patient population is homogeneous in terms of ethnicity. Admittedly, this constitutes an inherent selection bias that cannot be avoided in retrospective studies.
A certain probability of cancer in patients without microscopic confirmation is a prerequisite for a survival comparison of patients with pathological proof of disease. In our cohort, this probability was generally high because of the ethnically uniform population and a consistent diagnostic workup including F18‐FDG PET‐CT, which is in line with international recommendations for pulmonary nodules.39 Therefore, we believe that the current analysis contributes to the discussion of most suitable care and management of patients with presumed early stage lung cancer.
In our cohort of early stage lung cancer patients, OS and CSS depend on CCI rather than on pathological confirmation of disease. This corroborates the notion that the risk of overtreatment is low in ethnically uniform patient populations if the diagnostic workup consistently includes F18‐FDG PET‐CT, resulting in a high pretreatment probability of cancer. Nevertheless, the results from the current analysis do not support the idea of substituting tissue‐based diagnosis of disease in general.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1. Overall survival in T1 patients with or without pathological proof of disease did not differ significantly (n = 99; logrank P: 0.516).
Figure S2. Cancer‐specific survival in T1 patients with or without pathological proof of disease did not differ significantly (n = 99; logrank P: 0.231).
Figure S3. Overall survival in T2 patients with or without pathological proof of disease did not differ significantly (n = 48; logrank P: 0.168).
Figure S4. Cancer specific survival in T2 patients with or without pathological proof of disease did not differ significantly (n = 48; logrank P: 0.090).
Figure S5. Overall survival in T1 patients independent of Charleson Comorbidity Index (n = 99; logrank P: 0.062).
Figure S6. Overall survival in T2 patients independent of Charleson Comorbidity Index (n = 48; logrank P: 0.099).
Figure S7. Cancer specific survival in T1 patients independent of Charleson Comorbidity Index (n = 99; logrank P: 0.081).
Figure S8. Cancer specific survival in T2 patients independent of Charleson Comorbidity Index (n = 48; logrank P: 0.349).
Table S1. Univariate and multivariate analysis of prognostic factors for overall and cancer‐specific survival.
References
- 1. Malvezzi M, Carioli G, Bertuccio P et al European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol 2017; 28: 1117–23. [DOI] [PubMed] [Google Scholar]
- 2. Mortani Barbosa EJ Jr. Lung cancer screening overdiagnosis: Reports of overdiagnosis in screening for lung cancer are grossly exaggerated. Acad Radiol 2015; 22: 976–82. [DOI] [PubMed] [Google Scholar]
- 3. Verstegen NE, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: Comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011; 101: 250–4. [DOI] [PubMed] [Google Scholar]
- 4. Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early‐stage lung cancer. N Engl J Med 1999; 341: 1198–205. [DOI] [PubMed] [Google Scholar]
- 5. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non‐small‐cell lung cancer: A population‐based time‐trend analysis. J Clin Oncol 2010; 28: 5153–9. [DOI] [PubMed] [Google Scholar]
- 6. Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high‐risk cancer operation: A national study. J Am Coll Surg 2007; 205: 729–34. [DOI] [PubMed] [Google Scholar]
- 7. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: A population‐based matched‐pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011; 101: 240–4. [DOI] [PubMed] [Google Scholar]
- 8. Lagerwaard FJ, Verstegen NE, Haasbeek CJ et al Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: 348–53. [DOI] [PubMed] [Google Scholar]
- 9. Hiraki T, Mimura H, Gobara H et al CT fluoroscopy‐guided biopsy of 1,000 pulmonary lesions performed with 20‐gauge coaxial cutting needles: Diagnostic yield and risk factors for diagnostic failure. Chest 2009; 136: 1612–7. [DOI] [PubMed] [Google Scholar]
- 10. Laurent F, Montaudon M, Latrabe V, Begueret H. Percutaneous biopsy in lung cancer. Eur J Radiol 2003; 45: 60–8. [DOI] [PubMed] [Google Scholar]
- 11. Louie AV, Palma DA, Dahele M, Rodrigues GB, Senan S. Management of early‐stage non‐small cell lung cancer using stereotactic ablative radiotherapy: Controversies, insights, and changing horizons. Radiother Oncol 2015; 114: 138–47. [DOI] [PubMed] [Google Scholar]
- 12. Cerfolio RJ, Bryant AS. Survival of patients with true pathologic stage I non‐small cell lung cancer. Ann Thorac Surg 2009; 88: 917–22. [DOI] [PubMed] [Google Scholar]
- 13. Rutter CE, Corso CD, Park HS et al Increase in the use of lung stereotactic body radiotherapy without a preceding biopsy in the United States. Lung Cancer 2014; 85: 390–4. [DOI] [PubMed] [Google Scholar]
- 14. van Tinteren H, Hoekstra OS, Smit EF et al Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non‐small‐cell lung cancer: The PLUS multicentre randomised trial. Lancet 2002; 359: 1388–93. [DOI] [PubMed] [Google Scholar]
- 15. Aberle DR, Adams AM, Berg CD et al Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Iersel CA, de Koning HJ, Draisma G et al Risk‐based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch‐Belgian randomised lung cancer multi‐slice CT screening trial (NELSON). Int J Cancer 2007; 120: 868–74. [DOI] [PubMed] [Google Scholar]
- 17. Herder GJ, van Tinteren H, Golding RP et al Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18F‐fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. [DOI] [PubMed] [Google Scholar]
- 18. Swensen SJ, Silverstein MD, Edell ES et al Solitary pulmonary nodules: Clinical prediction model versus physicians. Mayo Clin Proc 1999; 74: 319–29. [DOI] [PubMed] [Google Scholar]
- 19. Gould MK, Donington J, Lynch WR et al Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e93S–e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K, American College of Chest Physicians . Treatment of non‐small cell lung cancer stage I and stage II: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 2007; 132 (3 Suppl): 234S–42S. [DOI] [PubMed] [Google Scholar]
- 21. Louie AV, Senan S, Patel P et al When is a biopsy‐proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer?: A decision analysis. Chest 2014; 146: 1021–8. [DOI] [PubMed] [Google Scholar]
- 22. Field JK, Smith RA, Aberle DR et al International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012; 7: 10–9. [DOI] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 24. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol 2004; 57: 1288–94. [DOI] [PubMed] [Google Scholar]
- 25. Shaikh T, Churilla TM, Murphy CT et al Absence of pathological proof of cancer associated with improved outcomes in early‐stage lung cancer. J Thorac Oncol 2016; 11: 1112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wurstbauer K, Deutschmann H, Kopp P, Sedlmayer F. Radiotherapy planning for lung cancer: Slow CTs allow the drawing of tighter margins. Radiother Oncol 2005; 75: 165–70. [DOI] [PubMed] [Google Scholar]
- 27. Wurstbauer K, Deutschmann H, Dagn K et al DART‐bid (dose‐differentiated accelerated radiation therapy, 1.8 Gy twice daily)‐‐a novel approach for non‐resected NSCLC: Final results of a prospective study, correlating radiation dose to tumor volume. Radiat Oncol 2013; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Baardwijk A, Wanders S, Boersma L et al Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non‐small‐cell lung cancer. J Clin Oncol 2010; 28: 1380–6. [DOI] [PubMed] [Google Scholar]
- 29. Zehentmayr F, Wurstbauer K, Deutschmann H et al DART‐bid: Dose‐differentiated accelerated radiation therapy, 1.8 Gy twice daily: High local control in early stage (I/II) non‐small‐cell lung cancer. Strahlenther Onkol 2015; 191: 256–63. [DOI] [PubMed] [Google Scholar]
- 30. Temming S, Kocher M, Stoelben E et al Risk‐adapted robotic stereotactic body radiation therapy for inoperable early‐stage non‐small‐cell lung cancer. Strahlenther Onkol 2018; 194: 91–7. [DOI] [PubMed] [Google Scholar]
- 31. Fleckenstein J, Boda‐Heggemann J, Siebenlist K et al Non‐coplanar VMAT combined with non‐uniform dose prescription markedly reduces lung dose in breath‐hold lung SBRT. Strahlenther Onkol 2018; 194: 815–23. [DOI] [PubMed] [Google Scholar]
- 32. Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F et al Planning benchmark study for SBRT of early stage NSCLC : Results of the DEGRO Working Group Stereotactic Radiotherapy. Strahlenther Onkol 2017; 193: 780–90. [DOI] [PubMed] [Google Scholar]
- 33. Guckenberger M, Andratschke N, Alheit H et al Definition of stereotactic body radiotherapy: Principles and practice for the treatment of stage I non‐small cell lung cancer. Strahlenther Onkol 2014; 190: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Ameri A, Malhotra P, Thygesen H et al Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015; 89: 27–30. [DOI] [PubMed] [Google Scholar]
- 35. Woody NM, Stephans KL, Andrews M et al A histologic basis for the efficacy of SBRT to the lung. J Thorac Oncol 2017; 12: 510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mellemgaard A, Luchtenborg M, Iachina M et al Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J Thorac Oncol 2015; 10: 272–9. [DOI] [PubMed] [Google Scholar]
- 37. Firat S, Byhardt RW, Gore E. The effects of comorbidity and age on RTOG study enrollment in stage III non‐small cell lung cancer patients who are eligible for RTOG studies. Int J Radiat Oncol Biol Phys 2010; 78: 1394–9. [DOI] [PubMed] [Google Scholar]
- 38. Tammemagi CM, Neslund‐Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer 2003; 103: 792–802. [DOI] [PubMed] [Google Scholar]
- 39. Meyer M, Vliegenthart R, Henzler T et al Management of progressive pulmonary nodules found during and outside of CT lung cancer screening studies. J Thorac Oncol 2017; 12: 1755–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overall survival in T1 patients with or without pathological proof of disease did not differ significantly (n = 99; logrank P: 0.516).
Figure S2. Cancer‐specific survival in T1 patients with or without pathological proof of disease did not differ significantly (n = 99; logrank P: 0.231).
Figure S3. Overall survival in T2 patients with or without pathological proof of disease did not differ significantly (n = 48; logrank P: 0.168).
Figure S4. Cancer specific survival in T2 patients with or without pathological proof of disease did not differ significantly (n = 48; logrank P: 0.090).
Figure S5. Overall survival in T1 patients independent of Charleson Comorbidity Index (n = 99; logrank P: 0.062).
Figure S6. Overall survival in T2 patients independent of Charleson Comorbidity Index (n = 48; logrank P: 0.099).
Figure S7. Cancer specific survival in T1 patients independent of Charleson Comorbidity Index (n = 99; logrank P: 0.081).
Figure S8. Cancer specific survival in T2 patients independent of Charleson Comorbidity Index (n = 48; logrank P: 0.349).
Table S1. Univariate and multivariate analysis of prognostic factors for overall and cancer‐specific survival.


