Abstract
Background
Extracellular matrix (ECM) is remodeled during carcinogenesis. An abundant constituent of ECM is collagen. Type I collagen is secreted by fibroblasts, is important for tumor growth and epithelial‐mesenchymal transition, and may also be secreted by cancer cells. However, the role and function of cancer‐derived Type I collagen in the tumor microenvironment remains unclear.
Methods
We used immunohistochemistry and Western blot to detect Type I collagen expression in non‐small cell lung cancer (NSCLC) and esophageal squamous cell carcinoma (ESCC) cell lines, respectively. We assessed the migration and adhesion capability of these cells in vivo by inhibiting Type I collagen in tumors. Relevant data were extracted from a large cohort study of The Cancer Genome Atlas to analyze messenger RNA levels. Protein expression of Type I collagen was further determined in tumor tissues of patients using tissue microarray.
Results
Cancer cell lines secreted Type I collagen. The molecular weight of cancer‐derived Type I collagen was different from that secreted by cancer‐associated fibroblasts and normal fibroblasts. Expression levels of COL1A1 and COL1A2 (subtypes of Type I collagen) messenger RNA in NSCLC and ESCC tumors were higher than in normal tissues, but were not associated with tumor node metastasis stages. Low expression of Type I collagen was significantly associated with poor overall survival and cancer cell differentiation.
Conclusion
NSCLC and ESCC cells could produce Type I collagen endogenously, revealing the potential functions of Type I collagen in cancer development. Cancer‐derived Type I collagen was associated with overall survival and cancer cell differentiation.
Keywords: Type I collagen, extracellular matrix, esophagus cancer, non‐small cell lung cancer, tumor microenvironment
Introduction
In the tumor microenvironment, the extracellular matrix (ECM) plays an important role in tumorigenesis and tumor development. Activation of the ECM is a landmark event for the formation of a tumor from injured tissue and cancer cells.1, 2 Neoplastic cells grow, expand, and metastasize, eventually resulting in cancer. This is regarded as speciation, which is a heterogeneous and adaptive process involving tumor microenvironment remodeling.3, 4
The major component of the ECM is collagen, of which the most abundant is Type I collagen. Type I collagen is essential to maintain the structure of interstitial spaces, skin, gut, and breast, as well as defining the mechanical properties of tissues, including stiffness and porosity. Type I collagen either promotes or represses cell migration and positioning, depending on different contexts, such as cancer cell adhesion, proliferation, and differentiation.5, 6, 7 Type I collagen‐dense ECM can even drive the progression of estrogen receptor 1 (ERα+) breast cancer by potentially altering hormonal signals.8 In addition, Type I collagen promotes embryonic stem cell growth,9 and contributes to the formation of solid cord‐like assemblies from microvascular endothelial cells after adopting an in vitro spindle‐shaped morphology.10
It is widely accepted that Type I collagen is produced by stromal fibroblasts.11, 12, 13 Tumor metalloproteinases (MMPs) degrade Type I collagen into Type I procollagen peptides, such as the amino‐terminal propeptide (PINP) and carboxy‐terminal propeptide (PICP), during cancer development. The levels of these propeptides are associated with the prognosis of patients with ovarian cancer and could be detected in serum, cyst fluid, and ascetic fluid.14, 15 In 2008, Palmieri et al. demonstrated that the procollagen I COOH‐terminal fragment (C3) could induce the expression of VEGF and C‐X‐C motif chemokine receptor 4 (CXCR4), as well as activating MMP‐2 and MMP‐9.1 Type I collagen is crucial for the invasion and metastasis of ovarian cancer cells, as it promotes the migration of cancer cells by interacting with α2β1‐integrin.16
However, few researchers have investigated the source of Type I collagen, for instance, what it is made from and where it comes from. “Plasticity” is a feature of the tumor microenvironment during cancer development.17, 18, 19 During the process, stromal cells, including fibroblasts, macrophages, and other non‐cancer cells, are recruited to the nearby tumor microenvironment. Over the past few decades, the mainstream point of view has been that tumors secrete MMPs to degrade Type I collagen in the ECM. However, whether cancer could produce its own Type I collagen remains unclear. Few researchers have considered that Type I collagen could be derived from tumors. Only one study has shown the endogenous production of Type I collagen by cancer cells.20 In our previous study, we used a three‐dimensional culture to show that cancer cells could generate Type I collagen, which could affect tumor growth.21 In the present study, we verified that Type I collagen could be secreted by cancer cells, and further investigated the role and clinical significance of Type I collagen during cancer development. The results of in vitro and in vivo experiments, together with relevant clinical data and biological information databases, suggest that cancer‐derived Type I collagen is vital for tumor growth and has significant clinical implications for patients during cancer development.
Methods
Clinical specimens
Samples of lung tumor tissues were collected from resection specimens provided by Sun Yat‐sen University Cancer Center (Guangzhou, China).
Mice
Cancer cells were subcutaneously injected into three to four‐week‐old nude male mice via one of their flanks. The Institutional Animal Care and Use Committee (IACUC) of Sun Yat‐sen University approved all animal use protocols. All applicable institutional guidelines of GDPU for the care and use of animals were followed.
Immunohistochemistry
Immunohistochemistry (IHC) was performed following instructions on the Cell Signaling Technology Inc. website (Danvers, MA, USA).
Masson's trichrome staining
Tissues slides were deparaffinized and rehydrated according to standard pathological techniques.
Primary cell culture
Lung tumor tissue and normal lung tissue were cut into fine pieces for primary culture.
Further details of each of these stages are available in Appendix S1.
Collection of cell culture medium
Details are shown in the legends to the Supplementary Figures.
Western blotting
Western blotting was conducted following the standard protocol advised on the Cell Signaling Technology Inc. website.
Wound healing and Transwell invasion assays
A Type I collagen inhibitor (procollagen C‐proteinase, Cat: K383367) was purchased from Selleck Chemicals Inc. (Houston, TX, USA). Wound healing and invasion assays were then performed using cells treated with and without procollagen C‐proteinase. Further details are available in Appendix S1.
Cell colony formation assay
Colonies of cultured cancer cells were quantified to assess cancer cell colony formation. Cancer cells (5 × 103 cells) were cultured in 60 mm dishes. After three to four weeks, cell colonies were counted. The experiment was repeated three times. The results are presented as mean values ± standard deviation.
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Pearson's chi‐square test was used to compare the clinicopathological features of tumors in Type I collagen‐positive and negative patients. Continuous data were analyzed using a t‐test. Kaplan–Meier plots and log rank tests were used for survival analysis. A P value < 0.05 was considered statistically significant.
Results
Collagen expression was found in lung cancer nests after Masson's trichrome staining
Most researchers believe that Type I collagen is produced by fibroblasts. Our results confirmed this conclusion (Fig 1a); however, we also observed collagen expression in the normal vascular wall of the lung (Fig 1b) and in several clinical samples from cancer nests after Masson's trichrome staining (Fig 1c, arrows). Interestingly, further investigation revealed that collagen was expressed in different subtypes of cancer, including lung squamous and adenocarcinoma (Fig 1d,e, respectively).
Figure 1.
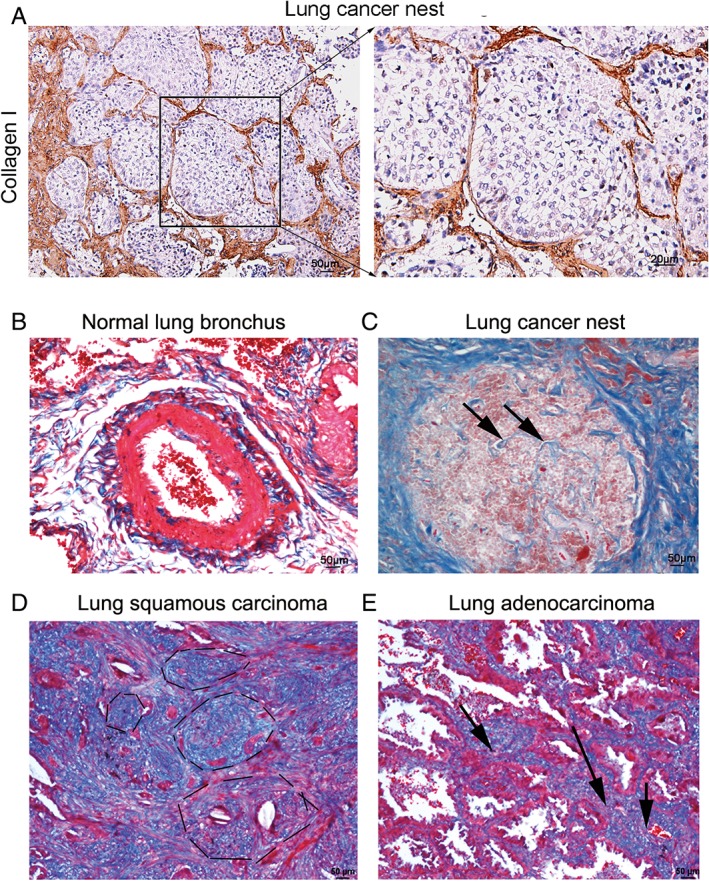
Masson's staining revealed partial expression of Type I collagen in lung cancer nests. (a) Type I collagen expression was detected in non‐small cell lung cancer tissue using immunohistochemistry staining (brown) and was mainly located in the extracellular matrix (left), with little located in the tumor, as shown by high power microscope field (right, 400×, bar = 25 μm). (b) After Type I collagen was stained blue with Masson's staining, we observed that the bronchus was encircled by Type I collagen (arrows), indicating that Type I collagen was expressed in normal lung tissue. (c) We also observed several blue lines in the lung cancer nest. (d,e) Similar findings were observed in lung squamous and lung adenocarcinoma.
Type I, but not Type III or IV collagen is expressed in lung cancer nests
To further explore which type of collagen is expressed in cancer nests, IHC was performed using antibodies recognizing Type I, III, and IV collagen. The results showed that Type I collagen, but not Type III or IV, was present in lung cancer nests (Fig 2). We also observed the expression of Type I collagen in the ECM (Fig 1a). Similar results were observed in IHC analysis of xenograft samples: Type I collagen was markedly expressed in cancer nests, while Type III and IV were undetectable (Fig 2). The results revealed that in certain types of lung cancer, the expression of Type I collagen was significantly higher than that of Type III and IV. These preliminary findings revealed that the major type of collagen expressed in lung cancer tissue is Type I.
Figure 2.
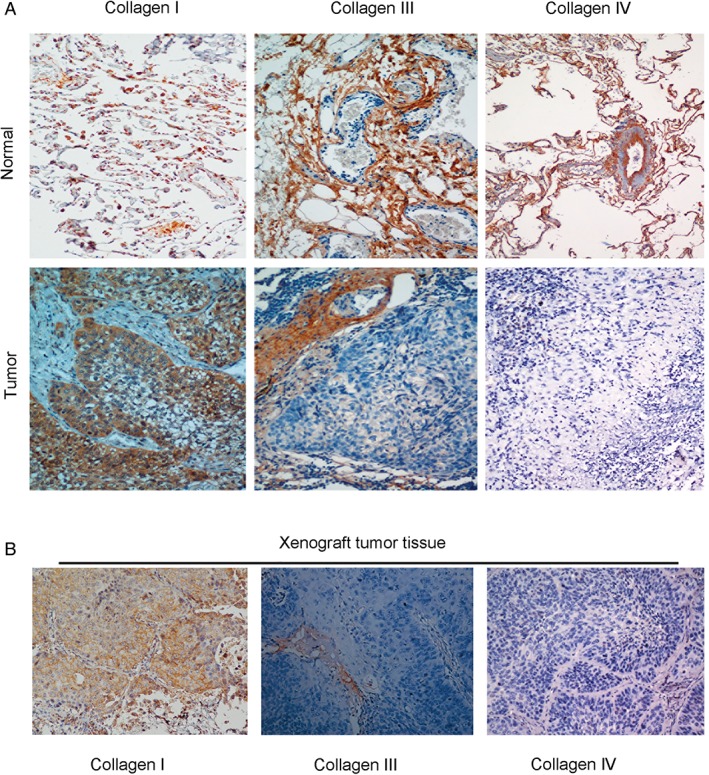
Type I collagen, but not Type III or IV, was identified in lung cancer nests. (a) To explore which type of collagen was expressed in the lung cancer nests, we conducted immunohistochemistry on the tumor tissue. Type I collagen expression was found in human lung tumor tissues. (b) We further assessed xenograft tissue sections from mice transplanted with lung cancer cells using antibodies recognizing Type I, III, and IV collagen. Under 200× magnification, Type I collagen was observed in the cancer nest, while the expression of Type III and IV was barely observed.
Lung cancer cells secrete Type I collagen
To further investigate whether Type I collagen could be derived from cancer cells in addition to fibroblasts, we conducted Western blotting to detect the potential expression of Type I collagen in four kinds of lung cancer cell lines, including squamous carcinoma and adenocarcinoma (SCC35, SCC37, SCC210011, and AD212102). The results confirmed the expression of Type I collagen in lung cancer cell lines (Fig 3a). To verify the specificity of the antibody used in Western blotting, we detected Type I collagen protein extracted from mouse tail, the principal component of which is Type I collagen. The results showed that the antibodies were sensitive and specific for Type I collagen from mouse tail and displayed a ladder of molecular weights in Western blotting results (Fig 3b). These results are consistent with findings from our previous study that Type I collagen messenger RNA (mRNA) could be expressed in lung cancer cell lines, as determined by quantitative real‐time PCR (qRT‐PCR). To clearly observe the expression of Type I collagen in both cancer cells and fibroblasts, we built a cell culture model as follows: fresh tumor tissues were obtained from the clinical department of the Sun Yat‐sen University Cancer Center, which were cultured after being washed with phosphate buffered saline and cut into fine pieces. The primary tumor tissues contained both cancer cells and fibroblasts. After 15–25 days, growth of the cancer cells and fibroblasts in dishes with 3.7% paraformaldehyde was terminated. IHC was then conducted using the antibodies recognizing Type I collagen. The results showed that cancer cells and fibroblasts co‐existed in the dishes. Tumor cells were identified using an antibody recognizing cytokeratin, a high molecular weight cancer marker. We observed that not only fibroblasts, but also cancer cells produced Type I collagen (Fig 3c).
Figure 3.
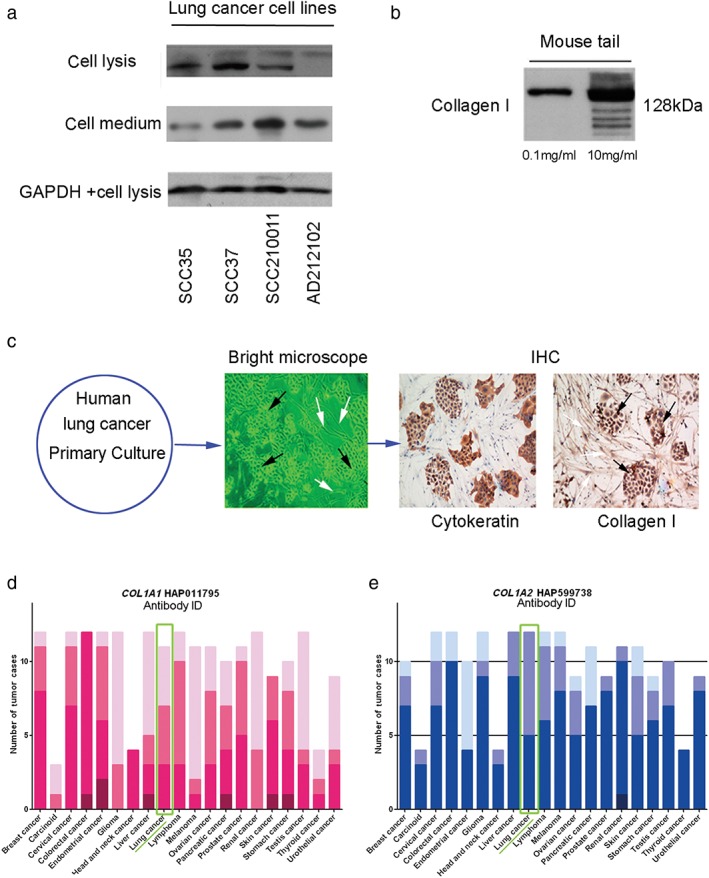
Lung cancer cell lines produce Type I collagen. (a) Western blotting results show that non‐small cell cancer cell lines could produce Type I collagen, which could be detected in the culture medium. Several cell lines expressed two types of Type I collagen, but their identities are unknown. The second protein band might represent a small degraded fraction derived from Type I collagen. (b) To confirm the specificity of the antibody, we examined extracts of mouse tail, which mainly comprise Type I collagen. The ladder band of Type I collagen displayed different molecular weights. The molecular weight of Type I collagen was approximately 128 kDa and other ladder bands were degradation products. (c) Primary cancer cell culture with immunohistochemistry (IHC) was used to analyze cancer cells and fibroblasts derived from tumor tissue. Type I collagen was expressed in both cancer cells and fibroblasts (400×). (d,e) Data were extracted from the protein atlas database to analyze the protein expression of Type I collagen in various cancer types using antibodies recognizing COL1A1 and COL1A2. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.  H,
H,  M,
M,  L,
L,  Not,
Not,  H,
H,  M,
M,  L,
L,  Not.
Not.
Furthermore, we used a bioinformatic platform (www.proteinatlas.org) to analyze the expression of two subtypes of Type I collagen, COL1A1 and COL1A2. The results showed that both COL1A1 and COL1A2 existed in cancer tissue,22 and the proteins were expressed in various types of cancer, including lung cancer (Fig 3d,e), as shown in the green line frame.
Inhibiting cancer cell‐derived Type I collagen promoted cancer cell invasion and suppressed tumor clone formation
To determine whether cancer‐derived Type I collagen has an effect on cancer cell activity, we reduced the activity of Type I collagen using its inhibitor, procollagen C‐proteinase. The results of wounding healing assay demonstrated that inhibition of Type I collagen in A549 cells promoted the invasive ability of cancer cells (Fig 4a,b). Fewer clones formed from cancer cells in the group treated with procollagen C‐proteinase than in the control group (Fig 4c). However, Transwell migration assay showed increased migration of cancer cells across the wells after treatment with procollagen C‐proteinase (Fig 4d). These findings indicate that cancer‐derived Type I collagen might promote tumor clone formation while suppressing cancer cell invasion.
Figure 4.
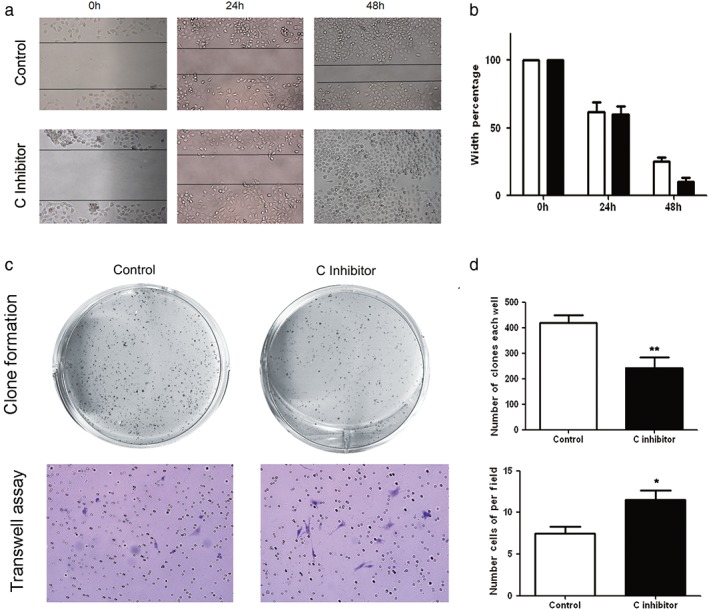
Inhibition of cancer‐derived Type I collagen suppressed tumor clone formation while increasing the invasive ability of cancer cells. (a,b) Wound‐healing assays show that cell motility and invasion were markedly increased in an A549 cell line treated with a Type I collagen inhibitor. Representative images were taken at 0, 24, and 48 hours after scratching. (c, top) After Type I collagen was inhibited, a significant reduction in the number of tumor clones was observed on the culture dishes, indicating that the Type I collagen inhibitor significantly suppressed tumor clone formation. (c, bottom) Cancer cell invasion was also promoted when Type I collagen was inhibited. The results are presented as the mean ± standard deviation. (d) Column charts of the statistical results (P, *< 0.05, **< 0.01).  Control,
Control,  C inhibitor.
C inhibitor.
High expression levels of COL1A1 and COL1A2 messenger RNA in NSCLC and ESCC
Type I collagen from the ECM is widely believed to promote tumor growth, whereas the function of cancer‐derived Type I collagen in cancer development remains unclear. In the present study, we confirmed that Type I collagen was also secreted by tumors; therefore, we further investigated whether the mRNA expression of Type I collagen was associated with cancer stage. We collected data from The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov) of 501 patients with esophageal squamous cell carcinoma (ESCC) and 535 patients with non‐small cell lung cancer (NSCLC).23, 24, 25, 26 We analyzed and compared the mRNA expression levels of COL1A1 and COL1A2 in tumors and normal tissue samples. As expected, the mRNA levels of these two subtypes of Type I collagen were both significantly higher in tumor tissue than in normal tissue in all types of cancer studied, including lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and ESSC (all P < 0.0005) (Fig 5a). Nevertheless, they were not associated with cancer stage (group 1: stages I and II, group 2: stages III and IV, all P > 0.05) (Fig 5b).
Figure 5.
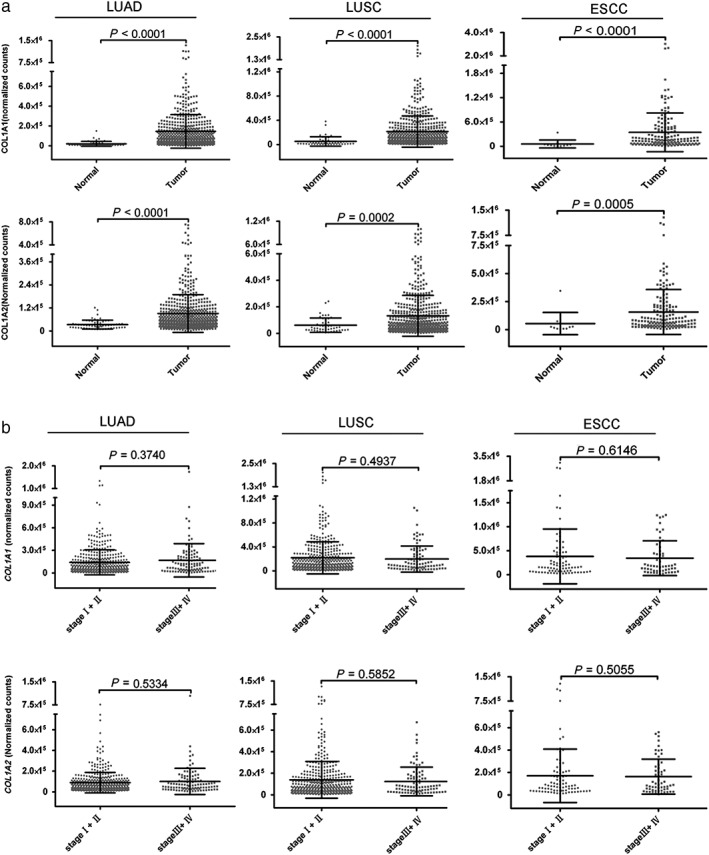
The expression of Type I collagen messenger RNA (mRNA) was higher in tumor tissue than in normal tissue. (a) Clinical data of 501 patients with esophageal squamous cell carcinoma (ESCC) and 535 patients with lung cancer (LUAD and LUSC) from The Cancer Genome Atlas were collected, and the mRNA expression level of Type I collagen (COL1A1 and COL1A2) in these patients was analyzed. The level of mRNA expression of Type I collagen was significantly higher in tumor tissue than in normal tissue (P < 0.0001). (b) mRNA expression of Type I collagen was not associated with cancer stage (P > 0.05).
Clinical significance of Type I collagen expression in ESCC
To determine whether Type I collagen could also be derived from other types of cancer cells, we conducted Western blotting to detect Type I collagen in seven ESCC cell lines. Type I collagen expression was detected in all seven ESCC cancer cell lines (Fig 5a). We continued to explore whether the Type I collagen derived from cancer cells was the same as that secreted by cancer‐associated fibroblasts (CAFs) and normal fibroblasts (NFs). Western blotting results confirmed the expression of Type I collagen in the ESCC cell lines (Fig 6a) and identified a different molecular weight of ESCC cancer cells from that secreted by CAFs and NFs (Fig 6b). The reason for the difference could be that Type I collagen comprises various degradation products in cancer cells and fibroblasts (CAFs and NFs). The specificity and quality of the Type I collagen antibody was confirmed in the previous experiment (Fig 3b).
Figure 6.
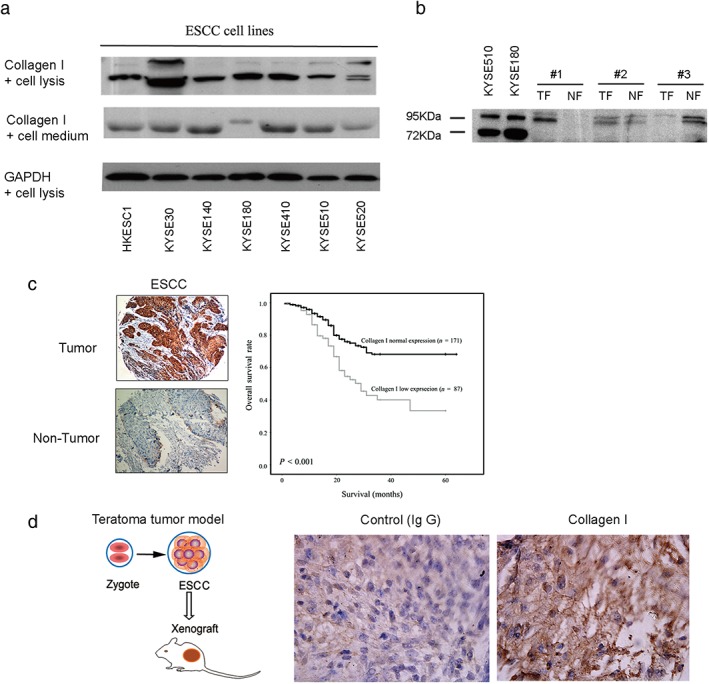
Type I collagen is secreted by esophageal squamous cell carcinoma (ESSC) and has clinical significance. (a) Type I collagen was determined in ESCC cells lines using Western blotting. (b) Comparison between cancer‐derived and fibroblast‐derived Type I collagen revealed that they have different molecular weights. Cancer‐derived Type I collagen was represented by 72 kDa and 95 kDa bands, while the tumor fibroblast (TF) and normal fibroblast (NF)‐derived samples only show the 95 kDa band. They both expressed a 120 kDa band (data not shown). (c) A tissue microarray assay using immunohistochemistry shows that Type I collagen was significantly overexpressed in the tumor tissues of 258 patients with ESCC from Lin County (Henan province, China) compared to non‐tumor tissues. Kaplan–Meier analysis showed that low expression levels of Type I collagen were significantly associated with poor overall survival and cell differentiation. (d) Considering that stem cells are related to cell differentiation, we used a teratoma tumor model made from human embryonic stem cell lines in vivo to verify that Type I collagen was present in situ in cancer stem‐like cells, which maintain a differentiation state. All values were expressed as the average of three biological replicates. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IgG, immunoglobulin.
To further explore whether the protein expression of Type I collagen derived from cancer is related to cancer stage, we conducted tissue microarray analysis in a retrospective cohort of 258 patients with ESCC. The analysis focused on Type I collagen expression inside tumors rather than in the ECM (as shown by the representative image in Fig 6c, left). Interestingly, in some patients, Type I collagen expression was significantly lower in tumor tissues than in adjacent non‐tumor tissues (87/258, 33.7%; P < 0.001). Correlation analysis showed that low Type I collagen expression in ESCC tumors was significantly associated with cancer cell differentiation (P = 0.0058). No significant association was found between downregulation of Type I collagen and other clinicopathological parameters (P > 0.05, Pearson χ2 test) (Table 1).
Table 1.
Clinical correlation of cancer‐derived collagen I expression in 258 of primary ESCCs
| Collagen I expression | P | |||
|---|---|---|---|---|
| Clinical Feature | Number | Normal | Low | |
| Gender | 0.792 | |||
| Male | 115 | 75 (65.2%) | 40 (34.8%) | |
| Female | 143 | 96 (67.1%) | 47 (32.9%) | |
| Age | 0.427 | |||
| ≤ 60 | 146 | 100 (68.5%) | 46 (31.5%) | |
| > 60 | 112 | 71 (63.4%) | 41 (36.6%) | |
| Tumor invasion§ ‡ | 0.879 | |||
| T1 | 19 | 9 (47.4%) | 10 (52.6%) | |
| T2 | 73 | 37 (51%) | 36 (49.3%) | |
| T3 | 157 | 83 (53%) | 74 (47.1%) | |
| Lymph node Metastasis | 0.507 | |||
| N0 | 150 | 102 (68.0%) | 48 (32.0%) | |
| N1 | 108 | 69 (63.9%) | 39 (36.1%) | |
| TNM stage | 0.479 | |||
| Early stage (I–II) | 177 | 120 (67.8%) | 57 (32.2%) | |
| Advanced stage (III–IV) | 81 | 51 (63.0%) | 30 (37.0%) | |
| Tumor size (cm3)†, ‡ | 0.134 | |||
| ≤ 5 | 30 | 17 (57.7%) | 13 (43.3%) | |
| > 5 | 172 | 121 (70.3%) | 51 (29.7%) | |
| Cell differentiation | 0.0058 | |||
| Well (I–II) | 31 | 22 (71.0%) | 9 (29.0%) | |
| Moderate (III) | 166 | 118 (71.1%) | 48 (28.9%) | |
| Poor (IV) | 58 | 28 (48.3%) | 30 (51.7%) | |
Tumor size was measured and obtained by 0.5 × the length × (the width).2
Partial data was not available, thus all statistics were based on informative data.
Invasion was defined by results of final pathological analysis. Bold text indicates a statistically significant difference.
ESCC, esophageal squamous cell carcinoma; TNM, tumor node metastasis.
Univariate survival analysis suggested that downregulated expression of Type I collagen was positively and significantly correlated with the overall survival of patients with ESSC (log rank = 15.0; P < 0.001) (Fig 6c, Table 2). The mean overall survival times in Type I collagen‐positive (n = 171) and Type I collagen‐negative (n = 87) patients with ESCC were 49.6 (95% confidence interval [CI] 46.0–53.2) and 34.5 (95% CI 29.1–39.8) months, respectively. Multivariable Cox regression analysis was used to assess the hazard ratios (0.740 [0.566–0.870]) and the results indicated that cancer cell‐derived Type I collagen could be a potential prognostic marker to predict the overall survival of patients with ESCC (P < 0.05) (Table 2).
Table 2.
Univariate and multivariate analysis of overall survival rate and clinicopathological parameters in 258 pairs of primary ESCCs
| Univariate† | Multivariate‡ | ||
|---|---|---|---|
| Factor | P | HR (95% Cl) | P |
| Gender | 0.707 | 1.029 (0.619–1.711) | 0.911 |
| Male | |||
| Female | |||
| Age | 0.366 | 1.009 (0.980–1.039) | 0.543 |
| ≤60 | |||
| >60 | |||
| Tumor invasion§ | 0.031 | 2.619 (0.198–3.803) | 0.851 |
| T1 | |||
| T2 | |||
| T3 | |||
| Lymph node Metastasis | 0.000 | 0.967 (0.367–2.546) | 0.945 |
| N0 | |||
| N1 | |||
| TNM stage | 0.000 | 1.669 (0.622–4.479) | 0.309 |
| Early stage (I–II) | |||
| Advanced Stage (III‐IV) | |||
| Tumor size (cm3)¶,†† | 0.039 | 2.432 (0.747–7.911) | 0.139 |
| ≤ 5 | |||
| > 5 | |||
| Cell differentiation | 0.000 | 1.808 (1.139–2.870) | 0.012 |
| Well (I–II) | |||
| Moderate (III) | |||
| Poor (IV) | |||
| Collagen I | 0.000 | 0.740 (0.566–0.870) | 0.027 |
| Negative | |||
| Positive | |||
By Kaplan–Meier.
By Cox proportional‐hazards regression model.
Tumor size was measured and obtained by 0.5 × the length × (the width).2
Partial data was not available, thus all statistics were based on informative data.
Invasion was defined by results of final pathological analysis. Bold text indicates a statistically significant difference.
CI, confidence interval; ESCC, esophageal squamous cell carcinoma; TNM, tumor node metastasis.
As mentioned above, we noted that low levels of Type I collagen in ESCC were significantly associated with cancer cell differentiation. To explore whether the expression of Type I collagen in cancer stem cells in vivo is related to cell differentiation, we generated embryonic carcinomas by transferring embryonic stem cell lines from human blastocysts into SCID mice to create a tumor model27 and used this model to determine whether Type I collagen was expressed in cancer stem‐like cells. IHC analysis confirmed the existence of Type I collagen in embryonic carcinoma (Fig 6d), indicating that cancer‐derived Type I collagen was expressed during the process of cancer stem cell growth.
Discussion
A number of previous studies have demonstrated that Type I collagen is important for cancer development. It promotes cancer cell survival and prostate cancer cell proliferation via the activation of relevant cell signals.28, 29, 30 Type I collagen is also implicated in the induction of epithelial‐mesenchymal transition in carcinoma cell lines from the lung31 and breast.32 Type I collagen could downregulate E‐cadherin in pancreatic cancer by inducing the disruption of E‐cadherin33 and SMADS.34 Type I collagen promotes pancreatic cancer metastasis by activating c‐Jun NH2‐terminal kinase 1 and upregulating N‐cadherin levels.35 However, nearly all of these studies were conducted based on the fact that Type I collagen is secreted by fibroblasts. In our experiments, we confirmed that Type I collagen, but not Type III and IV, is expressed in cancer nests by using tumor sections, tumor xenografts, and cancer cells derived from patients. We validated the expression of Type I collagen in cancer cell lines and in their culture medium using Western blotting. We further confirmed this using primary cell culture and embryonic carcinoma as a tumor model, when stained with antibodies recognizing Type I collagen antibody (Fig 6).
Type I collagen was secreted not only by cancer cells but also by fibroblasts. We performed a comparison and found that cancer‐derived Type I collagen has a different molecular weight from that of fibroblast‐derived (CAF and NF) Type I collagen; however, we did not obtain their amino acid sequences. Our previous study showed that the stimulation of fibroblast culture supernatant could lead to the overexpression of cancer‐derived Type I collagen.21
Cancer‐derived and fibroblast‐derived Type I collagen could affect the tumor microenvironment, although the underlying mechanisms need to be explored. Type I collagen generated by cancer contributes to tumor clone formation. Previous studies have claimed that the excessive production of Type I collagen is not caused by increased collagen synthesis by stromal fibroblasts but instead is endogenously produced by breast cancer cells.36 Our findings are consistent with this theory. Considering the ubiquity of Type I collagen, it was meaningful to verify the existence of cancer‐derived Type I collagen in this pilot study.
We observed the expression of Type I collagen in both cancer cells and fibroblasts using primary cell culture. However, although we focused on tumor nests rather than tissues adjacent to the tumor, we were still unable to distinguish cancer‐derived Type I collagen from fibroblast‐derived Type I collagen.
In summary, our results suggest the novel concept that cancer cells produce Type I collagen endogenously. By inhibiting Type I collagen, we found that cancer‐derived Type I collagen could facilitate cancer cell adhesion by modulating the ECM to promote tumor clone formation. Low expression of Type I collagen was significantly associated with cancer cell differentiation, and correlated with poor prognosis in 258 patients with ESCC. These findings provide new insights into the role of cancer‐derived Type I collagen in cancer development and its clinical value in cancer therapy. Type I collagen produced by cancer cells is a promising target to improve cancer diagnosis and treatment. Further studies are warranted to verify other functions of Type I collagen in tumors.
Disclosure
No authors report any conflict of interest.
Supporting information
Appendix S1. Materials and methods
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (Grant ID 81773118, 81472336, and 31471290), the Research and Capacity Building for Public Welfare of Guangdong Province (Grant ID 2015A030302086 and 2014A020212313), and the Technology Planning Project of Guangdong Province (Grant ID 2014B020212012). We thank all members of Prof. XY Guan's team from the University of Hong Kong for providing constructive suggestions for this study.
References
- 1. Palmieri D, Astigiano S, Barbieri O et al Procollagen I COOH‐terminal fragment induces VEGF‐A and CXCR4 expression in breast carcinoma cells. Exp Cell Res 2008; 314: 2289–98. [DOI] [PubMed] [Google Scholar]
- 2. Cretu A, Brooks PC. Impact of the non‐cellular tumor microenvironment on metastasis: Potential therapeutic and imaging opportunities. J Cell Physiol 2007; 213: 391–402. [DOI] [PubMed] [Google Scholar]
- 3. Duesberg P, Mandrioli D, McCormack A, Nicholson JM. Is carcinogenesis a form of speciation? Cell Cycle 2011; 10: 2100–14. [DOI] [PubMed] [Google Scholar]
- 4. Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011; 147: 992–1009. [DOI] [PubMed] [Google Scholar]
- 5. Kokenyesi R, Murray KP, Benshushan A, Huntley ED, Kao MS. Invasion of interstitial matrix by a novel cell line from primary peritoneal carcinosarcoma, and by established ovarian carcinoma cell lines: Role of cell‐matrix adhesion molecules, proteinases, and E‐cadherin expression. Gynecol Oncol 2003; 89: 60–72. [DOI] [PubMed] [Google Scholar]
- 6. Ritty TM, Herzog J. Tendon cells produce gelatinases in response to type I collagen attachment. J Orthop Res 2003; 21: 442–50. [DOI] [PubMed] [Google Scholar]
- 7. Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro‐adhesive and chemotactic activities of thrombospondin‐1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin‐like growth factor‐1 and CD98. J Biol Chem 1999; 274 (16): 11408. [DOI] [PubMed] [Google Scholar]
- 8. Barcus CE, O'Leary KA, Brockman JL et al Elevated collagen‐I augments tumor progressive signals, intravasation and metastasis of prolactin‐induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res 2017; 19: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen SS, Revoltella RP, Papini S et al Multilineage differentiation of rhesus monkey embryonic stem cells in three‐dimensional culture systems. Stem Cells 2003; 21: 281–95. [DOI] [PubMed] [Google Scholar]
- 10. Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem 2003; 278: 327–34. [DOI] [PubMed] [Google Scholar]
- 11. Steinberg J. Collagen turnover and the growth state in 3T6 fibroblast cultures. Lab Invest 1978; 39: 491–6. [PubMed] [Google Scholar]
- 12. Steinberg J. The turnover of collagen in fibroblast cultures. J Cell Sci 1973; 12: 217–34. [DOI] [PubMed] [Google Scholar]
- 13. Kauppila S, Stenbäck F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol 1998; 186: 262–8. [DOI] [PubMed] [Google Scholar]
- 14. Santala M, Risteli J, Risteli L et al Synthesis and breakdown of fibrillar collagens: Concomitant phenomena in ovarian cancer. Br J Cancer 1998; 77: 1825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santala M, Simojoki M, Risteli J, Risteli L, Kauppila A. Type I and III collagen metabolites as predictors of clinical outcome in epithelial ovarian cancer. Clin Cancer Res 1999; 5: 4091–6. [PubMed] [Google Scholar]
- 16. Fishman DA, Kearns A, Chilukuri K et al Metastatic dissemination of human ovarian epithelial carcinoma is promoted by alpha2beta1‐integrin‐mediated interaction with type I collagen. Invasion Metastasis 1998; 18: 15–26. [DOI] [PubMed] [Google Scholar]
- 17. Friedl P, Wolf K. Plasticity of cell migration: A multiscale tuning model. J Cell Biol 2010; 188: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaggioli C, Hooper S, Hidalgo‐Carcedo C et al Fibroblast‐led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 2007; 9: 1392–400. [DOI] [PubMed] [Google Scholar]
- 19. Sanz‐Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol 2010; 22: 690–6. [DOI] [PubMed] [Google Scholar]
- 20. Zhu GG, Kauppila A, Risteli L, Mäkinen M, Stenbäck F, Risteli J. Immunohistochemical study of type I collagen and type I pN‐collagen in benign and malignant ovarian neoplasms. Cancer 1995; 75: 1010–7. [DOI] [PubMed] [Google Scholar]
- 21. Li, J. , Li X, Lan T et al. [Type I collagen secreted by lung cancer cells promotes cancer cell growth in a three‐ dimensional culture system]. Nan Fang Yi Ke Da Xue Xue Bao 2014;34:1129–34. (In Chinese.) [PubMed] [Google Scholar]
- 22. Uhlén M et al Proteomics. Tissue‐based map of the human proteome. Science 2015; 347: 1260419. [DOI] [PubMed] [Google Scholar]
- 23. Giordano TJ. The cancer genome atlas research network: A sight to behold. Endocr Pathol 2014; 25: 362–5. [DOI] [PubMed] [Google Scholar]
- 24. The Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Cancer Genome Atlas Research Network, Analusis Working Group, Asan University et al Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res 2008; 100: 133–58. [DOI] [PubMed] [Google Scholar]
- 28. Cheng Z, Gao W, Fan X et al Extracellular signal‐regulated kinase 5 associates with casein kinase II to regulate GPIb‐IX‐mediated platelet activation via the PTEN/PI3K/Akt pathway. J Thromb Haemost 2017; 15: 1679–88. [DOI] [PubMed] [Google Scholar]
- 29. Kiefer JA, Farach‐Carson MC. Type I collagen‐mediated proliferation of PC3 prostate carcinoma cell line: Implications for enhanced growth in the bone microenvironment. Matrix Biol 2001; 20: 429–37. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz MA, Assoian RK. Integrins and cell proliferation: Regulation of cyclin‐dependent kinases via cytoplasmic signaling pathways. J Cell Sci 2001; (114): 2553–60. [DOI] [PubMed] [Google Scholar]
- 31. Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial‐to‐mesenchymal transition in lung cancer cells via transforming growth factor‐beta signaling. Am J Respir Cell Mol Biol 2008; 38: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I‐induced membrane‐type 1‐matrix metalloproteinase expression and matrix metalloproteinase‐2 activation in the metastatic progression of breast carcinoma. Lab Invest 1997; 76: 651–60. [PubMed] [Google Scholar]
- 33. Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E‐cadherin‐mediated cell‐cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res 2006; 66: 4662–71. [DOI] [PubMed] [Google Scholar]
- 34. Imamichi Y, König A, Gress T, Menke A. Collagen type I‐induced Smad‐interacting protein 1 expression downregulates E‐cadherin in pancreatic cancer. Oncogene 2007; 26: 2381–5. [DOI] [PubMed] [Google Scholar]
- 35. Bracke ME, Boterberg T, Bruyneel EA, Mareel MM. Collagen invasion assay. Methods Mol Med 2001; 58: 81–9. [DOI] [PubMed] [Google Scholar]
- 36. Al‐Adnani MS, Kirrane JA, McGee JO. Inappropriate production of collagen and prolyl hydroxylase by human breast cancer cells in vivo. Br J Cancer 1975; 31: 653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Materials and methods


