Abstract
Background
The mechanism underlying tumor spread through air spaces (STAS) has not been well studied. We investigated the role of tumor stromal cells in the pathogenesis of STAS from a pathological perspective and evaluated the prognostic significance of tumor stromal cells and STAS in postoperative patients with lung adenocarcinoma.
Methods
We retrospectively analyzed 208 postsurgical patients with stage I–IIIA lung adenocarcinoma. The presence of STAS was evaluated by hematoxylin and eosin staining. The expression of α‐smooth muscle actin (SMA)‐positive cancer‐associated fibroblasts (CAFs) and CD204‐positive tumor‐associated macrophages (TAMs) was analyzed by immunohistochemistry. A logistic regression model was applied to confirm the predictive factors of STAS. Survival analysis was performed to evaluate the effect of α‐SMA‐positive CAFs, CD204‐positive TAMs, and STAS on prognosis. A nomogram was generated to evaluate the prognosis of postoperative patients.
Results
Logistic regression suggested that the expression of α‐SMA‐positive CAFs (P < 0.001) and the number of CD204‐positive TAMs (P < 0.001) were related to the presence of STAS. The multivariate Cox proportional hazards model suggested that STAS (P = 0.004), α‐SMA‐positive CAFs (P < 0.001), and CD204‐positive TAMs (P < 0.001) were independent risk factors for prognosis. Harrell's c‐indexes for overall and recurrence‐free survival prediction based on nomograms were 0.84 (95% confidence interval 0.76–0.91) and 0.82 (95% confidence interval 0.76–0.89), respectively.
Conclusions
The presence of STAS was associated with high expression of α‐SMA and CD204 in lung adenocarcinoma. Nomograms including STAS and stromal cells as variables are recommended as practical models to evaluate the prognosis of lung adenocarcinoma patients.
Keywords: Lung cancer, prognosis, STAS, stromal cell, tumor spread through air spaces
Introduction
Non‐small cell lung cancer (NSCLC) is one of the leading causes of cancer‐associated death worldwide, with high incidence and mortality rates.1 The high degree of malignancy and the heterogeneity of cancer cells have led researchers to focus on the study of malignant cells present in the epithelial compartment.2 Recent studies have reported important variations in recurrence‐free survival (RFS) and overall survival (OS) rates after radical resection, even among stage I lung adenocarcinoma patients, suggesting the existence of high‐risk subgroups.3, 4, 5, 6 This heterogeneity in survival outcomes and histology motivated the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) to propose a new multidisciplinary lung adenocarcinoma classification system in 2011.7 This classification scheme recognizes five different patterns of lung adenocarcinoma growth (i.e. histological subtypes): lepidic, acinar, papillary, solid, and micropapillary.7
Along with the publication of the IASLC/ATS/ERS classification, a few new morphological features have been identified that appear to be prognostically important, including spread through air spaces (STAS).8 STAS is defined as the spread of lung cancer cells into air spaces in the lung parenchyma beyond the edge of the main tumor and can be evaluated by hematoxylin and eosin (H&E) staining. STAS occurs less frequently in lepidic‐predominant adenocarcinomas and more frequently in micropapillary and solid‐predominant adenocarcinomas. A growing number of independent studies have validated STAS as a predictor of recurrence and survival in lung adenocarcinoma.8, 9, 10, 11, 12, 13 However, the mechanisms underlying the presence of STAS have not been well studied.
Several recent studies demonstrated that the biological behavior of cancer cells is largely influenced by the tumor microenvironment.14, 15, 16, 17, 18 The tumor microenvironment is composed of not only cancer cells, but also several kinds of stromal cells, including fibroblasts; macrophages; inflammatory cells, such as neutrophils and lymphocytes; and newly formed microvessels. These stromal cells can influence the proliferation, survival, and invasion of cancer cells and create a specific tumor microenvironment.19, 20, 21, 22, 23, 24
Cancer‐associated fibroblasts (CAFs) and tumor‐associated macrophages (TAMs) are the major cellular components of the tumor microenvironment. Studies show that co‐cultivation and co‐injection of cancer cells with tumor‐promoting CAFs or TAMs leads to enhanced invasiveness or implantation of malignant cells.25, 26, 27 Furthermore, tumor‐promoting CAFs and TAMs release various growth factors, cytokines, and proteinases that create a favorable microenvironment for tumor progression.27, 28, 29 Alpha smooth muscle actin (α‐SMA) and CD204 are representative markers of tumor‐promoting CAFs and TAMs in resected specimens.21, 30, 31, 32 Therefore, we hypothesized that the expression of α‐SMA and CD204 may be related to the presence of STAS.
The purpose of this study was to investigate the roles of tumor stromal cells in the pathogenesis of STAS from a pathological perspective and to evaluate the prognostic significance of tumor stromal cells and STAS in patients with lung adenocarcinoma.
Methods
Patients and clinicopathological data
Data from 312 patients with lung cancer treated by radical surgical resection at the Second Affiliated Hospital of Soochow University between January 2009 and December 2014 were retrospectively analyzed. The inclusion criteria were as follows: (i) patients treated by radical resection; (ii) pathologically confirmed stage I–IIIA lung adenocarcinoma; and (iii) complete follow‐up information. Patients who received neoadjuvant radiochemotherapy were excluded. Finally, a total of 208 patients were included in the study. Two pathologists separately reviewed the H&E stained slides of 208 resected specimens. If there were controversies or discordance in the pathological diagnoses, consensus was reached by discussion. Lung adenocarcinomas were classified according to the IASLC/ATS/ERS classification7 and staged according to the eighth edition of the tumor node metastasis (TNM) Classification for Lung Cancer.33 The presence of STAS was identified by routine H&E staining.
The Institutional Review Board of the Second Affiliated Hospital of Soochow University approved the study. The requirement for informed patient consent was waived because of the retrospective nature of the study.
Immunohistochemical staining
The presence of α‐SMA‐positive CAFs and CD204‐positive TAMs in consecutive slides from resected samples was evaluated by immunohistochemical staining for α‐SMA and CD204 in lung adenocarcinoma specimens. Sections (4 μm thick) were deparaffinized with xylene, rehydrated, and antigen‐retrieved in a microwave oven for 20 minutes. The slides were then washed three times in phosphate buffered saline (PBS) and immersed in a 0.3% hydrogen peroxide solution in methanol for 15 minutes to inhibit endogenous peroxidase activity. After washing the slides three times in PBS, nonspecific binding was blocked by preincubation with 2% normal swine serum in PBS (blocking buffer) for 30 minutes at room temperature. For α‐SMA immunohistochemical staining, individual slides were incubated overnight at 4°C with anti‐human α‐SMA rabbit polyclonal antibody (PA5‐22251; Thermo Fisher Scientific, Rockford, IL, USA) at a final dilution of 1:200 in blocking buffer. Tissue sections were also stained with a mouse anti‐human CD204 antibody (Scavenger Receptor Class A‐E5; TransGenic Inc., Kobe, Japan) at a final dilution of 1:400 in blocking buffer. Slides were subsequently incubated with EnVision (Dako, Denmark A/S, Glostrup, Denmark) for one hour at room temperature. The slides were then washed three times with PBS and incubated with EnVision (Dako) for one hour at room temperature, and after extensive washing with PBS, the color reaction was developed in 2% 3, 3‐diamino‐benzidine in 50 mM Tris‐buffer (pH 7.6) containing 0.3% hydrogen peroxidase. Finally, the sections were counterstained with Meyer hematoxylin, dehydrated, and mounted.
Assessment of α‐smooth muscle actin (SMA) expression in cancer‐associated fibroblasts (CAFs) and CD204 in tumor‐associated macrophages (TAMs)
Two independent pathologists unaware of clinical patient data reviewed the stained slides. To score the expression of α‐SMA in CAFs, four high power fields in tumor stroma were randomly selected for each slide. According to previous studies, the staining index was calculated as a product of staining intensity (negative = 0, weak = 1, moderate = 2, and strong = 3) multiplied by staining extent (0% = 0, 1–10% = 1, 11–50% = 2, and > 50% = 3). A final score of 0–2 indicated α‐SMA‐negative CAFs, and a score of > 2 indicated α‐SMA‐positive CAFs.34, 35
To estimate the expression of CD204 in TAMs, the four areas showing the highest CD204‐positive TAM infiltration within a section were selected, and the number of CD204‐positive TAMs in tumor stroma was counted under a light microscope at ×400 magnification (0.0625 mm2/field). The count was recorded as the average number of CD204‐positive TAMs for each case. High expression was defined as an average number of CD204‐positive TAMs > 8.32
Statistical analysis
Correlation analysis was performed using the χ2 test and a logistic regression model. OS and RFS were calculated using the Kaplan–Meier method, and differences between groups were analyzed using the log‐rank test. Cox proportional hazards multivariate models were used to identify independent predictors. All P values reported were two‐sided, and the significance level was set at < 0.05. Analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Description of baseline features
The clinicopathological features of 208 patients are shown in Table S1. Among the enrolled patients, 51.9% were female and 56.3% were non‐smokers. The mean age of the patients was 63 (range: 31–81) years. There were 127 patients (61.1%) with stage I disease, 50 (24.0%) with stage II disease, and 31 (14.9%) with stage IIIA disease. The distribution of predominant histological patterns was as follows: lepidic in 14 patients (6.7%), acinar in 64 (30.8%), papillary in 95 (45.7%), solid in 28 (13.5%), and micropapillary in 7 patients (3.4%). STAS was present in the histopathological slides of 107 patients (51.4%). A representative image of an H&E stained case for STAS is shown in Figure 1. In the adenocarcinoma specimens, 84 cases (40.4%) of high α‐SMA‐positive CAFs and 110 cases (52.9%) with a high number of CD204‐positive TAMs were identified. Representative images of immunohistochemical staining for α‐SMA and CD204 are shown in Figure 2.
Figure 1.
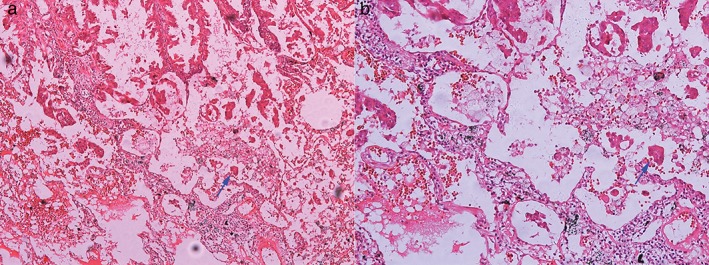
Spread through air spaces (STAS) in the alveolar cavity: (a) original magnification, ×100; and (b) original magnification, ×400.
Figure 2.
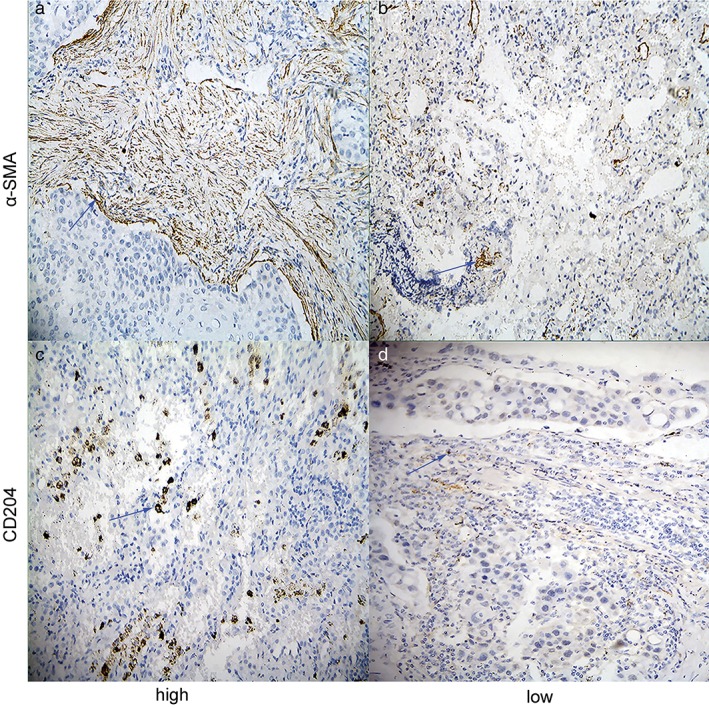
Immunohistochemical staining of α‐smooth muscle actin (SMA) and CD204 in lung adenocarcinomas (original magnification, ×400). Representative images of (a) high stromal and (b) low stromal α‐SMA‐positive cases and (c) high stromal and (d) low stromal CD204‐positive cases.
Associations between the presence of spread through air spaces (STAS) and clinicopathological factors, α‐SMA, and CD204
The associations between clinicopathological variables, α‐SMA‐positive CAFs, and CD204‐positive TAMs and the presence of STAS are shown in Table 1. The presence of STAS was significantly associated with higher clinical stage (odds ratio [OR] 12.300, 95% confidence interval [CI] 4.049–37.369; P < 0.001), larger tumor diameter (OR 2.907, 95% CI 1.654–5.107; P < 0.001), lymph node metastasis (OR 5.723, 95% CI 3.051–10.734; P < 0.001), and micropapillary histological type (OR 10.800, 95% CI 0.997–116.998; P < 0.001). STAS was more likely to be present in resected specimens with a higher frequency of α‐SMA‐positive CAFs (OR 4.096, 95% CI 2.256–7.436; P < 0.001) and a higher number of CD204‐positive TAMs (OR 2.567, 95% CI 1.517–4.656; P < 0.001). Together these results suggested that cancer‐associated stromal cells were related to the occurrence of STAS. In addition, the presence of CD204‐positive TAMs was significantly correlated with smoking status (P < 0.001).
Table 1.
Associations between the presence of STAS and clinicopathological features, α‐SMA‐positive CAFs, and CD204‐positive TAMs
| Variables | STAS, No. of patients | |||
|---|---|---|---|---|
| Negative | Positive | OR (95% CI) | P | |
| Age, year | 0.183 | |||
| ≤ 65 | 46 | 39 | 1 | |
| > 65 | 39 | 68 | 1.458 (0.837–2.541) | |
| Gender | 0.218 | |||
| Male | 48 | 60 | 1 | |
| Female | 53 | 47 | 1.410 (0.816–2.434) | |
| Smoking history | 0.541 | |||
| No | 59 | 58 | 1 | |
| Yes | 42 | 49 | 1.187 (0.686–2.055) | |
| Clinical stage | < 0.001 | |||
| I | 82 | 45 | 1 | |
| II | 15 | 35 | 4.252 (2.099–8.611) | |
| IIIA | 4 | 27 | 12.300 (4.049–37.369) | |
| Tumor diameter (cm) | < 0.001 | |||
| ≤ 3 | 65 | 36 | 1 | |
| > 3 | 41 | 66 | 2.907 (1.654–5.107) | |
| Lymph node metastasis | < 0.001 | |||
| No | 82 | 46 | 1 | |
| Yes | 19 | 61 | 5.723 (3.051–10.734) | |
| Histological subtypes | 0.005 | |||
| Lepidic | 9 | 5 | 1 | |
| Acinar | 42 | 22 | 0.943 (0.281–3.158) | |
| Papillary | 38 | 57 | 2.700 (0.840–8.680) | |
| Solid | 11 | 17 | 2.782 (0.735–10.524) | |
| Micropapillary | 1 | 6 | 10.800 (0.997–116.998) | |
| α‐SMA‐positive CAFs | < 0.001 | |||
| Low | 77 | 47 | 1 | |
| High | 24 | 60 | 4.096 (2.256–7.436) | |
| CD204‐positive TAMs | 0.001 | |||
| Low | 60 | 38 | 1 | |
| High | 41 | 69 | 2.657 (1.517–4.656) | |
Significant P value shown in bold.
CAFs, cancer‐associated fibroblasts; CI, confidence interval; OR, odds ratio; STAS, spread through air spaces; TAMs, tumor‐associated macrophages.
Prognostic impact of STAS, α‐SMA‐positive CAFs, and CD204‐positive TAMs in patients with stage I–IIIA lung adenocarcinoma
Figure 3 shows the OS and RFS curves of patients with stage I–IIIA lung adenocarcinoma according to the presence of STAS (P < 0.001), the scoring of α‐SMA‐positive CAFs (P < 0.001), and the number of CD204‐positive TAMs (P < 0.001). The log‐rank test revealed a significant difference between the survival curves of the two groups categorized by either variable. Univariate analysis identified nine significant risk factors for OS: gender, smoking history, clinical stage, tumor diameter, lymph node metastasis, histological subtype, α‐SMA‐positive CAFs, CD204‐positive TAMs, and STAS‐positivity. Seven significant risk factors for recurrence were identified: clinical stage, tumor diameter, lymph node metastasis, histological subtype, α‐SMA‐positive CAFs, CD204‐positive TAMs, and STAS positivity (Table 2). Multivariate analysis with the Cox regression model showed that the presence of STAS (hazard ratio [HR] 3.390, 95% CI 1.925–5.968; P < 0.001), and high frequencies of α‐SMA‐positive CAFs (HR 4.782, 95% CI 2.781–8.226; P < 0.001) and CD204‐positive TAMs (HR 4.633, 95% CI 2.668–8.044; P < 0.001) were statistically significant independent predictors for OS, whereas the presence of STAS (HR 1.759, 95% CI 1.096–2.824; P = 0.019) and high frequencies of α‐SMA‐positive CAFs (HR 5.561, 95% CI 3.364–9.195; P < 0.001) and CD204‐positive TAMs (HR 2.236, 95% CI, 1.458–3.427; P < 0.001) were statistically significant independent predictors for RFS (Table 3).
Figure 3.
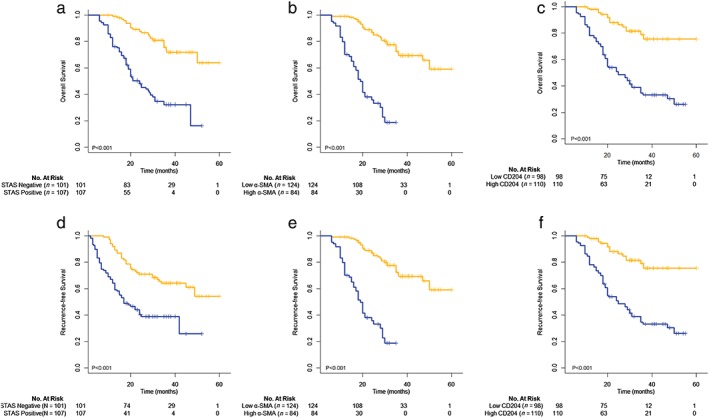
Overall survival curves according to the presence of (a) spread through air spaces (STAS) (P < 0.001), (b) scoring of α‐smooth muscle actin (SMA)‐positive cancer‐associated fibroblasts (CAFs) (P < 0.001) and (c) the number of CD204‐positive tumor‐associated macrophages (TAMs) (P < 0.001). Recurrence‐free survival curves (d) according to the presence of STAS (P < 0.001), (e) scoring of α‐SMA‐positive CAFs (P < 0.001) and (f) the number of CD204‐positive TAMs (P < 0.001). ( ) STAS Negative (n = 101) (
) STAS Negative (n = 101) ( ) STAS positive (n = 107), (
) STAS positive (n = 107), ( ) low α‐SMA (n = 124) (
) low α‐SMA (n = 124) ( ) high α‐SMA (n = 84), (
) high α‐SMA (n = 84), ( ) low CD204 (n = 98) (
) low CD204 (n = 98) ( ) high CD204 (n = 110), (
) high CD204 (n = 110), ( ) STAS negative (n = 101) (
) STAS negative (n = 101) ( ) STAS positive (n = 107), (
) STAS positive (n = 107), ( ) low α‐SMA (n = 124) (
) low α‐SMA (n = 124) ( ) high α‐SMA (n = 84), and (
) high α‐SMA (n = 84), and ( ) low CD204 (n = 98) (
) low CD204 (n = 98) ( ) high CD204 (n = 110).
) high CD204 (n = 110).
Table 2.
Univariate analysis using the Cox proportional‐hazards regression model for overall and recurrence‐free survival
| Variables | Overall survival | Recurrence‐free survival | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age, year | 0.373 | 0.108 | ||
| ≤ 65 | 1 | 1 | ||
| > 65 | 1.223 (0.786–1.903) | 1.412 (0.927–2.150) | ||
| Gender | 0.029 | 0.076 | ||
| Male | 1 | 1 | ||
| Female | 1.619 (1.050–2.497) | 1.438 (0.962–2.151) | ||
| Smoking history | 0.010 | 0.101 | ||
| No | 1 | 1 | ||
| Yes | 1.747 (1.140–2.678) | 1.397 (0.937–2.082) | ||
| Tumor diameter | < 0.001 | < 0.001 | ||
| ≤ 3 | 1 | 1 | ||
| > 3 | 2.555 (1.634–3.996) | 3.076 (2.007–4.714) | ||
| Lymph node metastasis | < 0.001 | < 0.001 | ||
| No | 1 | 1 | ||
| Yes | 3.639 (2.315–5.719) | 3.854 (2.531–5.868) | ||
| Clinical stage | < 0.001 | < 0.001 | ||
| I | 1 | 1 | ||
| II | 2.525 (1.469–4.340) | 2.843 (1.745–4.632) | ||
| IIIA | 20.874 (10.935–39.848) | 16.484 (9.343–29.082) | ||
| Histological subtypes | < 0.001 | 0.025 | ||
| Lepidic | 1 | 1 | ||
| Acinar | 1.221 (0.420–3.548) | 1.280 (0.494–3.315) | ||
| Papillary | 1.427 (0.507–4.013) | 1.469 (0.584–3.696) | ||
| Solid | 1.915 (0.625–5.861) | 1.805 (0.617–3.982) | ||
| Micropapillary | 2.219 (0.810–8.832) | 2.061 (1.711–4.477) | ||
| α‐SMA‐positive CAFs | < 0.001 | < 0.001 | ||
| Low | 1 | 1 | ||
| High | 7.031(4.272–11.571) | 8.185(5.105–13.123) | ||
| CD204‐positive TAMs | <0.001 | 0.001 | ||
| Low | 1 | 1 | ||
| High | 4.628 (2.684–7.980) | 2.077 (1.367–3.157) | ||
| STAS | < 0.001 | < 0.001 | ||
| Negative | 1 | 1 | ||
| Positive | 4.552 (2.771–7.478) | 2.688 (1.762–4.101) | ||
Significant P value shown in bold.
CAFs, cancer‐associated fibroblasts; CI, confidence interval; HR, hazard ratio; TAMs, tumor‐associated macrophages; STAS, spread through air spaces.
Table 3.
Multivariate analysis using the Cox proportional‐hazards regression model for overall and recurrence‐free survival
| Variables | Overall survival | Recurrence‐free survival | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.844 | –– | ||
| Male | 1 | –– | ||
| Female | 1.085 (0.481–2.450) | –– | ||
| Smoking history | 0.412 | –– | ||
| No | 1 | –– | ||
| Yes | 1.406 (0.623–3.175) | –– | ||
| Tumor diameter | 0.201 | 0.010 | ||
| ≤ 3 | 1 | 1 | ||
| > 3 | 1.385 (0.841–2.281) | 1.840 (1.154–2.933) | ||
| Lymph node metastasis | < 0.001 | 0.021 | ||
| No | 1 | 1 | ||
| Yes | 1.549 (0.919–2.612) | 1.756 (1.090–2.828) | ||
| Histological subtypes | 0.038 | 0.709 | ||
| Lepidic | 1 | 1 | ||
| Acinar | 1.565 (0.524–4.671) | 1.237 (0.467–3.277) | ||
| Papillary | 1.063 (0.366–3.089) | 1.154 (0.450–2.961) | ||
| Solid | 2.055 (0.654–6.455) | 1.380 (0.470–4.050) | ||
| Micropapillary | 3.578 (0.974–13.139) | 2.231 (0.614–5.101) | ||
| α‐SMA‐positive CAFs | < 0.001 | < 0.001 | ||
| Low | 1 | 1 | ||
| High | 4.782 (2.781–8.226) | 5.561 (3.364–9.195) | ||
| CD204‐positive TAMs | < 0.001 | < 0.001 | ||
| Low | 1 | 1 | ||
| High | 4.633 (2.668–8.044) | 2.236 (1.458–3.427) | ||
| STAS | < 0.001 | 0.019 | ||
| Negative | 1 | 1 | ||
| Positive | 3.390 (1.925–5.968) | 1.759 (1.096–2.824) | ||
Significant P value shown in bold.
CAFs, cancer‐associated fibroblasts; CI, confidence interval; HR, hazard ratio; STAS, spread through air spaces; TAMs, tumor‐associated macrophages.
Subgroup analysis of the prognostic roles of α‐SMA and CD204 based on STAS status
As shown in Figure 4, patients with a high frequency of α‐SMA‐positive CAFs had poorer OS (P < 0.001) and RFS (P < 0.001) than those with low expression levels in both STAS‐positive and STAS‐negative groups. However, only patients in the STAS‐positive group with high CD204 expression had poorer OS (P < 0.001) and RFS (P < 0.001) than those with low CD204 expression (P = 0.37 and 0.95, respectively).
Figure 4.
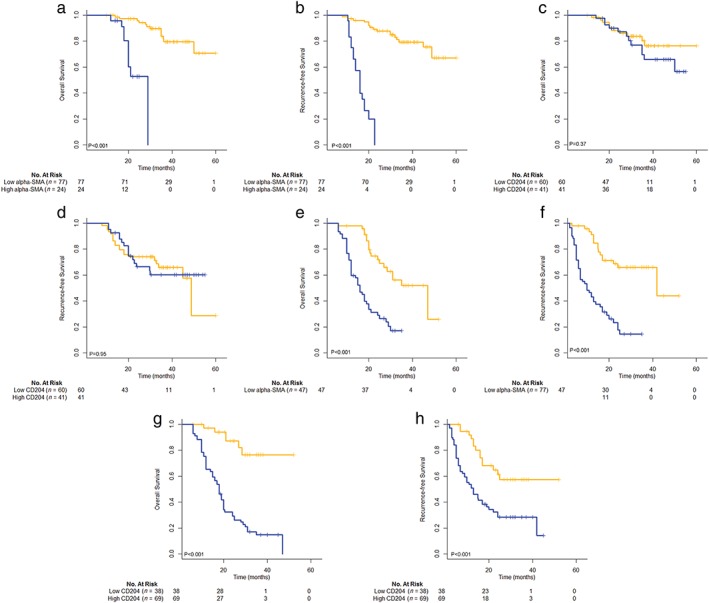
Subgroup analysis of the prognostic role of α‐smooth muscle actin (SMA) and CD204 based on spread through air space (STAS) status. Overall and recurrence‐free survival curves of STAS‐negative patients according to (a,b) the scoring of α‐SMA‐positive cancer‐associated fibroblasts (CAFs) (both P < 0.001) and (c,d) the number of CD204‐positive tumor‐associated macrophages (TAMs) (P = 0.37 and P = 0.95, respectively); and STAS‐positive patients according to (e,f) the scoring of α‐SMA‐positive CAFs (both P < 0.001) and (g,h) the number of CD204‐positive TAMs (both P < 0.001). ( ) Low alpha‐SMA (n = 77) (
) Low alpha‐SMA (n = 77) ( ) high alpha‐SMA (n = 24), (
) high alpha‐SMA (n = 24), ( ) low alpha‐SMA (n = 77) (
) low alpha‐SMA (n = 77) ( ) high alpha‐SMA (n = 24), (
) high alpha‐SMA (n = 24), ( ) low CD204 (n = 60) (
) low CD204 (n = 60) ( ) high CD204 (n = 41), (
) high CD204 (n = 41), ( ) low CD204 (n = 60) (
) low CD204 (n = 60) ( ) high CD204 (n = 41), (
) high CD204 (n = 41), ( ) low alpha‐SMA (n = 47) (
) low alpha‐SMA (n = 47) ( ) high (
) high ( ) alpha‐SMA (n = 60), (
) alpha‐SMA (n = 60), ( ) low alpha‐SMA (n = 77) (
) low alpha‐SMA (n = 77) ( ) high alpha‐SMA (n = 24), (
) high alpha‐SMA (n = 24), ( ) low CD204 (n = 38) (
) low CD204 (n = 38) ( ) high CD204 (n = 69), and low CD204 (n = 38) (
) high CD204 (n = 69), and low CD204 (n = 38) ( ) high CD204 (n = 69).
) high CD204 (n = 69).
Nomograms for predicting prognosis of lung adenocarcinoma
To predict the OS and RFS of patients with lung adenocarcinoma, two nomograms based on clinical risk factors including STAS, α‐SMA, and CD204 as variables were established by the multivariate Cox regression model according to the significant independent risk factors for OS and RFS (Fig 5a,b). Nomograms can be interpreted by adding the points assigned to each variable, which is indicated at the top of the scale. The total points can be converted to predict the five‐year probability of death and recurrence or metastasis for a patient in the lowest scale.36 The Harrell's c‐indexes for OS and RFS prediction were 0.84 (95% CI 0.76–0.91) and 0.82 (95% CI 0.76–0.89), respectively. Calibration curves for the two nomograms (Fig 5c,d) showed no deviations from the reference line and no need for recalibration.
Figure 5.
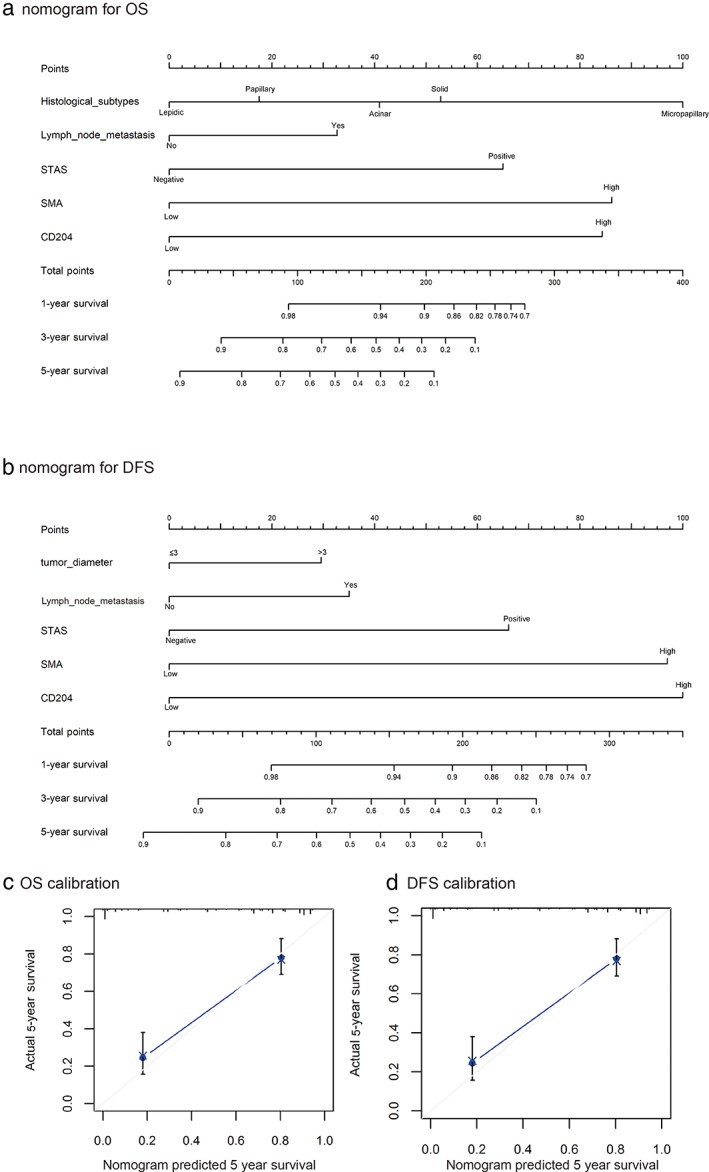
Nomograms of the results of prognostic models using clinicopathological characteristics and pretreatment inflammatory biomarkers to predict (a) overall survival (OS) and (b) recurrence‐free survival (RFS) of patients with lung adenocarcinoma. Nomograms can be interpreted by summing up the points assigned to each variable, indicated at the top of the scale. The total points can be converted to predict the five‐year probability of death and recurrence or metastasis for a patient in the lowest scale. Harrell's c‐indexes for OS and RFS prediction were 0.84 (95% confidence interval [CI] 0.76–0.91) and 0.82 (95% CI 0.76–0.89), respectively. Calibration curves for five‐year (c) OS and (d) RFS using nomograms with clinicopathological characteristics and pretreatment inflammatory biomarkers are shown. The x‐axis represents the nomogram‐predicted probability of survival and the y‐axis represents actual survival. The reference line is 45°and indicates perfect calibration. SMA, smooth muscle actin; STAS, spread through air space.
Discussion
It is still debatable whether STAS is an in vivo effect in any instance or potentially an artifact induced by cutting though a tumor with a knife.37 However, until now, all studies on STAS have shown that this novel morphologic feature is of high prognostic value. STAS has been identified as a risk factor for OS and RFS in all stages of lung adenocarcinoma,11, 12 and as an independent predictor of recurrence in patients with stage I lung adenocarcinoma that have undergone limited surgical resection.8 STAS also correlates with more aggressive growth patterns of lung adenocarcinoma, ranging from papillary‐predominant to micropapillary‐predominant and solid‐predominant adenocarcinomas,12, 13 similar to the results of our analysis (Table 1). Our study results showed that STAS was present in 51.4% of lung adenocarcinoma cases. This was consistent with the data reported by Warth et al., who reported that STAS was present in 50.6% of patients.38
Wislez et al. showed that neutrophils can induce tumor shedding and the aerogenous spread of lung adenocarcinoma with bronchioalveolar carcinoma features, which is related to shorter survival.39 However, the roles of other tumor‐associated stromal cells in the development of STAS have not been fully explored based on the new pathological classification of lung adenocarcinoma. CAFs and TAMs are the major cellular components of the tumor microenvironment and can orchestrate cancer dissemination and metastasis.40, 41 Our results indicate that the presence of STAS is significantly associated with a higher frequency of α‐SMA‐positive CAFs and a higher number of CD204‐positive TAMs. The subgroup analysis results indicated that α‐SMA‐positive CAFs play an important prognostic role in postoperative lung adenocarcinoma patients, regardless of the presence of STAS in resected specimens. However, CD204‐positive TAMs only significantly predicted prognosis in the presence of STAS. Therefore basic research is required to further illustrate the mechanism of whether CD204‐positive TAMs can promote the occurrence of STAS.
Despite substantial diagnostic and therapeutic improvements in the last two decades, the overall five‐year survival rate of lung cancer patients remains < 15%.42 This fact has shifted the focus of cancer treatment from an incomplete view including only malignant cells to a comprehensive view including both tumor cells and stromal cells in the tumor microenvironment. Stromal cells play prominent roles in tumor initiation, progression, and metastasis through the secretion of soluble factors, such as growth factors or inflammatory chemokines. In addition, they are involved in remodeling the tumor extracellular matrix and tumor metabolism, the regulation of the motility and stemness of cancer cells, and the preparation of the metastatic niche. Therefore, focusing on stromal cells as therapeutic targets in the treatment of lung adenocarcinomas could be valuable. Hitherto, several drugs targeting the tumor stroma are either under study or in clinical use. CSF1R inhibitors significantly suppress the recruitment of tumor‐associated macrophages and inhibit tumor growth.43 Imatinib can inhibit the activity of CAFs by interfering with platelet‐derived growth factor signaling,44 and the VEGF trap can prevent tumor metastasis by inhibiting angiogenesis. Zhang et al. found that imatinib also can inhibit macrophage M2‐like polarization both in vitro and in vivo, which contributes to its anti‐metastatic function in lung cancer.45 In addition, Shinya et al. found that targeting platelet‐derived growth factor signaling between cancer cells undergoing EMT and CAFs was a promising therapeutic target to inhibit cancer progression and improve patient prognosis.46 Our results showed that CAFs and TAMs were significantly related to STAS and may be effective therapeutic targets in patients with STAS. In addition, to help clinicians categorize these patients according to risk factors and to identify high‐risk patients, two nomograms based on clinical risk factors and STAS, α‐SMA, and CD204 were established. The results showed that Harrell's c‐indexes for OS and RFS prediction of 0.84 (95% CI 0.76–0.91) and 0.82 (95% CI 0.76–0.89), respectively. Nomograms based on OS and DFS can be recommended as practical models to evaluate the prognosis of lung adenocarcinoma patients.
The present study had several limitations. First, because of the retrospective nature of the study, performance and selection bias were inevitable. Second, the study included patients from a single institution. Third, basic research on the mechanism underlying STAS was not included. Further multicenter studies with larger patient cohorts may address these limitations, and basic research is required to further illustrate the mechanism by which the tumor microenvironment promotes the progression of STAS.
In conclusion, the presence of STAS was associated with high expression of α‐SMA and CD204 in lung adenocarcinoma. Nomograms including STAS and stromal cells as variables are recommended as practical models to evaluate the prognosis of lung adenocarcinoma patients.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Patient characteristics.
Acknowledgments
This study was supported by the Jiangsu Provincial Commission of Health and Family Planning (Grant no. H201521), the Natural Science Foundation of Jiangsu Province (Grant no. BK20161224), and the Youth Science and Technology Project of Suzhou Health and Family Planning Commission (Grant no. KJXW2016016). We wish to thank International Science Editing for editing this manuscript.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Micke P, Ostman A. Tumour‐stroma interaction: Cancer‐associated fibroblasts as novel targets in anti‐cancer therapy? Lung Cancer 2004; 45(Suppl 2): S163–75. [DOI] [PubMed] [Google Scholar]
- 3. Ryo M, Junji Y, Genichiro I et al Prognostic impact of histology on early‐stage non‐small cell lung cancer. Chest 2011; 140: 135–45. [DOI] [PubMed] [Google Scholar]
- 4. Sakao Y, Miyamoto H, Sakubara M et al Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: The impact of nonbronchioloalveolar carcinoma components. Ann Thorac Surg 2007; 83: 209–14. [DOI] [PubMed] [Google Scholar]
- 5. Rena O, Papalia E, Ruffini E et al Stage I pure bronchioloalveolar carcinoma: Recurrences, survival and comparison with adenocarcinoma of the lung. Eur J Cardiothorac Surg 2003; 23: 409–14. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Wu J, Tan Q, Zhu L, Gao W. Why do pathological stage IA lung adenocarcinomas vary from prognosis? A clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinomas based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013; 8: 1196–202. [DOI] [PubMed] [Google Scholar]
- 7. Travis WD, Brambilla E, Noguchi M et al International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadota K, Nitadori JI, Sima CS et al Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences following limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015; 10: 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morimoto J, Nakajima T, Suzuki H et al Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016; 152: 64–72.e1. [DOI] [PubMed] [Google Scholar]
- 10. Onozato ML, Kovach AE, Yeap BY et al Tumor Islands in resected early‐stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013; 37: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiov Th 2016; 23: 567–72. [DOI] [PubMed] [Google Scholar]
- 12. Warth A, Muley T, Kossakowski C et al Prognostic impact of intra‐alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 2015; 39: 793–801. [DOI] [PubMed] [Google Scholar]
- 13. Warth A, Muley T, Harms A et al Clinical relevance of different papillary growth patterns of pulmonary adenocarcinoma. Am J Surg Pathol 2016; 40: 818–26. [DOI] [PubMed] [Google Scholar]
- 14. Balkwill FR, Charles KA, Mantovani A et al Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7: 211–7. [DOI] [PubMed] [Google Scholar]
- 15. Kalluri R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003; 3: 422–33. [DOI] [PubMed] [Google Scholar]
- 16. Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease‐1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 2005; 120: 303–13. [DOI] [PubMed] [Google Scholar]
- 17. Olaso E, Salado C, Egilegor E et al Proangiogenic role of tumor‐activated hepatic stellate cells in experimental melanoma metastasis. Hepatology 2003; 37: 674–85. [DOI] [PubMed] [Google Scholar]
- 18. Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel RS. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. P Natl Acad Sci USA 1991; 88: 6028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishii G, Sangai T, Ito T et al In vivo and in vitro characterization of human fibroblasts recruited selectively into human cancer stroma. Int J Cancer 2005; 117: 212–20. [DOI] [PubMed] [Google Scholar]
- 20. Ishii G, Ochiai A, Neri S et al Phenotypic and functional heterogeneity of cancer‐associated fibroblast within the tumor microenvironment. Adv Drug Delivery Rev 2016; 99 (Pt B): 186–96. [DOI] [PubMed] [Google Scholar]
- 21. Ito M, Ishii G, Nagai K, Maeda R, Nakano Y, Ochiai A. Prognostic impact of cancer‐associated stromal cells in patients with stage I lung adenocarcinoma. Chest 2012; 142: 151–8. [DOI] [PubMed] [Google Scholar]
- 22. Noguchi M, Shimosato Y. The development and progression of adenocarcinoma of the lung. Cancer Treat Res 1995; 72: 131–42. [DOI] [PubMed] [Google Scholar]
- 23. Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Prognostic impact of intratumoral vascular invasion in non‐small cell lung cancer patients. Thorax 2010; 65: 1092–8. [DOI] [PubMed] [Google Scholar]
- 24. Kaseda K, Ishii G, Aokage K et al Identification of intravascular tumor microenvironment features predicting the recurrence of pathological stage I lung adenocarcinoma. Cancer Sci 2013; 104: 1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagemann T, Wilson JL, Burke F et al Ovarian cancer cells polarize macrophages toward a tumor‐associated phenotype. J Immunol 2006; 176: 5023–32. [DOI] [PubMed] [Google Scholar]
- 26. Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co‐cultivation with macrophages is due to TNF‐alpha dependent up‐regulation of matrix metalloproteases. Carcinogenesis 2004; 25: 1543–9. [DOI] [PubMed] [Google Scholar]
- 27. Orimo A, Gupta PB, Sgroi DC et al Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF‐1/CXCL12 secretion. Cell 2005; 121: 335–48. [DOI] [PubMed] [Google Scholar]
- 28. Ono M. Molecular links between tumor angiogenesis and inflammation: Inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 2008; 99: 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Wever O, Demetter P, Mareel M et al Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123: 2229–38. [DOI] [PubMed] [Google Scholar]
- 30. Cirri P, Chiarugi P. Cancer associated fibroblasts: The dark side of the coin. Am J Cancer Res 2011; 1: 482–97. [PMC free article] [PubMed] [Google Scholar]
- 31. Xing F, Saidou J, Watabe K et al Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci 2010; 15: 166–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohtaki Y, Ishii G, Nagai K et al Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol 2010; 5: 1507–15. [DOI] [PubMed] [Google Scholar]
- 33. Detterbeck FC, Boffa DJ, Kim AW et al The 8th edition Lung Cancer Stage Classification. Chest 2017; 151: 193–203. [DOI] [PubMed] [Google Scholar]
- 34. Du H, Chen D, Zhou Y et al Fibroblast phenotypes in different lung diseases. J Cardiothorac Surg 2014; 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y, Zou L, Zhang Y et al Transforming growth factor‐β1 and α‐smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I–IIIA nonsmall cell lung cancer after curative resection. Tumor Biol 2014; 35: 6707–13. [DOI] [PubMed] [Google Scholar]
- 36. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26: 1364–70. [DOI] [PubMed] [Google Scholar]
- 37. Thunnissen E, Blaauwgeers HJ, de Cuba EM et al Ex vivo artifacts and histopathologic pitfalls in the lung. Arch Pathol Lab Med 2016; 140: 212–20. [DOI] [PubMed] [Google Scholar]
- 38. Warth A, Muley T, Kossakowski CA et al Prognostic impact of intra‐alveolar tumor spread in pulmonary adenocarcinomas. Am J Surg Pathol 2015; 39: 793–801. [DOI] [PubMed] [Google Scholar]
- 39. Wislez M, Antoine M, Rabbe N et al Neutrophils promote aerogenous spread of lung adenocarcinoma with bronchioloalveolar carcinoma features. Clin Cancer Res 2007; 13: 3518–27. [DOI] [PubMed] [Google Scholar]
- 40. Paulsson J, Micke P. Prognostic relevance of cancer‐associated fibroblasts in human cancer. Semin Cancer Biol 2014; 25: 61–8. [DOI] [PubMed] [Google Scholar]
- 41. Linde N, Casanova‐Acebes M, Sosa MS et al Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 2018; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cufer T, Ovcaricek T, Brien MO et al Systemic therapy of advanced non‐small cell lung cancer: Major‐developments of the last 5‐years. Eur J Cancer 2013; 49: 1216–25. [DOI] [PubMed] [Google Scholar]
- 43. Ries C, Cannarile M, Hoves S et al Targeting tumor‐associated macrophages with anti‐CSF‐1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014; 25: 846–59. [DOI] [PubMed] [Google Scholar]
- 44. Kinoshita K, Nakagawa K, Hamada J et al Imatinib mesylate inhibits the proliferation‐stimulating effect of human lung cancer‐associated stromal fibroblasts on lung cancer cells. Int J Oncol 2010; 37: 869–77. [DOI] [PubMed] [Google Scholar]
- 45. Yaoa Z, Zhanga J, Zhang B et al Imatinib prevents lung cancer metastasis by inhibiting M2‐like polarization of macrophages. Pharmacol Re 2018; 133: 121–31. [DOI] [PubMed] [Google Scholar]
- 46. Neri S, Miyashita T, Hashimoto H et al Fibroblast‐led cancer cell invasion is activated by epithelial‐mesenchymal transition through platelet‐derived growth factor BB secretion of lung adenocarcinoma. Cancer Lett 2017; 395: 20–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics.


