Abstract
The classical pathway of complement (CP) can mediate C3 opsonization of Ags responsible for the costimulation and activation of cognate B lymphocytes. In this manner, the complement system acts as a bridge between the innate and adaptive immune systems critical for establishing a humoral response. However, aberrant complement activation is often observed in autoimmune diseases in which C3 deposition on self-antigens may serve to activate self-reactive B cell clones. In this study, we use BIVV009 (Sutimlimab), a clinical stage, humanized mAb that specifically inhibits the CP-specific serine protease C1s to evaluate the impact of upstream CP antagonism on activation and proliferation of normal and autoimmune human B cells. We report that BIVV009 significantly inhibited complement-mediated activation and proliferation of primary human B cells. Strikingly, CP antagonism suppressed human Ig–induced activation of B cells derived from patients with rheumatoid arthritis. These results suggest that clinical use of CP inhibitors in autoimmune patients may not only block complement-mediated tissue damage, but may also prevent the long-term activation of autoimmune B cells and the production of autoantibodies that contribute to the underlying pathologic condition of these diseases.
Introduction
B cells play an essential role in host defense by producing Abs that neutralize invading pathogens and target them for destruction. Specificity of mature B cells to self is limited through negative selection that includes clonal deletion, receptor editing, and induction of anergy in cells with sufficient BCR affinity to self-ligands (1–5). Nevertheless, a limited number of self-reactive B cell clones advance through negative selection. In addition, somatic hypermutations upon Ag-dependent activation of mature B cells may occasionally generate de novo specificity to self-ligands. Thus, autoreactive B cells are often found in the circulation and are even thought to be physiological (6, 7). Further activation of such self-specific B cells may drive clonal expansion and provoke the production of pathogenic autoantibodies, resulting in autoimmune disorders.
Activation of peripheral B cells during an immune response is a finely tuned mechanism that requires several signals to promote proliferation and differentiation of the selected clone. Ag recognition by the BCR initiates the transition of a quiescent naive B cell to an activated state. The fate of the activated B cell depends on additional signals received from costimulatory receptors such as CD40 (8), TLRs (9), and cytokine receptors (10) as well as the B cell coreceptor complex, a multimeric assembly consisting of CD81, CD19, and the complement receptor 2 (CR2; or CD21). The activating and growth-promoting effects of C3-split products on B cells has been demonstrated (11). Mechanistically, C3-split products deposited on the target Ag bind to CD21, lowering the threshold of BCR activation by several orders of magnitude (12) and providing a powerful survival stimulus (13–16). Therefore, ligation of BCR to a complement-opsonized cognate self-antigen may result in the survival of an autoreactive clone that can lead to the development of autoimmunity. Indeed, complement-opsonized autoantigens have been shown to break B cell anergy (17).
Aberrant complement pathway activation has been demonstrated in many autoimmune disorders, particularly in diseases associated with pathogenic autoantibodies (7). Binding of C1 complex, the triggering mechanism of the classical pathway of complement (CP), to an immune complex containing a self-antigen and an autoantibody results in the formation of the CP C3 convertase, C4b2a. Subsequent cleavage of complement proteins C3 and C5 results in the following: 1) generation of C3a and C5a, anaphylatoxins that attract and activate effector immune cells to the site of Ab binding/complement activation; 2) deposition of C3 opsonins that mediate phagocytosis (18) and lymphocyte activation (15, 16); and, finally, 3) the formation of the membrane attack complex, a lytic pore that disrupts the cellular membrane and leads to cellular destruction. Thus, complement components have long been an attractive target for drug development. Eculizumab, a humanized mAb targeting the downstream complement component C5, has proven to be safe and efficacious for patients with paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, and more recently, refractory myasthenia gravis (19, 20). However, C5 antagonism does not prevent C3-mediated pathologic conditions (18, 21), which could be addressed by targeting more proximally in the complement cascade.
We have previously described TNT003, a mouse mAb that blocks C1s activity and prevents the upstream activation of the CP (18, 22–25). In an in vitro model of cold agglutinin disease (CAD), TNT003 was shown to inhibit complement-dependent phagocytosis and lysis of RBCs induced by CAD patient autoantibodies (18, 26). In the present work, we studied the effect of C1s inhibition on the activation of primary human B cells using BIVV009 (Sutimlimab), the humanized form of TNT003, which was granted breakthrough therapy designation by the U.S. Food and Drug Administration for the treatment of primary CAD [clinical data reported elsewhere (27–30)]. We hypothesized that inhibition of complement deposition on the Ag would result in decreased activation of cognate B cells. In this review, we report that in a novel in vitro test system, BIVV009 prevents complement-enhanced activation and proliferation of normal primary human B cells and, furthermore, suppresses activation of IgG-reactive B cells from patients with rheumatoid arthritis.
Materials and Methods
Soluble complement proteins, Abs, and primary cells
Complement reagents, including pooled normal human serum (NHS), C3-immunodepleted serum (C3dpl), soluble C3d protein, and gelatin veronal buffer with Ca and Mg (GVB++), were purchased from Complement Technology (Tyler, TX). Serum was diluted to a final concentration with GVB++ and pretreated with complement inhibitor Abs for 30 min at 37°C before complement activation. C1s inhibitors TNT003 and BIVV009, anti-C5 Ab (no. A217; Quidel), and relevant isotype controls were used at 20–100 μg/ml. Primary PBMC from de-identified normal donors were purchased from AllCells (Alameda, CA) and STEMCELL Technologies (Vancouver, Canada). Primary PBMC from de-identified patients with rheumatoid arthritis were purchased from AllCells. No therapeutic modalities were used as exclusion criteria for samples from patients with rheumatoid arthritis.
Activation of primary human B cells by soluble agonists
B cells were activated by soluble anti-Ig (anti-human F[ab′]2 [Jackson ImmunoResearch Laboratories, West Grove, PA]) and C3d (Complement Technology); both were used at a final concentration of 2 μg/ml. Soluble agonists were simultaneously added to Fluo-4–loaded primary B cells immediately before the measurement of B cell activation by recording fluorescence at 488 nm with a SpectraMax i3 Multi-Mode Microplate Reader at 37°C (n ≥ 3).
Complement activation by Ig-coated plates
The schematic representation of the assay is shown in Fig. 2A. ELISA plates (3590; Corning, Corning, NY) for in vitro activation or proliferation assays (Fig. 2A) were coated with either 15 μg/ml endotoxin-free mouse IgG anti-human IgM (monoclonal mouse anti-human, no. orb216258; Biorbyt, Cambridge, U.K.), endotoxin-free, plasma-derived whole human IgM (16-16-090713; Athens Research & Technology, Athens, GA) or endotoxin-free, plasma-derived whole human IgG (1-001-A; R&D Systems, Minneapolis, MN) overnight at 4°C. Plates were washed with PBS and blocked with endotoxin-free 2% gelatin (tissue culture grade; MilliporeSigma, St. Louis, MO) for 1 h on a slow shaker at 37°C. Plates were subsequently washed with GVB++ buffer (Complement Technology). Complement was activated by either 5% NHS incubated in mouse anti-human plates, 2.5% NHS in human IgM plates, or 1.25% NHS in human IgG plates in the absence or presence of Abs inhibiting complement for 1.5 h at 37°C. C3dpl (Complement Technology) was used as a control at the same final concentrations. Human IgM plates were incubated with NHS, washed, and subjected to an additional coating step with a soluble anti-Ig for 30 min, followed by a wash with PBS. Plates with deposited complement were used for activation of primary B cells.
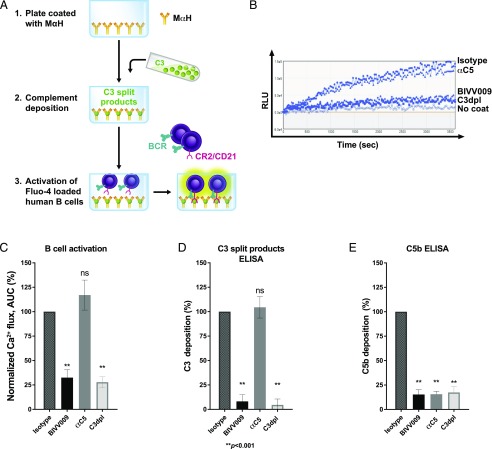
FIGURE 2.
C1s inhibition prevents complement-enhanced BCR activation in primary human B cells. (A) Schematic of an in vitro assay of B cell activation by BCR ligands and deposited complement. (1) Plates are coated with mouse anti-human Ab. (2) Complement is deposited on mouse anti-human–coated plates from either 5% NHS treated with 100 μg/ml human IgG4 (isotype), 100 μg/ml BIVV009 or anti-C5 Ab, or from 5% human C3dpl. (3) Activation of B cells loaded with Fluo-4 stain in mouse anti-human plates with deposited complement is measured as 488 nm fluorescence in a 96-well plate reader. (B) Representative Ca2+ flux traces of Fluo-4–stained primary normal human B cells incubated in mouse anti-human plates treated as described in (A). No coat refers to cells incubated in uncoated wells. (C) Normalized Ca2+ flux in cells from (B) (normalized to isotype control as in Fig. 1E). Data are mean values from independent experiments on B cells from 10 separate donors. (D) C3-split products ELISA of plates from (B). (E) C5b ELISA of plates from (B). Error bars are SEM. Statistics are one-way ANOVA comparison with isotype control. **p < 0.001. αC5, anti-C5; MαH, mouse anti-human.
Activation of primary human B cells by deposited complement
B cells were negatively isolated from frozen PBMC samples from normal human donors or from patients with rheumatoid arthritis (catalogue no. 17954; STEMCELL Technologies). B cell purity (CD19+ cells) was, on average, 88.4 ± 7.8%, as measured by flow cytometry. Negatively isolated B cells were maintained in RPMI 1640 media (Life Technologies, Carlsbad, CA) with 15% heat-inactivated serum (no. SH30070.03HI; HyClone) and antibiotics (no. 10378016; Life Technologies, Thermo Fisher Scientific, Waltham, MA). Human B cells were prestained with Fluo-4 (Life Technologies) per the manufacturer’s protocol and transferred to ELISA plates with activated complement. Fluorescence was measured at 488 nm with SpectraMax i3 Multi-Mode Microplate Reader at 37°C (n ≥ 3). Complement deposition on plates was confirmed by specific ELISAs.
Proliferation analysis of primary human B cells
Negatively isolated human B cells were prestained with CFSE (Tonbo Biosciences, San Diego, CA) and transferred to ELISA plates containing activated complement in the presence of 0.5–12.5 μg/ml TLR9 ligand CpG (InvivoGen, San Diego, CA), 0.01–0.1 μg/ml TLR7 ligand R848 (InvivoGen), or a combination of CpG and 6 ng/ml CD40L (ALX-522-110-C010; Enzo Life Sciences, Farmingdale, NY). After 1.5 h of incubation, cells were moved to U-bottom, 96-well plates for a long-term culturing in R15 media. On days 4 and 8, cells were fixed and stained with anti-CD19/APC and anti-CD21/PE Abs (BD Biosciences, San Jose, CA) and premixed with unconjugated latex beads (Spherotech, Lake Forest, IL). CD19/CD21 staining specifically identified B cells. CD19+/CFSElow gating was used to identify the proliferating B cell population. Latex beads were used as an internal reference for a cell count by flow cytometry. Cellular proliferation was measured on a BD Accuri C6 and expressed as percentage of CD19+/CFSElow cells relative to the total number of CD19+ cells. The number of acquired cells was normalized to the percentage of acquired beads. Percentage of proliferating cells was normalized to specified controls using the following formula: Value = 100 × ValueX/ValueIsotype.
ELISA analysis of complement deposition on plates
NHS-exposed plates used for activation and proliferation assays were analyzed for complement deposition. Plates were blocked with 1% casein for 30 min at room temperature and stained with either 10 μg/ml rabbit anti-human C3d (no. CL2904AP-1; Cedarlane Laboratories) or rabbit anti-human C5b (no. A227; Complement Technology) for 1 h on an orbital shaker. After three washes with PBS, cells were stained with an HRP-conjugated anti-rabbit secondary Ab for 1 h on an orbital shaker. Plates were washed and incubated with 100 μl TMB substrate/well (no. 34029; Thermo Fisher Scientific). HRP-developed signal was stopped after 5–15 min and measured with a SpectraMax i3 Multi-Mode Microplate Reader at 450 nm. Each point is the mean of at least three replicates. Errors are SEM.
Results
Soluble C3d enhances BCR-triggered activation of primary human B cells
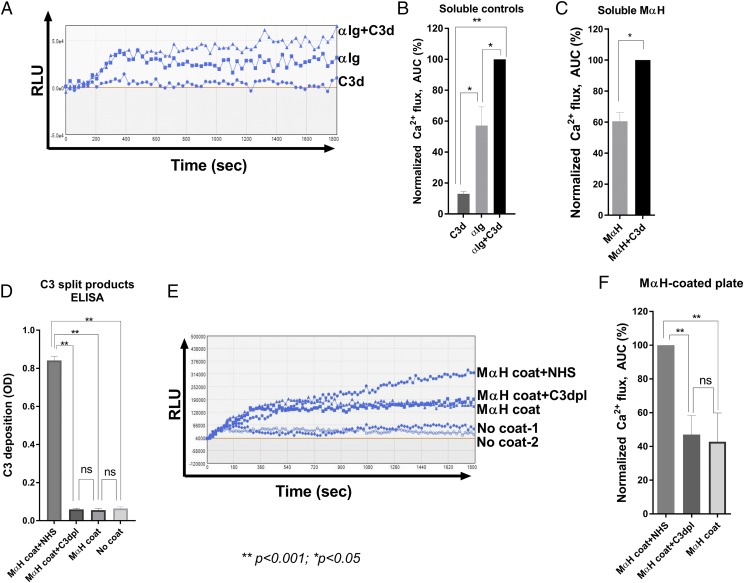
To assess the role of complement in modulating BCR-mediated signaling, we compared the response of primary human B cells stimulated with a BCR agonist and soluble complement C3d. We assessed B cell activation using a fluorescent Ca2+ reporter (17, 31). Healthy donor–derived, negatively isolated primary human B cells were preincubated with the cell-permeant Ca2+ dye, Fluo-4, and stimulated with either soluble C3d, a BCR cross-linking Ab (anti-Ig), or a combination of anti-Ig and C3d (Fig. 1A). Anti-Ig alone induced significant Ca2+ flux in primary human B cells, whereas soluble C3d alone did not. Costimulation with C3d and anti-Ig enhanced Ca2+ flux compared with either C3d or anti-Ig alone (Fig. 1A, 1B). These results are consistent with previous studies demonstrating the complement-enhanced BCR stimulation of peripheral blood–derived primary human B cells (17).
FIGURE 1.
Development of an in vitro assay to study complement-enhanced activation of primary human B cells. (A) Activation of B cells by soluble ligands. Representative Ca2+ flux traces of Fluo-4–stained cells induced with soluble complement C3d (2 μg/ml), BCR cross-linking Ab anti-Ig (2 μg/ml), or a combination of both. (B) Normalized Ca2+ flux calculated as the area under the curve (AUC) and normalized to anti-Ig+C3d. (C) Activation of B cells by soluble mouse anti-human or mouse anti-human+C3d. Ca2+ flux is normalized to mouse anti-human+C3d. (D) C3-split product ELISA of mouse anti-human–coated wells after complement activation from the following sources: 5% untreated NHS (NHS), 5% human C3dpl, no serum (mouse anti-human coat), or uncoated wells exposed to 5% NHS (no coat). (E) Activation of B cells by mouse anti-human plates with deposited complement. Representative Ca2+ flux traces of Fluo-4–stained primary normal human B cells incubated in wells coated with mouse anti-human (mouse anti-human coat) in wells coated with mouse anti-human and exposed to 5% NHS (mouse anti-human coat+NHS), 5% human C3dpl (mouse anti-human coat+C3dpl), or incubated in uncoated wells treated with 5% NHS (no coat 1, 2). (F) Normalized Ca2+ flux in cells treated as in (E). Data shown in (B)–(D) and (F) are mean values from at least three independent experiments with B cells from separate donors. Data in (F) are normalized using the following formula: Relative value = 100 × (ValueX−Valueno coat)/(Valuemouse anti-human+NHS−Valueno coat). Data in (B)–(D) and (F) are mean values. Error bars are SEM. Statistics were performed using a one-way ANOVA. *p < 0.05, **p < 0.001. αIg, anti-Ig; MαH, mouse anti-human; RLU, relative luminescence unit.
Development of an in vitro assay to study complement-enhanced activation of primary human B cells
In autoimmune diseases, autoantibody-induced complement deposition may serve as an adjuvant for autoreactive B cells that encounter C3d-bound Ag. To study the effects of Ab-mediated complement activation on the BCR Ag, we sought to establish a model in which the BCR agonist acts as both a BCR ligand and complement activator. We therefore evaluated IgG/IgM molecules raised against human BCR that would serve to activate human B cells via BCR and to activate complement. We identified a mouse monoclonal IgG raised against human IgM (mouse anti-human) that bound to and activated BCR on primary human B cells. As we observed with anti-Ig (Fig. 1B), mouse anti-human alone induced significant Ca2+ flux in primary human B cells, which was enhanced upon addition of soluble C3d (Fig. 1C). To evaluate its ability to activate complement, we coated ELISA plates with mouse anti-human, blocked, and then exposed wells to 5% NHS as a complement source. Mouse anti-human–mediated complement activation was assessed by ELISA for deposition of C3-split products in the well (Fig. 1D). Mouse anti-human–induced deposition of C3-split products from NHS (Fig. 1D; NHS), whereas C3 deposition was not detected in uncoated wells exposed to NHS (Fig. 1D; no coat), mouse anti-human–coated wells exposed to C3dpl (Fig. 1D), or in mouse anti-human–coated, untreated wells (Fig. 1D; mouse anti-human coat). Together, these results demonstrate that mouse anti-human alone activates normal primary human B cells and activates human complement.
We next evaluated Ca2+ flux in primary human B cells incubated in mouse anti-human–coated plates following exposure to NHS (Figs. 1E, 1F, 2A). Mouse anti-human–coated wells exposed to NHS potently activated primary human B cells (Fig. 1E, 1F). In contrast, mouse anti-human–coated wells exposed to C3dpl, or no serum, induced significantly lower activation of B cells (Fig. 1E, 1F). The uncoated wells exposed to NHS neither induced C3 deposition (Fig. 1D; no coat), nor induced Ca2+ flux in B cells (Fig. 1E; no coat) and were therefore used to determine the background signals for further experiments.
C1s inhibition prevents complement-enhanced BCR activation in primary human B cells
We next assessed the effect of complement inhibitors on complement-enhanced BCR activation of B cells (Fig. 2A). We pretreated NHS with either BIVV009, an anti-C5 Ab, or a human IgG4 isotype control Ab and exposed the treated sera to mouse anti-human–coated plates. C3dpl was used as a control for complement deposition. Wells exposed to the human IgG4 isotype control, Ab-treated serum induced a robust activation of primary human B cells, as measured by Ca2+ flux (Fig. 2B, 2C). In contrast, wells exposed to BIVV009-treated serum demonstrated a significantly reduced activation of primary human B cells, which was comparable with that observed in C3dpl (Fig. 2B, 2C,). Finally, wells exposed to the C5-inhibitor, Ab-treated serum induced a normal activation of primary human B cells comparable with that of isotype control (Fig. 2B, 2C). The control B cells that were incubated in uncoated wells (no mouse anti-human) are shown as no-coat control (Fig. 2B). To confirm the activity of the specific complement inhibitors, we checked complement deposition on the mouse anti-human–coated plates used for B cell stimulation. C3-split product deposition observed in wells exposed to NHS treated with the isotype control was comparable with the deposition observed in wells exposed to NHS treated with the C5 inhibitor (Fig. 2D). In contrast, wells exposed to NHS containing BIVV009 or C3dpl showed significantly reduced deposition of C3-split products. To ensure the activity of the C5 inhibitor, plates were stained for C5b, the first deposited cleavage product following C5 activation (Fig. 2E). NHS incubated with the isotype control Ab showed significant C5b deposition. In comparison, plates exposed to NHS containing BIVV009 or the C5 inhibitor showed significantly less C5b staining.
We confirmed the robustness of deposited C3 in augmenting BCR signaling using a different in vitro assay format.
We coated ELISA plates with plasma-purified human IgM and induced complement deposition by exposing them to NHS. After thoroughly washing the plates, we added soluble anti-Ig, which bound the IgM coat. In the final step, we incubated Fluo-4–preloaded B cells in the prepared plates and measured B cell activation as in previous experiments (Supplemental Fig. 1).
Similar to the results observed with mouse anti-human–coated plates, NHS treatment with the anti-C1s inhibitor TNT003 prevented complement-enhanced activation of primary human B cells (Supplemental Fig. 1A, 1B) and blocked C3 deposition on human IgM–coated plates (Supplemental Fig. 1C).
C3 activation enhances TLR-induced proliferation of human primary B cells
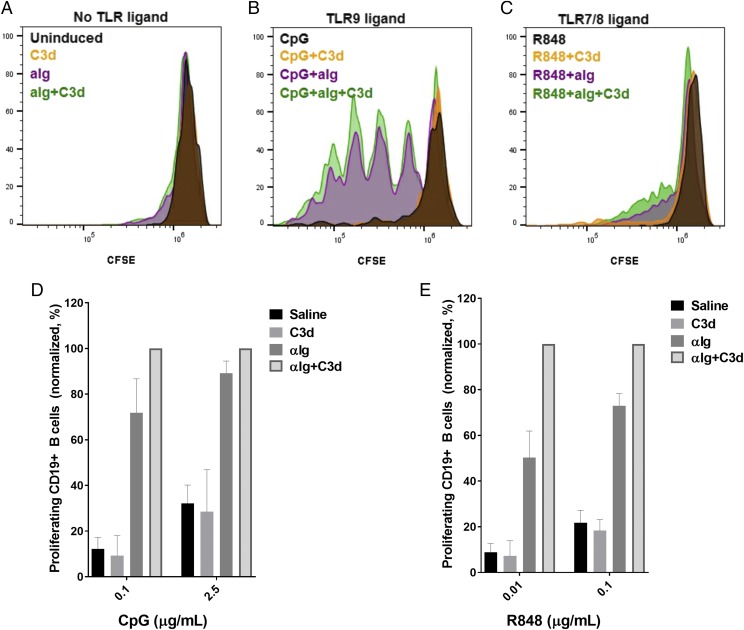
TLR signaling facilitates the immune response to foreign Ags by providing an essential costimulatory survival signal to activated B cells (9). In autoimmune conditions, TLR costimulation promotes survival and proliferation of autoimmune B cells (9, 32–34). TLR ligand stimulation induces proliferation of primary human B cells ex vivo (35, 36) and, thus, may be studied in our model. We therefore sought to test the effect of complement-enhanced BCR stimulation on TLR-induced proliferation of primary human B cells.
We preloaded B cells with the proliferation tracking dye CFSE and then stimulated them with either soluble C3d, anti-Ig, or in combination. The cells were then cultured for 4 d in either growth media alone (Fig. 3A), growth media containing the TLR9 agonist CpG (Fig. 3B, 3D), or the TLR7/8 agonist R848 (Fig. 3C, 3E). Cellular proliferation was assessed via flow cytometry by measuring the percent of CD19+/CFSElow cells. C3d stimulation alone showed little to no proliferation, comparable with that seen in untreated wells (Fig. 3A). Anti-Ig, alone or in combination with C3d, induced limited B cell division (Fig. 3A). In contrast, the TLR9 ligand CpG, in combination with anti-Ig, induced a robust cellular proliferation, whereas soluble C3d modestly enhanced CpG/anti-Ig–induced proliferation of primary human B cells (Fig. 3B, 3D). Although overall proliferation was lower than with CpG, we also observed a similar trend of C3d-enhanced, anti-Ig–induced proliferation with the TLR7/8 ligand R848 (Fig. 3C, 3E).
FIGURE 3.
C3 activation enhances TLR-induced proliferation of primary B cells. (A) Proliferation of CFSE-stained primary human B cells on day 4 poststimulation with saline (uninduced), C3d (2 μg/ml), anti-Ig (2 μg/ml), or a combination of both (2 μg/ml each). (B) Proliferation of B cells stimulated as in (A) in the presence of TLR9 ligand CpG (2.5 μg/ml). (C) Proliferation of B cells stimulated as in (A) in the presence of TLR7/8 ligand R848 (0.1 μg/ml). (D) Relative proliferation of B cells in the presence of 0.1 and 2.5 μg/ml CpG. (E) Relative proliferation of B cells in the presence of 0.01 and 0.1 μg/ml R848. Data shown in (D) and (E) are normalized to anti-Ig+C3d point in the presence of either 2.5 μg/ml CpG or 0.1 μg/ml R848. Data in (A)–(E) are mean values from independent experiments on B cells from three separate donors. αIg, anti-Ig.
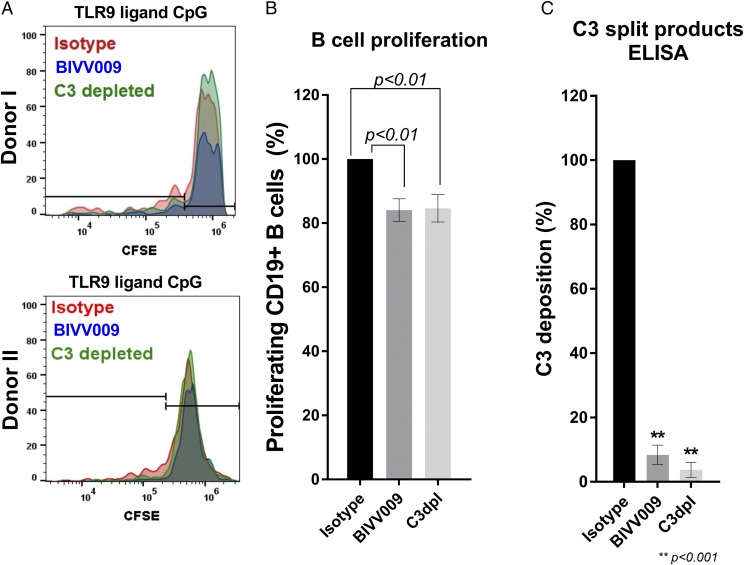
BIVV009 prevents complement-enhanced proliferation of normal primary human B cells
To assess the effect of complement on primary human B cell proliferation, we stimulated B cells using mouse anti-human–coated plates exposed to NHS in the presence of the TLR9 agonist CpG (Fig. 4A). After 90 min, cells were transferred to a U-bottom plate for long-term culturing (8 d). Mouse anti-human–coated plates with activated complement induced a moderate proliferation of 10–45% of total CD19+ B cells (Fig. 4A; Supplemental Fig. 2). Costimulation of B cells with mouse anti-human exposed to NHS enhanced cellular proliferation compared with C3dpl (Fig. 4A, 4B). Pretreatment of NHS with BIVV009 significantly decreased complement-enhanced proliferation, comparable with the level observed in C3dpl (Fig. 4A, 4B). Additionally, C3-split product deposition was significantly attenuated in the wells treated with BIVV009 compared with those treated with the isotype control Ab (Fig. 4C).
FIGURE 4.
BIVV009 prevents complement-enhanced proliferation of primary B cells. (A) Two representative flow cytometry plots of CD19+ B cell proliferation on day 8 after initial stimulation with CpG in mouse anti-human plate with activated complement deposited from the following sources: 5% NHS treated with 100 μg/ml human IgG4 (isotype), 5% NHS treated with 100 μg/ml BIVV009 (BIVV009), or 5% human C3dpl. Counting gates are shown. (B) Relative proliferation of CD19+CFSElow B cells normalized to an isotype control. Data shown are from independent experiments on B cells from 10 separate donors. (C) C3-split products ELISA of plates from (B). Statistics are one-way ANOVA. **p < 0.001.
BIVV009 prevents complement-enhanced activation of human IgG–reactive B cells from patients with rheumatoid arthritis
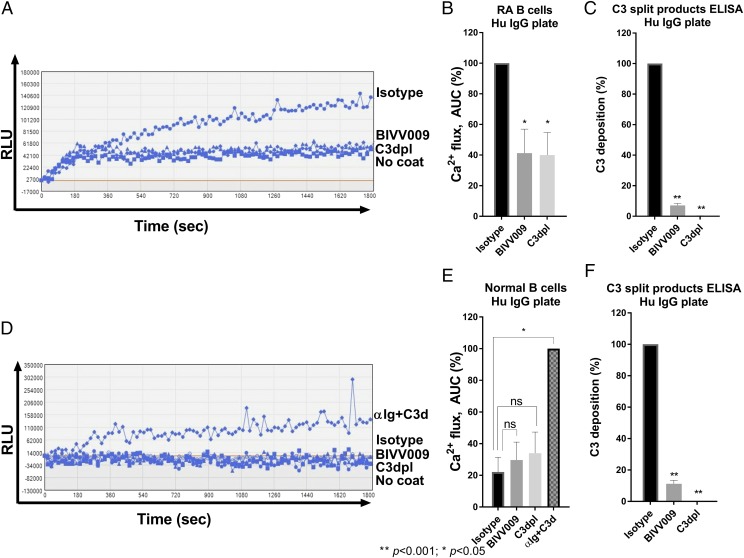
We next tested the effect of BIVV009 on the activation of B cells derived from patients with rheumatoid arthritis. B cell autoreactivity to human IgG is broadly found in rheumatoid arthritis and contributes to the development of human IgG–reactive autoantibodies known as rheumatoid factor (37–39). Because the in vitro assay we developed with normal human primary B cells used IgG as both a BCR stimulator and complement activator, we hypothesized that IgG autoreactive B cells from patients with rheumatoid arthritis may respond to human IgG in a similar plate-based format. We therefore evaluated the ability of human IgG–coated plates to induce Ca2+ flux in negatively isolated B cells from the peripheral blood of seven patients with rheumatoid arthritis (rheumatoid arthritis B cells).
In a similar format to the mouse anti-human assay, plates were coated with human IgG and subsequently exposed to NHS. Fluo-4–preloaded rheumatoid arthritis B cells were then added to the wells, and cellular activation was assessed by Ca2+ influx. We found that human IgG–coated plates exposed to NHS induced Ca2+ flux in rheumatoid arthritis B cells (Figs. 5A, 5B, 6). However, rheumatoid arthritis B cells exposed to human IgG–coated plates treated with NHS containing BIVV009 showed significantly reduced Ca2+ flux, comparable with that observed in C3dpl (Fig. 5A, 5B). Deposition of C3-split products was also decreased in BIVV009 and C3dpl-treated wells (Fig. 5C). In contrast, human IgG–coated plates exposed to NHS did not activate normal human B cells derived from four healthy donors (Fig. 5D, 5E), even though complement deposition was confirmed in these wells (Fig. 5F). Importantly, costimulation with anti-Ig and C3d induced a significant Ca2+ flux in B cells from these healthy donors, demonstrating that the cells were responsive to BCR stimulation (Fig. 5D, 5E).
FIGURE 5.
BIVV009 prevents complement-enhanced activation of human IgG–reactive B cells from patients with rheumatoid arthritis. (A) Representative Ca2+ flux traces of Fluo-4–stained rheumatoid arthritis B cells incubated in a human IgG plate with activated complement deposited from the following sources: 5% NHS treated with 100 μg/ml human IgG4 (isotype), 5% NHS treated with 100 μg/ml BIVV009 (BIVV009), or 5% human C3dpl. No coat refers to cells incubated in uncoated wells. (B) Relative Ca2+ flux normalized to isotype control as described in Fig. 1F. Data shown in (B) are from independent experiments on B cells from seven different patients with rheumatoid arthritis. (C) C3-split products ELISA of human IgG plates from (B). (D) Representative Ca2+ flux trace of Fluo-4–stained normal B cells incubated in human IgG plates treated as in (A). Anti-Ig+C3d is used as a positive control. (E) Relative Ca2+ flux normalized to the anti-Ig+C3d control as described in Fig. 1E. Data shown are from independent experiments on B cells from four separate normal donors. (F) C3-split products ELISA of human IgG plates from (E). Data in (B), (C), (E), and (F) are mean values. Statistics are a one-way ANOVA comparison with isotype control. *p < 0.05, **p < 0.001. αIg, anti-Ig; RLU, relative luminescence unit.
FIGURE 6.
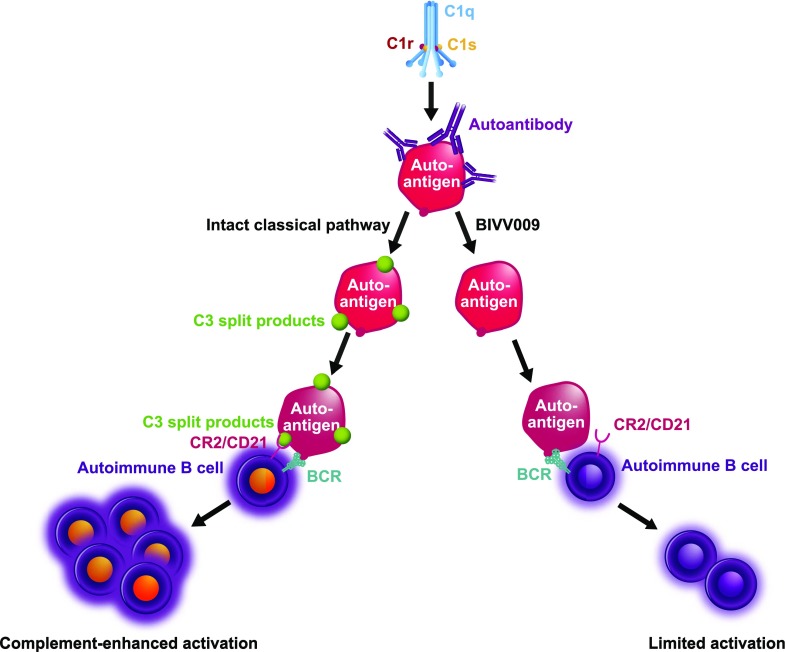
The CP inhibitor BIVV009 (Sutimlimab) prevents Ab-mediated, complement-enhanced activation of autoimmune human B cells. The C1 complex binds to the Ab-opsonized autoantigen and activates the CP cascade. Activation of the CP results in deposition of C3-split products on the surface of the autoantigen. A cognate autoimmune B cell recognizes autoantigen via the BCR and receives costimulation upon ligation of autoantigen-bound C3-split products to CR2 (CD21). This coincident signaling results in a complement-enhanced activation of autoimmune B cells. In the presence of BIVV009 (Sutimlimab), C3 deposition on autoantigens is inhibited. A cognate autoimmune B cell recognizes autoantigen via BCR alone, resulting in limited cellular activation and/or anergy.
We also tested rheumatoid arthritis B cells in the ex vivo proliferation assay. Similar to what was observed with normal B cells, soluble C3d increased the proliferation of anti-Ig–induced primary rheumatoid arthritis B cells in the presence of CpG (Supplemental Fig. 3B–D). However, we did not observe a complement-enhanced proliferation induced by human IgG in the presence of CpG (Supplemental Fig. 3E).
Discussion
Over the course of the evolution of the immune system, B lymphocytes have incorporated CRs that function to bridge innate and adaptive signaling mechanisms (40). Ag recognition by BCR concurrent with C3d/CR2 ligation leads to a level of cooperativity that drastically reduces the antigenic load required for cellular activation (12). In this manner, the complement system provides an independent confirmation of the foreign nature of the Ag to the adaptive immune system.
Complement deposition is observed in numerous diseases and conditions, often associated with pathological Abs that activate the CP (41). Indeed, complement deposition is used diagnostically in such diseases, including bullous pemphigoid (42), Ab-mediated rejection (43–45), and autoimmune hemolytic anemia (46, 47). The presence of complement-split products and autoantibody on target tissue or cells in these diseases suggests that complement deposition is a result of autoantibody-mediated CP activation on the autoantigen. We hypothesized that if complement stimulation is important for the continued expansion and replenishment of cognate autoreactive B cells (17, 48), then CP antagonism could inhibit autoimmune B cell activation.
In this experiment, we studied the impact of CP inhibition on Ab-mediated complement activation of primary human B cells using the C1s inhibitor BIVV009 (Fig. 6). We developed a novel in vitro assay to study complement-enhanced BCR signaling using a single mouse IgG raised against human IgM (mouse anti-human) capable of both binding to BCR and activating the CP (Fig. 2A). Using this system, we demonstrate that upstream (C1s) CP inhibition, in contrast to terminal pathway (C5) inhibition, blocks complement-enhanced activation of normal primary human B cells (Fig. 2B, 2C). Specifically, BIVV009 treatment of NHS efficiently reduced primary B cell activation to background levels (C3dpl; Fig. 2B, 2C). Importantly, attenuation of B cell activation was associated with reduced C3 deposition (Fig. 2D), suggesting that complement-enhanced B cell activation observed in our model system is a result of CR2 signaling through the B cell coreceptor.
To study the effect of activated complement on proliferation of B cells, we performed a flow cytometry analysis of CFSE-stained primary B cells and found that BCR and TLR ligand–induced cellular proliferation was augmented by the addition of soluble C3d (Fig. 3B–E). Using mouse anti-human as both a BCR ligand and complement activator, we found that BIVV009 significantly and reproducibly reduced the proliferation of CpG/BCR-stimulated B cells (Fig. 4A, 4B). This result suggests that CP inhibitors may limit the BCR and TLR-induced ymphoproliferation observed with autoimmune B cells (9, 32–34).
Numerous lines of evidence suggest that complement plays a pathological role in the development and progression of rheumatic diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (49, 50). Autoantibodies are a hallmark of both diseases and have been shown to activate the CP leading to anaphylatoxin generation and complement deposition (51–53). Rheumatoid factor (Igs that bind other Igs) is present in the serum of over 70% of patients with rheumatoid arthritis (37–39), suggesting that most patients with rheumatoid arthritis have autoreactive B cells that would be sensitive to human IgG stimulation and, therefore, amenable to interrogation in our ex vivo B cell activation model. Indeed, we found that primary rheumatoid arthritis B cells exhibited a robust Ca2+ flux when exposed to human IgG–coated plates pretreated with NHS (Fig. 5A). This Ca2+ flux was significantly attenuated in BIVV009-treated or C3dpl sera, demonstrating that B cells from patients with rheumatoid arthritis are sensitive to complement stimulation. Importantly, primary B cells obtained from healthy individuals showed no measurable human IgG–induced Ca2+ flux (Fig. 5D, 5E). Together, these results provide strong evidence that the complement-dependent Ca2+ flux we observed in rheumatoid arthritis B cells came from the activation of antirheumatoid factor B cell clones. Interestingly, we did not observe human IgG–mediated, complement-enhanced proliferation of rheumatoid arthritis B cells in the presence of CpG. However, unlike mouse anti-human, human IgG stimulates only IgG-reactive lymphocytes, which make up only a portion of the total B cells in the rheumatoid arthritis samples. Therefore, one potential explanation for why we did not observe proliferation of rheumatoid arthritis B cells in response to human IgG stimulation is that the number of viable IgG-sensitive B cells several days poststimulation was insufficient to induce detectable proliferation in our system.
In addition to its direct effect on B cell activation, complement has been shown to play other roles critical to the development of a humoral immune response to Ag (48). Complement C3-split products on the surface of Ags and circulating immune complexes facilitate their transport and presentation to lymphocytes in the germinal center. Specifically, C3 opsonin–coated Ags are recognized by CR3 on the surface of subcapsular sinus macrophages that shuttle the Ag in a CR-dependent manner to B cells entering the lymph node (54, 55). These B cells in turn transport complement-coated Ag into the germinal center to follicular dendritic cells (FDC) via CR2 expressed on both cell types. Finally, Ag presentation by FDC to naive B cells is also complement dependent, in which FDCs display C3d-bound Ag to cognate B cells to trigger the BCR and CR2-mediated immune response (48, 56). The importance of CR2 signaling in the humoral immune response is supported by observations of a CR2-deficient individual, who presented with a reduced number of class-switched memory B cells and hypogammaglobulinemia (57). Thus, in addition to preventing the effects on direct B cell activation as demonstrated in this study, CP antagonism may interfere with other complement-dependent mechanisms of the humoral immune response and suppress the activation, propagation, and differentiation of autoimmune B cells in vivo.
Paradoxically, increased CP activity and congenital lack of CP activity are associated with SLE or SLE-like autoimmune conditions (58, 59). Patients with SLE with an intact CP have a drop in C4 and C3 levels (consumption) prior to onset of flares (60). This, as well as the presence of cell-bound complement activation products, such as C4d and C3d on the surface of RBCs, lymphocytes, and platelets in patients with SLE, suggests that increased CP activity drives the disease process (61). However, congenital deficiencies in CP components, most notably C1q deficiency, are among the most penetrant genetic risk factors for the development of lupus, in which 85–90% of individuals lacking C1q develop SLE-like symptoms (62, 63). The complement system, and the CP in particular, has been suggested to be responsible for the opsonization of immune complexes, apoptotic cells, and cellular debris for removal by phagocytes (64, 65). Therefore, it has been proposed that a nonfunctional CP leads to the increased exposure of proinflammatory intracellular self-antigens to cells of the immune system, resulting in an immune response that gives rise to autoantibodies against dsDNA, ribonuclear proteins, and other nuclear Ags (65). Interestingly, unlike the depletion of C1q, the inhibition of C1s had a small impact on the uptake of early apoptotic cells by human phagocytes in vitro, suggesting a critical role for the opsonic function (CP independent) of C1q in the removal of early apoptotic cells (66). Furthermore, 5-wk and 6-mo toxicology studies performed in adult cynomolgus macaques treated with BIVV009 demonstrated the complete inhibition of the CP throughout the course of each study. BIVV009-treated monkeys did not develop SLE or an autoimmune-like disease or symptoms, nor did BIVV009 affect levels of circulating immune complexes or induce the generation of autoantibodies against extractable nuclear Ags or dsDNA. These results suggest that pharmacological inhibition of C1s activity in a mature immune system may not be comparable with a congenital deficiency of C1q, in which in the latter case, CP enzymatic activity may protect against the development of autoimmunity in a developing immune system.
The data presented in this study provide evidence that inhibiting complement deposition on Ag can dampen activation of cognate B cells (Fig. 6). Long-term studies with BIVV009 treatment in autoimmune patients with autoantibody-mediated pathological conditions will provide an opportunity to test the hypothesis that C1s inhibition can alter the pathogenic humoral immune response in patients with complement-related autoimmune diseases.
Supplementary Material
The online version of this article contains supplemental material.
- CAD
- cold agglutinin disease
- C3dpl
- C3-immunodepleted serum
- CP
- classical pathway of complement
- CR2
- complement receptor 2
- FDC
- follicular dendritic cell
- GVB++
- gelatin veronal buffer with Ca and Mg
- NHS
- normal human serum
- SLE
- systemic lupus erythematosus.
Disclosures
P.A.N., E.L.R., T.S.B., and G.C.P. are employees of Bioverativ, a Sanofi company. T.S.B. is a shareholder of Sanofi. S.P. is a former employee of Bioverativ, a Sanofi company.
References
- 1.Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. 1991. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353: 765–769. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee D. 2006. Receptor editing in lymphocyte development and central tolerance. Nat. Rev. Immunol. 6: 728–740. [DOI] [PubMed] [Google Scholar]
- 3.Cambier J. C., Gauld S. B., Merrell K. T., Vilen B. J. 2007. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 7: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardemann H., Yurasov S., Schaefer A., Young J. W., Meffre E., Nussenzweig M. C. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 5.Yurasov S., Wardemann H., Hammersen J., Tsuiji M., Meffre E., Pascual V., Nussenzweig M. C. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson A., Diamond B. 2001. Autoimmune diseases. N. Engl. J. Med. 345: 340–350. [DOI] [PubMed] [Google Scholar]
- 7.Elkon K., Casali P. 2008. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 4: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker D. C. 1993. T cell-dependent B cell activation. Annu. Rev. Immunol. 11: 331–360. [DOI] [PubMed] [Google Scholar]
- 9.Pasare C., Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature 438: 364–368. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F., Browning J. L. 2002. BAFF: a fundamental survival factor for B cells. Nat. Rev. Immunol. 2: 465–475. [DOI] [PubMed] [Google Scholar]
- 11.Klaus G. G., Humphrey J. H. 1986. A re-evaluation of the role of C3 in B-cell activation. Immunol. Today 7: 163–165. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey P. W., Allison M. E., Akkaraju S., Goodnow C. C., Fearon D. T. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271: 348–350. [DOI] [PubMed] [Google Scholar]
- 13.Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA 81: 4510–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter R. H., Fearon D. T. 1989. Polymeric C3dg primes human B lymphocytes for proliferation induced by anti-IgM. J. Immunol. 143: 1755–1760. [PubMed] [Google Scholar]
- 15.Lyubchenko T., dal Porto J., Cambier J. C., Holers V. M. 2005. Coligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J. Immunol. 174: 3264–3272. [DOI] [PubMed] [Google Scholar]
- 16.Heyman B., Pilström L., Shulman M. J. 1988. Complement activation is required for IgM-mediated enhancement of the antibody response. J. Exp. Med. 167: 1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyubchenko T., Dal Porto J. M., Holers V. M., Cambier J. C. 2007. Cutting edge: complement (C3d)-linked antigens break B cell anergy. J. Immunol. 179: 2695–2699. [DOI] [PubMed] [Google Scholar]
- 18.Shi J., Rose E. L., Singh A., Hussain S., Stagliano N. E., Parry G. C., Panicker S. 2014. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood 123: 4015–4022. [DOI] [PubMed] [Google Scholar]
- 19.Legendre C. M., Licht C., Muus P., Greenbaum L. A., Babu S., Bedrosian C., Bingham C., Cohen D. J., Delmas Y., Douglas K., et al. 2013. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368: 2169–2181. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky R. A., Young N. S., Antonioli E., Risitano A. M., Schrezenmeier H., Schubert J., Gaya A., Coyle L., de Castro C., Fu C.-L., et al. 2008. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 111: 1840–1847. [DOI] [PubMed] [Google Scholar]
- 21.Roth A., Bommer M., Hottmann A., Herich-Terhorne D., Kuklik N., Veronika L., Schrezenmeier H., Dührsen U. 2015. Complement inhibition with eculizumab in patients with cold agglutinin disease (CAD): results from a prospective phase II trial (DECADE trial). Blood 126: 274. [Google Scholar]
- 22.Thomas K. A., Valenzuela N. M., Gjertson D., Mulder A., Fishbein M. C., Parry G. C., Panicker S., Reed E. F. 2015. An anti-C1s monoclonal, TNT003, inhibits complement activation induced by antibodies against HLA. Am. J. Transplant. 15: 2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peerschke E. I. B., Panicker S., Bussel J. 2015. Classical complement pathway activation in immune thrombocytopenia purpura: inhibition by a novel C1s inhibitor. Br. J. Haematol. 173: 942–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan B. P., Harris C. L. 2015. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14: 857–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasprick A., Holtsche M. M., Rose E. L., Hussain S., Schmidt E., Petersen F., Panicker S., Ludwig R. J. 2017. The anti-C1s antibody TNT003 prevents complement activation in the skin induced by bullous pemphigoid autoantibodies. J. Invest. Dermatol. 138: 458–461. [DOI] [PubMed] [Google Scholar]
- 26.Panicker S., Shi J., Rose E., Hussain S., Tom S., Strober W., Sloan S. R., Parry G., Stagliano N. 2013. TNT009, a classical complement pathway specific inhibitor, prevents complement dependent hemolysis induced by cold agglutinin disease patient autoantibodies. Blood 122: 42. [Google Scholar]
- 27.Eskandary F., Jilma B., Mühlbacher J., Wahrmann M., Regele H., Kozakowski N., Firbas C., Panicker S., Parry G. C., Gilbert J. C., et al. 2017. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am. J. Transplant. 18: 916–926. [DOI] [PubMed] [Google Scholar]
- 28.Derhaschnig U., Gilbert J., Jäger U., Böhmig G., Stingl G., Jilma B. 2016. Combined integrated protocol/basket trial design for a first-in-human trial. Orphanet J. Rare Dis. 11: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jilma B., Gilbert J. C., Panicker S., Parry G. C., Fillitz M., Thomas S., Sillaber C., Bartko J., Jaeger U. 2016. Chronic inhibition of complement C1s by TNT009 produces sustained, complete remission in patients with severe, transfusion-dependent cold agglutinin disease (CAD). Blood 128: 2435.27574188 [Google Scholar]
- 30.Jaeger U., D’Sa S., Schoergenhofer C., Bartko J., Sillaber C., Jilma-Stohlawetz P., Fillitz M., Schenk T., Patou G., Panicker S., et al. 2017. Long term efficacy, safety and PK/PD profile of the anti-C1s antibody (BIVV009) in primary cold agglutinin disease patients. Blood 130: 703. [Google Scholar]
- 31.Henson S. E., Smith D., Boackle S. A., Holers V. M., Karp D. R. 2001. Generation of recombinant human C3dg tetramers for the analysis of CD21 binding and function. J. Immunol. Methods 258: 97–109. [DOI] [PubMed] [Google Scholar]
- 32.Leadbetter E. A., Rifkin I. R., Hohlbaum A. M., Beaudette B. C., Shlomchik M. J., Marshak-Rothstein A. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607. [DOI] [PubMed] [Google Scholar]
- 33.Marshak-Rothstein A. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6: 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawlings D. J., Schwartz M. A., Jackson S. W., Meyer-Bahlburg A. 2012. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 12: 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruprecht C. R., Lanzavecchia A. 2006. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 36: 810–816. [DOI] [PubMed] [Google Scholar]
- 36.Nikitin P. A., Price A. M., McFadden K., Yan C. M., Luftig M. A. 2014. Mitogen-induced B-cell proliferation activates Chk2-dependent G1/S cell cycle arrest. PLoS One 9: e87299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steiner G., Smolen J. 2002. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 4(Suppl. 2): S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou C., Liao H., Chen Ch., Chen W., Wang H., Su K. 2007. The clinical application of anti-CCP in rheumatoid arthritis and other rheumatic diseases. Biomark. Insights 2: 165–171. [PMC free article] [PubMed] [Google Scholar]
- 39.Turesson C., Jacobsson L. T. H., Sturfelt G., Matteson E. L., Mathsson L., Rönnelid J. 2007. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann. Rheum. Dis. 66: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kieslich C. A., Morikis D. 2012. The two sides of complement C3d: evolution of electrostatics in a link between innate and adaptive immunity. PLOS Comput. Biol. 8: e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricklin D., Lambris J. D. 2013. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190: 3831–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otten J. V., Hashimoto T., Hertl M., Payne A. S., Sitaru C. 2014. Molecular diagnosis in autoimmune skin blistering conditions. Curr. Mol. Med. 14: 69–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colvin R. B. 2007. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J. Am. Soc. Nephrol. 18: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 44.Djamali A., Kaufman D. B., Ellis T. M., Zhong W., Matas A., Samaniego M. 2014. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am. J. Transplant. 14: 255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegall M. D., Chedid M. F., Cornell L. D. 2012. The role of complement in antibody-mediated rejection in kidney transplantation. Nat. Rev. Nephrol. 8: 670–678. [DOI] [PubMed] [Google Scholar]
- 46.Meulenbroek E. M., de Haas M., Brouwer C., Folman C., Zeerleder S. S., Wouters D. 2015. Complement deposition in autoimmune hemolytic anemia is a footprint for difficult-to-detect IgM autoantibodies. Haematologica 100: 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Packman C. H. 2015. The clinical pictures of autoimmune hemolytic anemia. Transfus. Med. Hemother. 42: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carroll M. C., Isenman D. E. 2012. Regulation of humoral immunity by complement. Immunity 37: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leffler J., Bengtsson A. A., Blom A. M. 2014. The complement system in systemic lupus erythematosus: an update. Ann. Rheum. Dis. 73: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 50.Trouw L. A., Haisma E. M., Levarht E. W. N., van der Woude D., Ioan-Facsinay A., Daha M. R., Huizinga T. W. J., Toes R. E. 2009. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 60: 1923–1931. [DOI] [PubMed] [Google Scholar]
- 51.Hay F. C., Nineham L. J., Perumal R., Roitt I. M. 1979. Intra-articular and circulating immune complexes and antiglobulins (IgG and IgM) in rheumatoid arthritis; correlation with clinical features. Ann. Rheum. Dis. 38: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okroj M., Heinegård D., Holmdahl R., Blom A. M. 2007. Rheumatoid arthritis and the complement system. Ann. Med. 39: 517–530. [DOI] [PubMed] [Google Scholar]
- 53.Kao A. H., Navratil J. S., Ruffing M. J., Liu C.-C., Hawkins D., McKinnon K. M., Danchenko N., Ahearn J. M., Manzi S. 2010. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 62: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez S. F., Lukacs-Kornek V., Kuligowski M. P., Pitcher L. A., Degn S. E., Turley S. J., Carroll M. C. 2010. Complement-dependent transport of antigen into B cell follicles. J. Immunol. 185: 2659–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prokopec K. E., Georgoudaki A.-M., Sohn S., Wermeling F., Grönlund H., Lindh E., Carroll M. C., Karlsson M. C. I. 2016. Cutting edge: marginal zone macrophages regulate antigen transport by B cells to the follicle in the spleen via CD21. J. Immunol. 197: 2063–2068. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez S. F., Lukacs-Kornek V., Kuligowski M. P., Pitcher L. A., Degn S. E., Kim Y.-A., Cloninger M. J., Martinez-Pomares L., Gordon S., Turley S. J., Carroll M. C. 2010. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 11: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thiel J., Kimmig L., Salzer U., Grudzien M., Lebrecht D., Hagena T., Draeger R., Voelxen N., Bergbreiter A., Jennings S., et al. 2012. Genetic CD21 deficiency is associated with hypogammaglobulinemia. [Published erratum appears in 2014 J. Allergy Clin. Immunol. 133: 604.] J. Allergy Clin. Immunol. 129: 801–810.e6. [DOI] [PubMed] [Google Scholar]
- 58.Truedsson L., Bengtsson A. A., Sturfelt G. 2007. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40: 560–566. [DOI] [PubMed] [Google Scholar]
- 59.Amano M. T., Ferriani V. P. L., Florido M. P. C., Reis E. S., Delcolli M. I. M. V., Azzolini A. E. C. S., Assis-Pandochi A. I., Sjöholm A. G., Farah C. S., Jensenius J. C., Isaac L. 2008. Genetic analysis of complement C1s deficiency associated with systemic lupus erythematosus highlights alternative splicing of normal C1s gene. Mol. Immunol. 45: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 60.Fernando M. M. A., Isenberg D. A. 2005. How to monitor SLE in routine clinical practice. Ann. Rheum. Dis. 64: 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsey-Goldman R., Li J., Dervieux T., Alexander R. V. 2017. Cell-bound complement activation products in SLE. Lupus Sci. Med. 4: e000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Schaarenburg R. A., Magro-Checa C., Bakker J. A., Teng Y. K. O., Bajema I. M., Huizinga T. W., Steup-Beekman G. M., Trouw L. A. 2016. C1q deficiency and neuropsychiatric systemic lupus erythematosus. Front. Immunol. 7: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macedo A. C. L., Isaac L. 2016. Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway. Front. Immunol. 7: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mevorach D., Mascarenhas J. O., Gershov D., Elkon K. B. 1998. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188: 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nauta A. J., Trouw L. A., Daha M. R., Tijsma O., Nieuwland R., Schwaeble W. J., Gingras A. R., Mantovani A., Hack E. C., Roos A. 2002. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 32: 1726–1736. [DOI] [PubMed] [Google Scholar]
- 66.Colonna L., Parry G. C., Panicker S., Elkon K. B. 2016. Uncoupling complement C1s activation from C1q binding in apoptotic cell phagocytosis and immunosuppressive capacity. Clin. Immunol. 163: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.