Abstract
Background
The relationships between coagulation factors and non‐small cell lung cancer (NSCLC) prognosis have been intensively studied. However, no previous study has investigated the combined effects of preoperative platelet (PLT), fibrinogen (FIB), and D‐dimer (D‐D) levels on the prognosis of NSCLC.
Methods
A multicenter prospective study was conducted over seven hospitals. A total of 395 patients diagnosed with operable NSCLC for the first time were included and followed‐up until disease progression or the end of the study. Baseline demographic and clinicopathological information, and preoperative coagulation test results were collected for each patient. Univariate and multilevel survival analyses were conducted using Cox regression and shared frailty models.
Results
Multilevel analyses revealed that there was a marginally significant association between elevated PLT level (> 215 × 109/L) and unfavorable progression‐free survival (PFS) (hazard ratio 2.42, P = 0.05), whereas preoperative FIB and D‐D were not significant prognostic factors for PFS (P = 0.31 and 0.30, respectively). Compared to patients with one elevation of the three coagulation factors, patients with at least two elevations of the three factors had a significantly higher risk of cancer progression (hazard ratio 4.62, P = 0.02).
Conclusion
The number of elevated preoperative coagulation factors may have a significant effect on PFS and could be used to predict the prognosis of NSCLC patients after surgery. Future studies are warranted to further investigate the interactions between these three coagulation factors.
Keywords: Coagulation, D‐dimer, fibrinogen, non‐small cell lung cancer, platelet
Introduction
Lung cancer is the most common malignant tumor in China. In 2015, 733 300 people were newly diagnosed with lung cancer, corresponding to an incidence rate of 53.4 per 100 000 people.1 Lung cancer is also the leading cause of cancer death in China. According to the Global Burden of Disease, over 13 million Disability‐Adjusted Life Years (DALYs) were lost as a result of lung cancer in China in 2016, accounting for 36.0% of global DALYs from lung cancer.2 Among the two major types of lung cancer, non‐small cell lung cancer (NSCLC) is the most common, constituting 85% of all cancer cases.3
Surgery is the best treatment option for many patients with NSCLC, but high incidence of local and distant relapse results in poor five‐year survival rates.4 In terms of predicting NSCLC prognosis, cancer stage at diagnosis, performance status, gender, and weight loss are the most widely accepted predictive factors.3 Other prognostic determinants include age, histologic subtype, and biologic markers, such as p53 gene mutation and K‐ras oncogene activation.3, 5, 6 Despite these conventional predictive factors, there is growing research interest in investigating the relationships between blood coagulation factors and NSCLC prognosis. Evidence from biological research has shown that tumor cells and the hemostatic system are highly interconnected. Tumor cells can activate systemic coagulation through multiple pathways and induce hemostatic and fibrinolytic abnormalities, which in turn contribute to cancer angiogenesis and metastasis.7
Previous research has shown that elevated platelet (PLT) count, fibrinogen (FIB) level, and D‐dimer (D‐D) level are all associated with poor prognosis in NSCLC patients,8, 9, 10, 11, 12, 13 but the results have been inconsistent.14, 15 The majority of these studies were retrospective single‐center cohorts with relatively small sample sizes. Furthermore, no previous study has investigated the combined effects of D‐D, FIB, and PLT levels on NSCLC prognosis. Therefore, we conducted a multicenter prospective cohort study to investigate both the individual and combined effects of preoperative PLT, FIB, and D‐D levels on progression‐free survival (PFS) in operable NSCLC patients.
Methods
Study design and patients
This study was conducted between April 2016 and December 2017 in seven Grade‐A tertiary hospitals located in Jiangsu province, China. Basic information of the seven hospitals is summarized in the Supplementary Appendix. Patients admitted to one of the seven hospitals that met the following criteria were included in the study: (i) diagnosed with NSCLC for the first time; (ii) without distant metastasis (I–III tumor node metastasis [TNM] stage); (iii) underwent surgery (sleeve lobectomy, segmentectomy resection, wedge resection, or pneumonectomy); and (iv) agreed to participate in the study and signed informed consent. The exclusion criteria were: (i) a diagnosis of other primary cancer, severe cardiovascular or respiratory disease, severe rheumatic disease, or severe hematological disease; (ii) severe postoperative complications within 30 days (including death); or a (iii) history of anticoagulant or antiplatelet drug use within two weeks before surgery.
The World Health Organization Classification of Lung Tumors (4th edition) was used for the histological classification of lung cancer,16 while lung cancer stages were determined in accordance with the International Association for the Study of Lung Cancer staging system.17 Blood samples were collected at three time points to measure PLT, FIB, and D‐D levels: one day after admission (preoperative), 72 hours after surgery, and one day before discharge from hospital. However, because of missing data (> 20%), only the preoperative coagulation test results were used for survival analyses. Other demographic and clinical information collected included: age, gender, anatomic location of the tumor, tumor size, lymphovascular invasion (LVI), visceral pleural invasion (VPI), and type of surgery. The Ethics Committee of Jiangsu Province Hospital approved this study.
Treatment and follow‐up
Adjuvant chemotherapy, radiotherapy, or chemoradiotherapy was prescribed to patients according to the Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version).18 Patient follow‐up was conducted at three‐month intervals until December 2017, disease progression, death, or loss to follow‐up. The median follow‐up duration was 13.2 (range: 3.0–18.5) months. At each follow‐up visit, patients underwent chest computed tomography (CT), abdominal ultrasound scan, and other examinations when necessary. If a patient did not show up for a scheduled follow‐up visit, a study nurse contacted the patient or his/her relatives.
PFS was chosen as the study endpoint and was defined as the interval from surgery to local or distant relapse, whichever occurred first. Survival time was considered as censored if the patients died, were lost to follow‐up, or were progression‐free at the end of the study.
Coagulation assays
Patients’ venous blood samples were obtained by trained nurses and immediately sent to each hospital's clinical laboratory. The laboratories in the seven hospitals used the same methods to measure blood PLT count (fluorescent nucleic acid stain method), plasma FIB (clotting method), and D‐D concentrations (immunochemical method). The clinical laboratories of the seven hospitals have all passed external quality assessment conducted by Health Commission of Jiangsu Province, indicating that their results are comparable.
Statistical analysis
Patients with missing clinicopathological and demographic data were not included in the analysis. The Markov chain Monte Carlo (MCMC) method was used when preoperative PLT, FIB, or D‐D values (42 in total) were missing.19 The median values of preoperative D‐D, FIB, and PLT were used as cutoff values to dichotomize them. Baseline characteristics were described using medians and quartiles for continuous variables and percentages for categorical variables. The Kaplan–Meier method was used to construct survival curves.20 Univariate survival analyses were conducted using the Cox proportional hazards regression model.21 Statistically significant potential confounders were included in the subsequent multivariate survival analyses as covariates. Two models were used in the multivariate survival analyses. In the first single‐level Cox regression model, we adjusted variables that were found to be statistically significant in univariate analyses. Considering the treatment level differences across hospitals, we used multilevel survival analysis in the second model. In the second multilevel model, both patient‐level covariates and hospital‐level variations were adjusted using the shared frailty model, which incorporates a random intercept into the Cox proportional hazards regression model.22 A P value < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline demographic and clinicopathological characteristics
We recruited 457 patients, 410 of which met the inclusion criteria and were included in the study. We further excluded 2 patients with unknown progression dates, 12 patients with missing clinicopathological data, and 1 patient without blood coagulation test results from the analyses. The baseline characteristics of the remaining 395 patients are summarized in Table 1 and the Supplementary Appendix. Among the 395 patients, 225 (56.96%) were men and 170 (43.04%) were women, at a median age of 64 years. The majority of the tumors were adenocarcinoma (83.29%), followed by squamous cell carcinoma (13.42%), and other histological types (3.29%). There were 341 (86.33%) patients at stage I–II and 54 (13.67%) at stage III. The medians of preoperative PLT, FIB, and D‐D values were 215 × 109/L, 2.75 g/L, and 0.20 mg/L, respectively. Except for age, gender, and histological type, all other clinicopathological patient characteristics were statistically different between the seven hospitals (Supplementary Appendix). Rates of disease progression were also significantly different between the seven hospitals (P < 0.001).
Table 1.
Demographic and other baseline characteristics of the 395 NSCLC patients
| Variables | Number of patients (%) |
|---|---|
| Age (years) | |
| Median | 64.00 |
| First and third quartiles | 56.00–69.00 |
| Gender | |
| Male | 225 (56.96%) |
| Female | 170 (43.04%) |
| Histological type | |
| Adenocarcinoma | 329 (83.29%) |
| Squamous cell carcinoma | 53 (13.42%) |
| Other | 13 (3.29%) |
| TNM stage | |
| I–II | 341 (86.33%) |
| III | 54 (13.67%) |
| Anatomic location | |
| Central type | 62 (15.70%) |
| Peripheral type | 333 (84.30%) |
| LVI | |
| Yes | 38 (9.62%) |
| No | 357 (90.38%) |
| VPI | |
| Yes | 55 (13.92%) |
| No | 340 (86.08%) |
| Surgery type | |
| Sleeve lobectomy | 326 (82.53%) |
| Wedge resection | 34 (8.61%) |
| Segmentectomy resection | 25 (6.33%) |
| Pneumonectomy | 10 (2.53%) |
| Tumor size (cm) | |
| Median | 2.00 |
| The first and third quartiles | 1.50–3.00 |
| D‐D level (mg/L)† | |
| Median | 0.20 |
| First and third quartiles | 0.10–0.39 |
| FIB level (g/L)† | |
| Median | 2.75 |
| The first and third quartiles | 2.32–3.45 |
| PLT counts (×109/L)† | |
| Median | 215 |
| First and third quartiles | 174–257 |
Preoperative levels, missing values were imputed using Markov chain Monte Carlo. D‐D, D‐dimer; FIB, fibrinogen; LVI, lymphovascular invasion; NSCLC, non‐small cell lung cancer; PLT, platelet; TNM, tumor node metastasis; VPI, visceral pleural invasion.
Survival analyses
During the follow‐up period, 24 patients experienced NSCLC recurrence. The one‐year PFS rates were 92.84% and 97.27% for patients with values of preoperative PLT > median and preoperative PLT ≤ median, 92.38% and 97.95% for patients with values of preoperative FIB > median and preoperative FIB ≤ median, and 93.20% and 96.92% for patients with values of preoperative D‐D > median and preoperative D‐D ≤ median, respectively. The results from univariate Cox regression indicated that elevated preoperative PLT count and FIB level were both significantly associated with poor outcomes (P = 0.03 and P < 0.01, respectively), whereas preoperative D‐D level was not associated with PFS (P = 0.06) (Fig 1). Other factors that were found to be statistically significant in the univariate analyses include gender (P = 0.02), anatomic location (P < 0.001), LVI (P = 0.01), VPI (P = 0.02), TNM stage (P < 0.001), and tumor size (P < 0.001).
Figure 1.
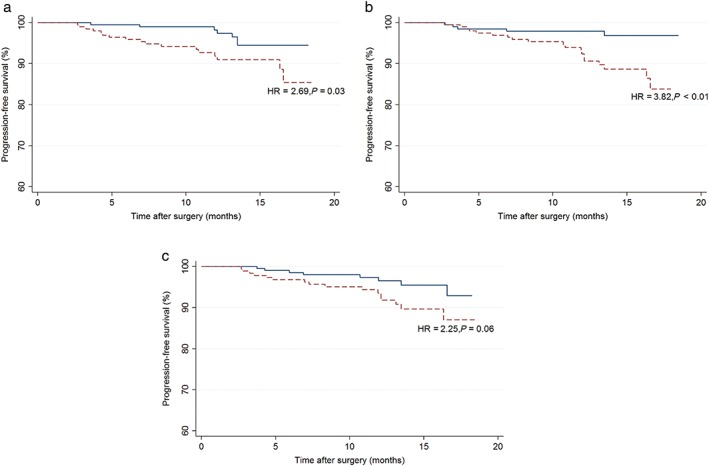
Kaplan–Meier plots for progression‐free survival according to preoperative (a) platelet (PLT) count ( ) PLT ≤ median, (
) PLT ≤ median, ( ) PLT > median; (b) fibrinogen (FIB) level (
) PLT > median; (b) fibrinogen (FIB) level ( ) FIB ≤ median, (
) FIB ≤ median, ( )FIB > median; and (c) D‐Dimer level, (
)FIB > median; and (c) D‐Dimer level, ( ) D‐D ≤ median, (
) D‐D ≤ median, ( ) D‐D > median. Hazard ratios (HRs) and P values were derived from univariate Cox regression.
) D‐D > median. Hazard ratios (HRs) and P values were derived from univariate Cox regression.
As shown in Tables 2 and 3, the results of the single‐level and multilevel models were consistent. The random effects in the multilevel model were statistically significant (P < 0.01), indicating that the multilevel model was suitable to fit our data. After adjusting for the potential confounders, preoperative D‐D was not significantly associated with patient outcome (hazard ratio [HR] 1.61, 95% confidence interval [CI] 0.66–3.93; P = 0.30). Preoperative FIB was also unrelated to PFS in the multilevel survival analysis (HR 1.79, 95% CI 0.59–5.43; P = 0.31). The preoperative PLT association with PFS was marginally significant in the multilevel model (HR 2.42, 95% CI 0.98–5.95; P = 0.05).
Table 2.
Survival analyses results of coagulation factors
| Variables | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) model 1† | P | Adjusted HR (95% CI) model 2‡ | P | |
|---|---|---|---|---|---|---|---|
| D‐D§ | |||||||
| ≤ 0.20 mg/L | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — | |
| > 0.20 mg/L | 2.25 (0.96–5.26) | 0.06 | 1.86 (0.75–4.60) | 0.18 | 1.61 (0.66–3.93) | 0.30 | |
| FIB§ | |||||||
| ≤ 2.75 g/L | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — | |
| > 2.75 g/L | 3.82 (1.43–10.23) | < 0.01 | 2.23 (0.76–6.54) | 0.14 | 1.79 (0.59–5.43) | 0.31 | |
| PLT§ | |||||||
| ≤ 215 × 109/L | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — | |
| > 215 × 109/L | 2.69 (1.11–6.48) | 0.03 | 2.32 (0.95–5.70) | 0.07 | 2.42 (0.98–5.95) | 0.05 | |
| PLT, FIB, and D‐D score | |||||||
| Every one point increase | 2.24 (1.41–3.56) | < 0.001 ¶ | 1.89 (1.14–3.16) | 0.01 ¶ | 1.76 (1.05–2.94) | 0.03 ¶ | |
| PLT, FIB, and D‐D score | |||||||
| Score 0 or 1 | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — | |
| Score 2 or 3 | 7.54 (2.25–25.27) | < 0.01 | 4.71 (1.34–16.52) | 0.02 | 4.62 (1.29–16.51) | 0.02 | |
Model 1: single‐level multivariate model; adjusted for gender, anatomic location, lymphovascular invasion (LVI), visceral pleural invasion (VPI), tumor node metastasis (TNM) stage, and tumor size.
Model 2: multilevel model; adjusted for gender, anatomic location, LVI, VPI, TNM stage, and tumor size.
Preoperative levels, missing values were imputed using Markov chain Monte Carlo.
P value for trend. Bold values (P<0.05) indicate statistical significance. CI, confidence interval; D‐D, D‐dimer; FIB, fibrinogen; HR, hazard ratio; PLT, platelet.
Table 3.
Survival analyses results of clinicopathological and other factors
| Variables | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI)† | P | Adjusted HR (95% CI)‡ | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — |
| Female | 0.28 (0.10–0.82) | 0.02 | 0.53 (0.16–1.71) | 0.29 | 0.46 (0.14–1.55) | 0.46 |
| TNM stage | ||||||
| Stage I–II | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — |
| Stage III | 7.93 (3.55–17.73) | < 0.001 | 5.48 (2.06–14.55) | < 0.001 | 4.76 (1.81–12.51) | < 0.01 |
| Anatomic location | ||||||
| Central type | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — |
| Peripheral type | 0.22 (0.10–0.50) | < 0.001 | 0.69 (0.27–1.77) | 0.43 | 0.89 (0.34–2.36) | 0.82 |
| LVI | ||||||
| Yes | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — |
| No | 0.31 (0.12–0.79) | 0.01 | 1.31 (0.44–3.88) | 0.63 | 1.07 (0.34–3.32) | 0.91 |
| VPI | ||||||
| Yes | ‐Reference‐ | — | ‐Reference‐ | — | ‐Reference‐ | — |
| No | 0.37 (0.16–0.87) | 0.02 | 1.11 (0.44–2.82) | 0.83 | 1.47 (0.54–4.02) | 0.45 |
| Tumor size | 1.35 (1.17–1.56)§ | < 0.001 | 1.11 (0.91–1.35)§ | 0.31 | 1.08 (0.87–1.34)§ | 0.49 |
| Hospital& | Adjusted hazard (95% CI) ¶ | |||||
| Hospital 1 | — | — | — | — | 1.15 (0.35–3.78) | 0.82 |
| Hospital 2 | — | — | — | — | 0.93 (0.24–3.54) | 0.91 |
| Hospital 3 | — | — | — | — | 0.58 (0.11–3.02) | 0.51 |
| Hospital 4 | — | — | — | — | 0.34 (0.08–1.49) | 0.15 |
| Hospital 5 | — | — | — | — | 0.74 (0.12–4.36) | 0.74 |
| Hospital 6 | — | — | — | — | 3.67 (1.26–10.66) | 0.02 |
| Hospital 7 | — | — | — | — | 1.80 (0.49–6.60) | 0.38 |
Only the variables that were statistically significant in univariate analyses were included in multivariate and multilevel analyses.
Single‐level multivariate model; adjusted for each other and platelet (PLT), fibrinogen (FIB), and D‐dimer (D‐D) score as a binary variable.
Multilevel model; adjusted for each other and PLT, FIB, and D‐D score as a binary variable.
Hazard ratio (HR) for every one unit increase in the variable.
Exponential of the random intercept in multilevel model; gender, tumor node metastasis (TNM) stage, anatomic location, lymphovascular invasion (LVI), visceral pleural invasion (VPI), tumor size and PLT, FIB and D‐D score (binary variable) were adjusted.
Bold values (P<0.05) indicate statistical significance. CI, confidence interval.
In order to investigate the combined effects of preoperative D‐D, FIB, and PLT levels on PFS, we further assigned each patient a score (0–3) according to the number of elevated preoperative coagulation factors: 0, patients with no elevation in the three preoperative coagulation factors; 1, patients with one elevation in the three preoperative coagulation factors; 2, patients with two elevations in any of the three preoperative coagulation factors; and 3, patients with all three preoperative coagulation factors elevated. The results of multilevel analyses revealed that the risk of relapse is 76% higher for every one point increase in the score (HR 1.76, 95% CI 1.05–2.94; P = 0.03) (Table 2). Specifically, compared to patients with scores of 0 or 1, the risk of relapse is 4.6 times higher for patients with scores 2 or 3 (HR 4.62, 95% CI 1.29–16.5; P = 0.02).
Except for TNM stage, which was significant in both single‐level multivariate models, other potential confounders, including gender, anatomic location, LVI, VPI, and tumor size were not significantly associated with outcome in multivariate models.
Discussion
The relationship between cancer and blood coagulation was first reported in 19th century. Since then, much attention has been devoted to this research field. Experimental studies have found that PLT contributes to cancer progression through both thrombin‐dependent and thrombin‐independent mechanisms.23 In thrombin‐dependent mechanisms, thrombin activated PLT can release a variety of growth factors, such as vascular endothelial growth factor,24 platelet‐derived growth factor,25 and transforming growth factor‐β,26 and therefore promote tumor cell angiogenesis and proliferation. In thrombin‐independent mechanisms, tumor cells can directly induce PLT activation and aggregation and then facilitate the formation of tumor cell‐platelet thrombi, which is believed to have the effect of preventing intravascular tumor cell elimination by natural killer (NK) cells.27 Some studies have shown that FIB can also protect tumor cells from NK cell‐mediated cytotoxicity by aggregating around tumor cells and forming dense fibrin layers.28, 29 In fact, FIB and PLT can interact with each other to protect tumor cells from NK cytotoxicity.30 FIB can enhance the adhesion of PLT to tumor cells, and PLT in turn can release thrombin and facilitate the aggregation of FIB.30 FIB can also serve as a scaffold to bind both vascular endothelial growth factor and fibroblast growth factor‐2 and augments their proliferative and angiogenesis effects.31 As a fibrin degradation product, D‐D is a sensitive indicator of coagulation and fibrinolysis activation.32 Despite its routine use as a predictor of venous thromboembolism in cancer patients, a growing number of studies have reported that a higher D‐D level is associated with tumor metastasis; however, the exact mechanisms are still unknown.33, 34 Some researchers have speculated that D‐D dimer is a global surrogate marker that indicates tumor aggressiveness.35
In the current study, the results of univariate analysis indicate that an elevated preoperative PLT count is significantly associated with a poor outcome. After adjusting for confounders, this relationship was marginally significant, which may suggest the independent prognostic role of PLT in NSCLC. In a study conducted by Kim et al., preoperative thrombocytosis (PLT > 400 × 109/L) was associated with a higher risk of recurrence among NSCLC patients.11 Li et al. revealed that advanced NSCLC patients with elevated PLT counts (> 200 × 109/L) had significantly poorer PFS compared to patients with PLT counts ≤ 200 × 109/L.12 In contrast, in studies conducted by Cakar et al. and Canova et al., thrombocytosis was not a significant prognostic factor of PFS.14, 15 However, in the study conducted by Canova et al., the PLT count was measured after chemotherapy rather than before treatment. In the study conducted by Cakar et al., both NSCLC and SCLC patients were included. These differences may explain the insignificant results of the two studies.
In the current study, we also found that patients with elevated preoperative FIB levels had a significantly higher risk of disease progression. However, this association was not confirmed in multilevel analysis. Inconsistent with our result, Sheng et al. and Jiang et al. both reported that NSCLC patients with preoperative hyperfibrinogenemia (≥ 4.0 g/L) had an increased risk of disease progression compared to patients without hyperfibrinogenemia.8, 10 Meanwhile, a recent study also revealed that preoperative hyperfibrinogenemia is significantly associated with disease progression among NSCLC patients.13 Nevertheless, the inconsistencies in results between our study and previous studies can at least partly be attributed to the short follow‐up time in our study. Only 24 patients had disease progression and over 90% of the patients were censored, therefore the statistical power of our study is low.
Jiang et al. reported that preoperative D‐D positivity (> 0.55 mg/L) is a significant and independent predictor for unfavorable disease‐free survival in NSCLC patients.8 A recent study conducted by Kaoru et al. also revealed that an elevated preoperative D‐D level (>1.0 mg/L) is significantly associated with poor recurrence‐free survival among stage I NSCLC patients.9 In our study, however, neither univariate nor multilevel analyses confirmed the prognostic significance of elevated preoperative D‐D. We believe this can be explained by the low statistical power of our study. Moreover, other more potent prognostic factors may have overshadowed its effect in the multilevel analyses.
To the best of our knowledge, this study is the first to investigate the combined effects of preoperative PLT, FIB, and D‐D levels on NSCLC prognosis. We found that the risk of cancer progression significantly increased for every one‐point increase in the number of elevated coagulation factors. Compared to patients with only one elevated coagulation factor, patients with at least two elevated coagulation factors had a significantly higher risk of cancer progression. Combining the individual and combined effects of preoperative PLT, FIB, and D‐D levels on NSCLC prognosis, we speculate that there may be some interactions between the three coagulation factors or any two of them. More studies with large sample sizes and longer follow‐up are warranted to further investigate the potential interactions between preoperative PLT, FIB, and D‐D among NSCLC patients. Meanwhile, the underlying mechanisms are largely unknown and require further study. Based on these results, we propose the combined use of preoperative PLT, FIB, and D‐D levels to predict the prognosis of operable NSCLC patients. As PLT, FIB, and D‐D levels are routinely measured before surgery for NSCLC patients, clinicians may also consider the combined use of the three coagulation factors in clinical practice.
The strengths of our study include a larger sample size compared to some previous studies, a multicenter and prospective study design, and the adoption of multilevel survival analysis. Nevertheless, some limitations need to be highlighted. First, as previously mentioned, the follow‐up duration of our study was short for an analysis of PFS and the sample size was insufficient, which decreased our statistical power to detect differences between different groups. We will consider extending the follow‐up time and including more cases in our further study. Secondly, because of missing data, only the preoperative PLT, FIB, and D‐D levels were used for survival analyses; therefore, the effects of postoperative PLT, FIB, and D‐D levels were not investigated. Thirdly, we did not collect information on the adjuvant therapies administered and adjust them in the analyses, which may have biased the results. Finally, coagulation assays were conducted in different laboratories and therefore systematic differences may exist. Nevertheless, we believe the differences were minor as all laboratories have passed external quality assessment and used the same methods for coagulation assays.
In the current multicenter prospective study conducted on operable NSCLC patients, we found that an elevated preoperative PLT level is significantly associated with poorer PFS. However, the prognostic significance of preoperative FIB and D‐D levels were not confirmed, partly a result of the short follow‐up duration in our study. More importantly, we found that the number of elevated preoperative coagulation factors may be a significant indicator of progression. We reason that there are potential interactions between these three coagulation factors that need further study. Researchers and clinicians may consider using preoperative PLT, FIB, and D‐D levels in combination when predicting the prognosis of NSCLC patients after surgery.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Basic information of the seven hospitals.
Table S2. Demographic and other baseline characteristics of the 395 non‐small cell lung cancer (NSCLC) patients stratified by the hospitals.
Acknowledgments
This study was supported by the Graduate Student's Research and Innovation Fund of Sichuan University (2018YJSY112).
Contributor Information
Liang Chen, Email: 2434794740@qq.com.
Jiayuan Li, Email: lijiayuan73@163.com.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Mortality Collaborators . Global, regional, and national under‐5 mortality, adult mortality, age‐specific mortality, and life expectancy, 1970‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1084–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ettinger DS, Akerley W, Bepler G et al Non‐small cell lung cancer. J Natl Compr Canc Netw 2010; 8: 740–801. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 5. Slebos RJ, Kibbelaar RE, Dalesio O et al K‐ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med 1990; 323: 561–5. [DOI] [PubMed] [Google Scholar]
- 6. Horio Y, Takahashi T, Kuroishi T et al Prognostic significance of p53 mutations and 3p deletions in primary resected non‐small cell lung cancer. Cancer Res 1993; 53: 1–4. [PubMed] [Google Scholar]
- 7. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: Biological and clinical aspects. J Thromb Haemost 2013; 11: 223–33. [DOI] [PubMed] [Google Scholar]
- 8. Jiang HG, Li J, Shi SB et al Value of fibrinogen and D‐dimer in predicting recurrence and metastasis after radical surgery for non‐small cell lung cancer. Med Oncol 2014; 31: 22. [DOI] [PubMed] [Google Scholar]
- 9. Kaseda K, Asakura K, Kazama A, Ozawa Y. Prognostic significance of preoperative plasma D‐dimer level in patients with surgically resected clinical stage I non‐small cell lung cancer: A retrospective cohort study. J Cardiothorac Surg 2017; 12: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer 2013; 133: 2720–5. [DOI] [PubMed] [Google Scholar]
- 11. Kim M, Chang H, Yang HC et al Preoperative thrombocytosis is a significant unfavorable prognostic factor for patients with resectable non‐small cell lung cancer. World J Surg Oncol 2014; 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Miao LY, Xiao YL, Cai HR, Zhang DP. Elevated platelets enhance cancer cell migration, promote hematogenous metastasis and associate with a poor prognosis in advanced non‐small cell lung cancer cases. Asian Pac J Cancer Prev 2014; 15: 139–43. [DOI] [PubMed] [Google Scholar]
- 13. Jiang LY, Zhang XF, Ding FB, Wang MS, Mei J, He Y. Prognostic implications of plasma fibrinogen and serum Creactive protein levels in non‐small cell lung cancer resection and survival. Trop J Pharm Res 2017; 16: 665–72. [Google Scholar]
- 14. Cakar B, Karaoglanoglu M, Sayici Y, Demirag GG, Yucel I. The prognostic value of thrombocytosis in newly diagnosed lung cancer patients: A retrospective analysis. J BUON 2011; 16: 677–81. [PubMed] [Google Scholar]
- 15. Canova S, Cicchiello F, Agustoni F et al Gemcitabine‐induced thrombocytosis as a potential predictive factor in non‐small cell lung cancer: Analysis of 318 patients. Tumori 2017; 103: 143–7. [DOI] [PubMed] [Google Scholar]
- 16. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 17. Vallieres E, Shepherd FA, Crowley J et al The IASLC Lung Cancer Staging Project: Proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 1049–59. [DOI] [PubMed] [Google Scholar]
- 18. Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015; 121 ((Suppl. 17)): 3165–81. [DOI] [PubMed] [Google Scholar]
- 19. Chen F, ed. Missing no more: Using the MCMC procedure to model missing data Proceedings of the SAS Global Forum Conference. SAS Institute Inc, Cary, NC 2013. [Cited 6 Apr 2018.] Available from URL: https://support.sas.com/resources/papers/proceedings13/436‐2013.pdf
- 20. Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics 1982; 38: 921–32. [PubMed] [Google Scholar]
- 21. Gill RD. Multistate life‐tables and regression models. Math Popul Stud 1992; 3: 259–76. [DOI] [PubMed] [Google Scholar]
- 22. Crowther MJ, Look MP, Riley RD. Multilevel mixed effects parametric survival models using adaptive Gauss‐Hermite quadrature with application to recurrent events and individual participant data meta‐analysis. Stat Med 2014; 33: 3844–58. [DOI] [PubMed] [Google Scholar]
- 23. Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease‐activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004; 104: 397–401. [DOI] [PubMed] [Google Scholar]
- 24. Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin‐induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A 1997; 94: 663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Kalen A, Risto O, Wahlstrom O. Time‐ and pH‐dependent release of PDGF and TGF‐beta from platelets in vitro. Platelets 2003; 14: 233–7. [DOI] [PubMed] [Google Scholar]
- 26. Takemoto A, Okitaka M, Takagi S et al A critical role of platelet TGF‐beta release in podoplanin‐mediated tumour invasion and metastasis. Sci Rep 2017; 7: 42186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palumbo JS, Talmage KE, Massari JV et al Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell‐mediated elimination of tumor cells. Blood 2005; 105: 178–85. [DOI] [PubMed] [Google Scholar]
- 28. Atagi S, Sone S, Fukuta K, Ogura T. Inhibition by fibrin coagulation of lung cancer cell destruction by human interleukin‐2‐activated killer cells. Jpn J Cancer Res 1992; 83: 1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen‐deficient mice. Cancer Res 2002; 62: 6966–72. [PubMed] [Google Scholar]
- 30. Zheng S, Shen J, Jiao Y et al Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci 2009; 100: 859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000; 96: 3772–8. [PubMed] [Google Scholar]
- 32. Adam SS, Key NS, Greenberg CS. D‐dimer antigen: Current concepts and future prospects. Blood 2009; 113: 2878–87. [DOI] [PubMed] [Google Scholar]
- 33. Blackwell K, Haroon Z, Broadwater G et al Plasma D‐dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol 2000; 18: 600–8. [DOI] [PubMed] [Google Scholar]
- 34. Diao D, Wang Z, Cheng Y et al D‐dimer: Not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One 2014; 9: e101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ay C, Dunkler D, Pirker R et al High D‐dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012; 97: 1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Basic information of the seven hospitals.
Table S2. Demographic and other baseline characteristics of the 395 non‐small cell lung cancer (NSCLC) patients stratified by the hospitals.


