Abstract
A preoperative chest computed tomography examination of the right breast in a 52‐year‐old woman with breast cancer revealed multiple nodules in both lungs. The nodule in the apical segment of the upper lobe of the right lung was larger, at a diameter of approximately 2.1 cm. The patient underwent resection of the right breast, followed by thoracoscopic wedge resection of four pulmonary nodules. Hematoxylin and eosin staining and immunohistochemistry showed that the nodules in the apical and anterior segments of the upper lobe and the paravertebral nodule in the lower lobe of the right lung were primary adenocarcinoma, and the subpleural nodule in the lower lobe of the right lung was infiltrated with inflammatory cells. Exon sequencing was conducted in the resected tissue samples and blood specimens. According to the characteristics of the somatic mutations, the nodule in the apical segment of the upper lobe of the right lung was primary lung adenocarcinoma, the nodule in the anterior segment of the upper lobe and the paravertebral nodule in the lower lobe of the right lung were intrapulmonary metastatic cancer, and the subpleural nodule in the lower lobe of the right lung indicated early stage tumor progression. This case provides new evidence that conducting gene detection in multiple tissue samples from patients who have undergone resection may assist to determine the relationship among multiple nodules in the lung to exclude lung metastasis of breast cancer.
Keywords: Gene mutation, intrapulmonary metastatic cancer, molecular detection, multiple primary lung cancer
Introduction
Multiple primary lung cancer (MPLC) refers to two or more primary malignant tumors occurring simultaneously or successively in the lung of the same patient, with no occurrence of N2/N3 lymph node or systemic metastasis. The distinction between MPLC and intrapulmonary metastatic cancer is of great importance to clinical treatment and prognosis. At present, clinical features, imaging, and pathological features are mainly used to comprehensively determine and distinguish MPLC and intrapulmonary metastatic cancer. However, if the pathological type and imaging features are similar, it is difficult to distinguish between the two. Recently, developments in gene mutation studies have provided a molecular basis for identifying MPLC and intrapulmonary metastatic cancer.
Case presentation
A 52‐year‐old Han Chinese woman was admitted to the Department of Thoracic Surgery of Beijing Friendship Hospital on 20 November 2017 after multiple nodules were detected in the right lung. The patient had a five‐year history of hypertension, denied any history of smoking and drinking, and had a family history of lung disease. On 2 November 2017, she had undergone reserved mastectomy for right breast cancer followed by axillary lymph node dissection at the Department of General Surgery of Beijing Friendship Hospital. Postoperative pathology showed infiltrating ductal carcinoma in the right breast and metastases in 1–20 right axillary lymph nodes. The chest computed tomography (CT) examination showed multiple nodules in both lungs; metastasis was not excluded. The nodule in the apical segment of the upper lobe of the right lung was larger but was not confirmed as malignant. Whole‐body positron emission tomography (PET)‐CT examination further showed that the nodule in the apical segment of the upper lobe of the right lung was larger; the lesion was considered to be malignant, thus indicating a strong possibility of peripheral lung cancer. No abnormal increase in F18 fluorodeoxyglucose (FDG) metabolism was found in the other pulmonary nodules and mediastinal and hilar lymph nodes. Follow‐up observation was recommended. It was difficult to preoperatively diagnose whether the multiple nodules of both lungs were primary lung cancer with intrapulmonary metastasis, or lung metastasis of breast cancer. The following two surgical programs were recommended after general discussion among the whole department:
Program 1: Video‐assisted thoracoscopic wedge resection of the nodule in the upper lobe of the right lung and wedge‐shaped resection of the nodule in the lower lobe of the right lung, followed by resection of the nodule in the upper lobe of the right lung and lymph node dissection, depending on the results of the intraoperative frozen section.
Program 2: Thoracoscopic excision of the nodule in the upper lobe of the right lung, mediastinal lymph node dissection, and dynamic observation of the remaining nodules.
Preoperative CT‐guided microcoil localization was conducted on the small nodules of the upper lobe of the right lung, and Program 1 was implemented on 24 November 2017. A total of four nodules were resected during the surgery: (i) a nodule in the apical segment of the upper lobe of the right lung, measuring 2.1 × 1.2 × 1.1 cm3; (ii) a nodule in the anterior segment of the upper lobe of the right lung approximately 0.4 cm in diameter; (iii) a subpleural nodule in the lower lobe of the right lung approximately 0.4 cm in diameter; and (iv) a paravertebral nodule in the lower lobe of the right lung approximately 0.6 cm in diameter (Fig 1). Postoperative pathology showed that the nodules in the apical and anterior segments of the upper lobe of the right lung and the paravertebral nodule in the lower lobe of the right lung were all primary lung adenocarcinoma. The subpleural nodule in the lower lobe of the right lung was infiltrated with inflammatory cells. The choice of surgical procedure was reasonable based on the postoperative pathological results.
Figure 1.
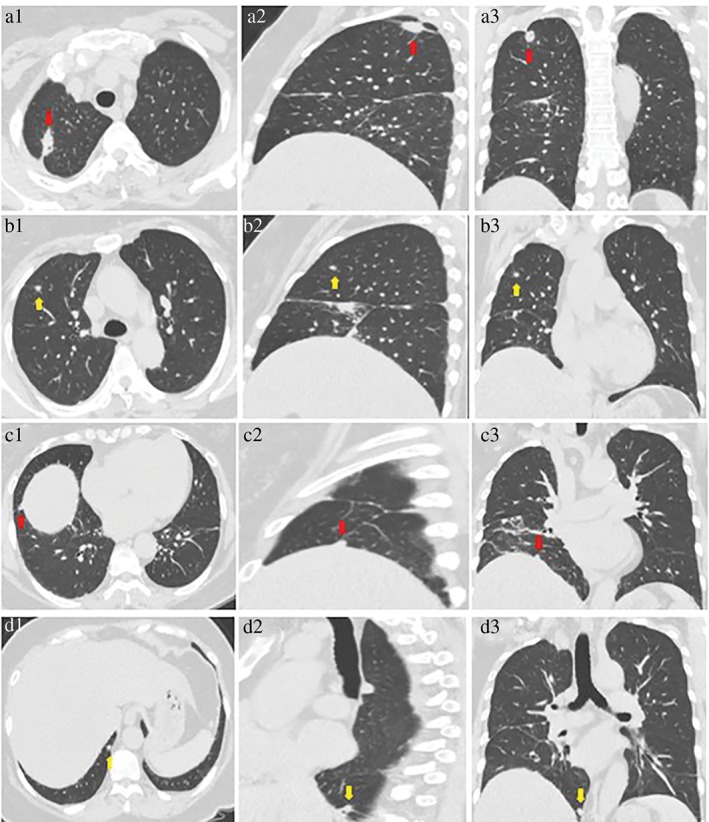
Three‐dimensional computed tomography image reconstruction of (a1–3) the 2.1 cm diameter nodule in the apical segment and the (b1–3) the 0.4 cm diameter nodule in the anterior segment of the upper lobe of the right lung; (c1–3) the 0.4 cm diameter subpleural nodule, and (d1–3) the 0.6 cm diameter paravertebral nodule in the lower lobe of the right lung.
Hematoxylin and eosin (HE) staining and immunohistochemistry were performed. HE staining of the three surgically resected pulmonary nodules showed adenocarcinoma infiltration in the lung tissue, and acinar was the dominant type (Fig 2a,b,d). The results of immunohistochemical detection showed aspartic proteinase A (Napsin A) (+), cytokeratin (CK) 7 (+), and thyroid transcription factor 1 (TTF‐1)(+). The positive Ki‐67 rate in the nodules of the apical segment of the upper lobe of the right lung (20%), the anterior segment of the upper lobe of the right lung (15%), and the paravertebral nodule in the lower lobe of the right lung (15%) indicated primary lung adenocarcinoma (Table 1). HE staining of the breast cancer specimen showed a glandular tubular structure and the lesion was ductal carcinoma in situ (Fig 2e,f). The results of immunohistochemical detection showed that estrogen receptor (ER) was approximately 80% strong (+), progesterone receptor (PR) approximately 30% strong (−) medium (+), Ki‐67 approximately 25% (+), E‐cadherin (+), CK8 (+), and CerbB‐2 (1+), which indicated breast infiltrating ductal carcinoma (Table 1). HE staining of the pulmonary inflammatory nodules showed benign micronodular hyperplasia in the stromal spindle cells (Fig 2c), and immunohistochemistry showed CK7 alveolar epithelium (+), CD31 blood vessel (+), Syn (−), CgA (−), and actin (−).
Figure 2.
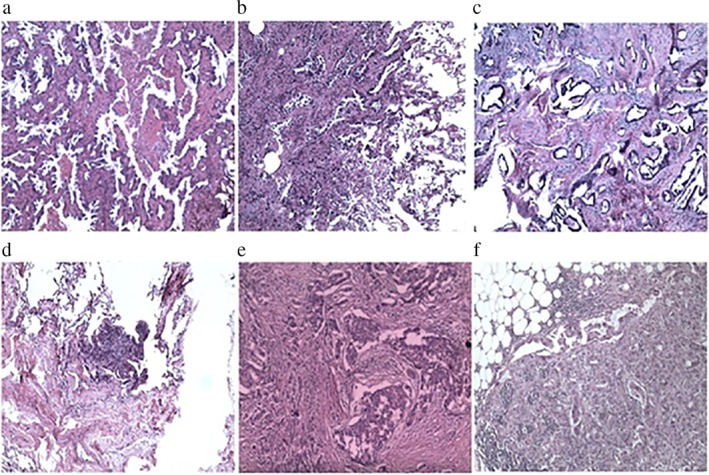
Hematoxylin and eosin (HE) staining of the nodule (a) in the apical segment and (b) the anterior segment of the upper lobe of the right lung. Adenocarcinoma infiltration was observed in the lung tissue, and acinar was the dominant type. (c) HE staining of the subpleural nodule slice in the lower lobe of the right lung. Inflammatory cell infiltration was observed in the lung tissue. (d) HE staining of the paravertebral nodule slice in the lower lobe of the right lung. Adenocarcinoma infiltration was observed in the lung tissue, and acinar was the dominant type. (e) HE staining of the lump slice in the right breast, with the breast infiltrating ductal carcinoma. The lesion was ductal carcinoma in situ. (f) HE staining of the lymph node slice in the right armpit.
Table 1.
Comparison of immunohistochemistry results
| Immunohistochemistry /Lesion | Nodule in the apical segment of the upper lobe of the right lung | Nodule in the anterior segment of the upper lobe of the right lung | Subpleural nodule in the lower lobe of the right lung | Paravertebral nodule in the lower lobe of the right lung | Breast lesion |
|---|---|---|---|---|---|
| CK20 | (−) | None | None | None | None |
| CK7 | (+) | (+) | Alveolar epithelium (+) | (+) | None |
| TTF‐1 | (+) | (+) | None | (+) | None |
| Napsin A | (+) | (+) | None | (+) | None |
| CK5/6 | (−) | (−) | None | (−) | Glandular epithelium (−) |
| P63 | Part(+) | (−) | None | (−) | (−) |
| Calponin | (−) | (−) | None | (−) | (−) |
| ER | (−) | (−) | None | (−) | Approximately 80% strong+ |
| PR | (−) | (−) | None | (−) | Approximately 30% strong–medium + |
| CerbB‐2 | (1+/2+) | Approximately 1+ | None | Approximately 1+ | 1+ |
| Ki‐67 | 20% (+) | Approximately 15% | Approximately 2 (+) | Approximately 15% | Approximately 25% (+) |
| E‐Cadherin | Membrane (+) | (+) | None | (+) | (+) |
| P120 | Membrane (+) | (+) | None | (+) | Membrane (+) |
| CK8 | (+) | (+) | None | (+) | (+) |
| MLH1 | (+) | None | None | None | None |
| MSH2 | (+) | None | None | None | None |
| MSH6 | (+) | None | None | None | None |
| PMS2 | (+) | None | None | None | None |
| P40 | None | (−) | None | (−) | None |
| Syn | None | None | (−) | None | None |
| CgA | None | None | (−) | None | None |
| CD31 | None | None | Blood vessel (+) | None | None |
| Actin | None | None | (−) | None | None |
| Vimentin | None | None | Focal cell (+) | None | None |
| HMB45 | None | None | (−) | None | None |
| GFAP | None | None | (−) | None | None |
Immunohistochemical detection was conducted in the nodules of the apical and anterior segments of the upper lobe of the right lung and subpleural and paravertebral nodules of the lower lobe of the right lung, and their relationships were compared. CgA, chromogranin A; CK, cytokeratin 20; E‐cadherin, epithelial cadherin; ER, estrogen receptor; Napsin A, aspartic proteinase A; PR, progesterone receptor; Syn, synapses; TTF‐1, thyroid transcription factor‐1.
Exon sequencing was conducted in the resected tissue samples and blood specimens, and the relationships among their somatic mutations were compared. Both TP53 and EGFR gene mutations were found in all three surgically resected pulmonary nodules, and their base alterations, amino acid alterations, and functional areas were the same. Although TP53 gene mutation existed in both the breast cancer lesion and the nodule in the upper lobe of the right lung, their base alterations, amino acid alterations, and functional areas were different. The frequencies of TP53 and EGFR mutations in the apical segment of the upper lobe of the right lung were 39.3% and 20%, respectively, which were significantly higher than in the other two nodules. Moreover, rare nucleophosmin (NPM1) mutation existed in both the subpleural nodule in the lower lobe of the right lung and the paravertebral nodule in the lower lobe of the right lung. On the first day after surgery, blood samples were collected for gene detection (Table 2).
Table 2.
Comparison of gene detection results
| Lesion/Gene | Mutant gene | Base alternation | Amino acid alternation | Functional area | Mutation frequency (%) | Pathological result |
|---|---|---|---|---|---|---|
| Nodule in the apical segment of the upper lobe of the right lung | TP53 | c.544delT | p.C182Afs*65 | EX5 | 39.3 | Primary adenocarcinoma of the lung |
| EGFR | c.2573T > G | p.L858R | EX21 | 20.0 | ||
| Nodule in the anterior segment of the upper lobe of the right lung | TP53 | c.544delT | p.C182Afs*65 | EX5 | 8.7 | Primary adenocarcinoma of the lung |
| EGFR | c.2573T > G | p.L858R | EX21 | 3.3 | ||
| Paravertebral nodule in the lower lobe of the right lung | TP53 | c.544delT | p.C182Afs*65 | EX5 | 2.0 | Primary adenocarcinoma of the lung |
| EGFR | c.2573T > G | p.L858R | EX21 | 1.9 | ||
| NPM1 | c.523GAT[6 > 5] | p.D175[6 > 5] | EX7 | 1.7 | ||
| Subpleural nodule in the lower lobe of the right lung | NPM1 | c.523GAT[6 > 5] | p.D175[6 > 5] | EX7 | 1.8 | Inflammatory cell infiltration |
| Breast lesion | TP53 | c.743G > A | p.R248Q | EX7 | 33.1 | Infiltrating ductal carcinoma |
| PTEN | c.1027‐2A > G | — | IVS8 | 31.3 | ||
| C11orf30 | c.3844G > C | p.E1282Q | EX21 | 14.3 | ||
| GATA3 | c.922‐3_922‐2delCA | — | IVS4 | 8.8 | ||
| GRIN2A | c.1124T > C | p.V375A | EX5 | 4.6 | ||
| FGFR1 | c.1549G > A | p.E517K | EX12 | 3.3 | ||
| BRD2 | c.478G > T | p.D160Y | EX4 | 2.4 | ||
| TOP1 | c.1149C > G | p.I383M | EX12 | 1.7 | ||
| NOTCH4 | c.689G > A | p.R230H | EX4 | 1.3 | ||
| CUL3 | c.610G > T | p.D204Y | EX5 | 1.1 | ||
| TSC2 | c.1073G > A | p.W358* | EX11 | 1.0 | ||
| KDM6A | c.1910C > G | p.S637C | EX16 | 1.0 | ||
| Blood specimen | H3F3C | c.312G > C | p.L104F | EX1 | 1.5 | — |
| FH | c.817G > A | p.A273T | EX6 | 1.0 |
Exon sequencing was conducted in the nodules in the apical and anterior segments of the upper lobe of the right lung and subpleural and paravertebral nodules in the lower lobe of the right lung, blood specimen, and breast lesion. The relationships among mutant genes, base alteration, amino acid alteration, functional area, and mutation frequency were compared.
Discussion
The probability of detecting pulmonary nodules by chest CT examination is high, as pulmonary multiple nodules are common and most are benign. The results of the National Lung Screening Trial in the United States showed that the probability of detecting a pulmonary nodule in a high‐risk population via chest CT screening was as high as 27.3%.1 The 2013 Fleischner Society guidelines recommend that for multiple ground‐glass nodules with prominent lesions, the major lesions need to be further treated. If CT examination confirms the presence of the lesion three months after the first examination, further diagnosis and treatment are suggested for larger lesions, especially for lesions with an internal solid component diameter of > 5 mm.2 A large number of clinical studies have reported the existence of several benign nodules around malignant nodules; the diameter of these small nodules is usually < 4 mm, indicating a low probability of malignancy.3 In this study, the patient had a history of right breast cancer, and whole‐body PET‐CT examination revealed a malignant larger nodule in the apical segment of the upper lobe of the right lung; no abnormal increase was found in FDG metabolism in the other pulmonary nodules or mediastinal and hilar lymph nodes. Follow‐up observation was recommended. The nodule in the upper lobe of the right lung was diagnosed as primary lung cancer in another hospital, and pulmonary lobectomy was suggested. The other nodules were considered benign lesions and continuous observation was suggested. However, this therapeutic regimen would have led to postoperative pathological staging and the incorrect therapeutic regimen. Therefore, several therapeutic regimens based on the patient's history and clinical characteristics were developed. The diagnosis of breast cancer was definite, but the possible relationships among the multiple nodules in the lung were as follows: (i) if all of the lesions were lung metastasis of breast cancer, surgery would produce no benefit; (ii) if the node in the apical segment of the upper lobe of the right lung was malignant and the other lesions were benign, pulmonary lobectomy along with mediastinal lymph node dissection was feasible; (iii) if the node in the apical segment of the upper lobe of the right lung was considered a malignant lesion and the remaining lesions were pulmonary metastases, surgery would produce no benefit; and (iv) if all of the pulmonary nodules were MPLC, lobectomy of the upper lobe of the right lung, mediastinal lymph node dissection, and wedge resection of the lower lobe of the right lung was feasible.
With the rapid development and application of molecular diagnostic technologies, understanding of non‐small‐cell lung cancer (NSCLC) has increased from the tissue level to the molecular level, and more tumor‐driven genes have successively been found. EGFR gene mutation is the most common mutation type in an Asian population with NSCLC.4 EGFR mutation in patients with NSCLC mainly occurs in the first four exons of the tyrosine kinase (TK) region (18–21). At present, more than 30 mutations have been found in the TK region, and the most common mutations occur in exons 19 and 21, at a rate of approximately 85%.5, 6 The probability of EGFR gene mutation in patients with NSCLC ranges from 30% to 60%, and EGFR gene mutation between primary and metastatic lesions is consistent.7, 8 TP53 gene mutation is also significant to the occurrence and development of NSCLC and the probability of mutation is approximately 40–60%, regardless of whether an EGFR mutation occurs.9 A study showed that the TP53 mutation rates in patients with NSCLC in the primary lung tumor and metastatic lymph nodes were 23.2% and 21.4%, respectively. The TP53 gene mutation had 92.9% correlation in the primary lung tumor and metastatic lymph nodes, and the TP53 gene mutation preceded lymph node metastasis and continued to play a role in tumor development.10
In our patient, both EGFR and TP53 gene mutations were found in the three malignant pulmonary nodules resected. Their base alterations, amino acid alterations, and functional areas were all the same, indicating that the cells showed the same clonality based on specific gene mutations. Although TP53 gene mutation occurred in both the breast cancer lesion and the nodule in the apical segment of the upper lobe of the right lung, the base alterations, amino acid alterations, and functional areas were different. The mutant genes in the breast cancer lesion were more significant than those in the nodule in the apical segment of the upper lobe of the right lung, indicating that the cells showed the same clonality based on specific gene mutations. The allele frequency of gene mutation could reflect the time sequence of mutant genes; the greater the allele frequency of individual somatic mutation, the earlier the mutation occurred.11 The TP53 and EGFR gene mutation frequencies in the nodule in the apical segment of the right upper lobe of the lung were 39.3% and 20%, respectively, which were significantly higher than in the other two nodules, indicating that the nodule in the apical segment of the upper lobe of the right lung occurred first. To sum up, the nodule in the apical segment of the upper lobe of the right lung was considered primary lung adenocarcinoma and the nodules in the anterior segment of the upper lobe and the paravertebral nodules in the lower lobe of the right lung were considered intrapulmonary metastatic cancer. In addition, NPM1 mutation occurred in the subpleural and paravertebral nodules in the lower lobe of the right lung. NPMI gene mutation is a molecular genetic anomaly with the highest detection rate in adult acute myeloid leukemia. NPMI can regulate the activities of the tumor suppressor genes p14 and p53 and participate in cell proliferation.12, 13, 14 However, NPMI gene mutation is not common in patients with NSCLC, suggesting that the subpleural nodules in the lower lobe of the right lung in this patient might indicate early stage tumor progression.
The latest tumor node metastasis (TNM) classification system proposes tailoring the TNM classification of multiple pulmonary sites of lung cancer to reflect the unique aspects of the four different patterns of presentation. Separate tumor nodule(s) (of the same histologic type) are classified on the basis of nodule location relative to the primary tumor site. Multifocal GG/L adenocarcinoma should be classified by the T category of the lesion with the highest T, along with the number of lesions (#) or simply (m) for multiple indicated in parentheses, and an N and M category that applies to all of the multiple tumor foci collectively.15 In a study cohort of clinical stage T4NXM0 patients, postoperative patients refused chemotherapy and chose oral TKI (gefitinib) treatment. After six months, complications of mild pulmonary fibrosis occurred and treatment was discontinued; however, the general condition of the patients was good, no signs of recurrence were found, and long‐term prognosis was pending further follow‐up. In patients with frequently occurring lung nodules, if pathological results cannot differentiate multiple primary lung cancer and intrapulmonary metastatic cancer, excision of the lesion would allow testing for TP53 and EGFR mutation. Changes in base alterations, amino acid alterations, and functional areas can indicate whether the lesions show the same clonality. Based on the order speculated by the frequency of allelic gene mutation, a judgment between primary tumor and metastasis could then be made. In our case, PET‐CT examination revealed a larger malignant nodule in the apical segment of the upper lobe of the right lung but no abnormal increase in FDG metabolism in the other pulmonary nodules or mediastinal and hilar lymph nodes. However, pathologic results revealed three malignant nodules. The reason for this discrepancy in results may be because pathological features significantly influence FDG uptake in solid‐type pulmonary adenocarcinomas. In particular, the fact that colloid/mucinous/lepidic adenocarcinomas have a significant tendency to generate false‐negative PET findings warrants attention.16
In conclusion, distinguishing two different pulmonary nodules, that is, primary lung cancer and intrapulmonary metastasis from MPLC in clinical practice is quite difficult. These two different diagnoses significantly affect the treatment and prognosis of patients. Pathology is still the gold standard for the diagnosis of lung cancer; however, the staging and classification of lung cancer are challenging. In this study, determining the homology and pluralism by conducting gene detection of tissue samples and analysis of the characteristics of their somatic mutations was effective and accurate. For patients with multiple pulmonary nodules, complete resection of all ipsilateral lesions is recommended during surgery if possible. Gene detection should be conducted in all resected tissue samples, and the staging and tumor origin need to be clearly defined.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This article was supported by 215 high‐level personnel from the Beijing Municipal Commission of Health and Family Planning. We thank Dr. Dan Tian for reviewing the paper.
References
- 1. National Lung Screening Trial Research Team , Church TR, Black WC et al Results of initial low‐dose computed tomographic screening for lung cancer. N Engl J Med 2013; 368: 1980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gould MK, Donington J, Lynch WR et al Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (Suppl. 5): e93S–e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swensen SJ, Jett JR, Hartman TE et al CT screening for lung cancer: Five‐year prospective experience. Radiology 2005; 235: 259–65. [DOI] [PubMed] [Google Scholar]
- 4. Cao C, Lu S, Sowa A et al Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs. Cancer Lett 2008; 266: 249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burgess TL, Sun J, Meyer S et al Biochemical characterization of AMG 102: A neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther 2010; 9: 400–9. [DOI] [PubMed] [Google Scholar]
- 6. Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non‐small cell lung cancer – Search and destroy. Eur J Cancer 2006; 42: 17–23. [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto S, Takahashi K, Iwakawa R et al Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 2006; 119: 1491–4. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K, Kohno T, Matsumoto S et al Clonal and parallel evolution of primary lung cancers and their metastases revealed by molecular dissection of cancer cells. Clin Cancer Res 2007; 13: 111–20. [DOI] [PubMed] [Google Scholar]
- 9. Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol 2011; 2011: 583929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang YL, Wu CT, Shih JY, Lee YC. Comparison of p53 and epidermal growth factor receptor gene status between primary tumors and lymph node metastases in non‐small cell lung cancers. Ann Surg Oncol 2011; 18: 543–50. [DOI] [PubMed] [Google Scholar]
- 11. Lu X, Xu Q, Wang J, Bi J, Wang Z, Li Y. Allele frequency of somatic mutations in individuals reveals signatures of cancer‐related genes. Acta Biochim Biophys Sin (Shanghai) 2015; 47: 657–60. [DOI] [PubMed] [Google Scholar]
- 12. Schneider F, Hoster E, Unterhalt M et al NPM1 but not FLT3‐ITD mutations predict early blast cell clearance and CR rate in patients with normal karyotype AML (NK‐AML) or high‐risk myelodysplastic syndrome (MDS). Blood 2009; 113: 5250–3. [DOI] [PubMed] [Google Scholar]
- 13. Falini B, Mecucci C, Tiacci E et al Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–66. [DOI] [PubMed] [Google Scholar]
- 14. Thiede C, Koch S, Creutzig E et al Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006; 107: 4011–20. [DOI] [PubMed] [Google Scholar]
- 15. Detterbeck FC, Nicholson AG, Franklin WA et al The IASLC Lung Cancer Staging Project: Summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol 2016; 11: 639–50. [DOI] [PubMed] [Google Scholar]
- 16. Lococo F, Galeone C, Formisano D et al 18F‐fluorodeoxyglucose positron emission tomographic scan in solid‐type p‐stage‐I pulmonary adenocarcinomas: What can produce false‐negative results? Eur J Cardiothorac Surg 2017; 51: 667–73. [DOI] [PubMed] [Google Scholar]


