Abstract
Background
Mediastinal cystic lesions account for approximately 15–20% of all mediastinal masses and are difficult to differentiate because of similar imaging manifestation. The aim of this study was to differentiate mediastinum cystic lesions through endoscopic ultrasound‐guided‐fine needle aspiration (EUS‐FNA) and parameters from cyst‐fluid analysis.
Methods
Over a period of eight years, 37 patients suspected with mediastinal cystic lesions were assessed. Cyst fluid was collected via EUS‐FNA and further examined using cytological and biochemical techniques. Definitive diagnosis was established based on cytology, surgical pathology, and/or clinical follow‐up.
Results
Based on the final pathological reports or long‐term follow‐up, 19 patients were diagnosed with benign cysts, 14 with benign or malignant tumors, 2 with tuberculosis, 1 with an abscess, and 1 with a pancreatic pseudocyst. Computed tomography or magnetic resonance imaging mistakenly distinguished eight cases as solid masses (27.03%), but EUS revealed cystic characteristics. Carcinoembryonic antigen and lactate dehydrogenase (LDH) were evaluated from the cyst fluid obtained by EUS‐FNA. There was no statistically significant difference in carcinoembryonic antigen values between benign and malignant cysts; however the average LDH value in the malignancy group was significantly higher than in the benign group.
Conclusion
EUS‐FNA showed great potential for differentiating mediastinal lesions by combining imaging manifestation and cytological examination. The elevated LDH value from cyst fluid chemical analysis could be used as an auxiliary indicator for diagnosing malignancy.
Keywords: Cystic lesion, diagnosis, EUS‐FNA, mediastinum
Introduction
Cystic masses of the mediastinum are a heterogenous group of lesions including congenital abnormalities, infection, and neoplasms, and account for 15–20% of all mediastinal masses.1, 2 Congenital cysts include foregut duplication cysts, cysts of the thymus, pericardial cysts, and diverticula. Common mediastinal cystic lesions include abscesses, tuberculosis, mediastinal pancreatic pseudocysts, cystic tumors, and cystic degeneration of solid tumors. In addition, 6–15% of primary mediastinal masses are composed of foregut duplication cysts, which result from the abnormal budding of the ventral part of the endodermic primitive foregut and can be further classed as esophageal duplication, bronchogenic, and neureneric.3, 4 They are typically located adjacent to or within the esophagus, trachea, and bronchus, and the inner cystic wall is lined by columnar and/or squamous and ciliated detached mucosa.
Most patients with mediastinal cysts are asymptomatic. However, when the adjacent structures are compressed or ruptured by mediastinal cysts, this will result in mediastinitis, with symptoms including chest pain, cough, dyspnea, dysphagia, and stridor.2 Although the traditional therapy is surgical removal of the cysts, whether surgery should be performed for benign or asymptomatic cysts has become controversial.5, 6 In general, computed tomography (CT) and magnetic resonance imaging (MRI) are efficient to identify these cystic lesions. On CT, the cystic lesion shows a well‐defined, thin, smooth wall with homogeneous attenuation. On MRI, foregut cysts usually present with high signal intensity on T2‐weighted and T1‐weighted variables.1, 7 However, for cysts with thick proteinaceous contents, such images could lead to a 30–80% chance of misdiagnosis as solid mass lesions.1, 8, 9 Endoscopic ultrasound‐guided‐fine needle aspiration (EUS‐FNA) offers a minimally invasive pathway to acquire tissues and to identify the pathological type prior to surgery.10 Furthermore, EUS‐FNA offers the advantages of reaching the lower mediastinum and aortopulmonary windows that endobronchial ultrasound transbronchial aspiration (EBUS) cannot, and thus is recommended to analyze tuberculosis lymphadenopathy and stage mediastinal malignancies.11, 12 As recommended, prophylactic antibiotics should be used before an EUS‐FNA procedure to prevent mediastinitis.13 However, obtaining adequate tissue from the cystic wall to make a diagnosis of foregut duplication cysts by EUS‐FNA remains challenging. Analysis of cystic fluid had been reported to play an important role in differentiating pancreatic lesions via biochemical analysis of various tumor markers, such as adenosine deaminase and lactate dehydrogenase (LDH).14, 15 The use of biochemical analysis of cystic fluid to differentiate mediastinal cystic masses has not been reported; thus, the purpose of this study was to find an alternative approach based on EUS‐FNA to evaluate mediastinal cysts. We hope that examination of cystic fluid may offer a method to differentiate between benign and malignant mediastinal lesions.
Methods
In this retrospective study, we observed 37 patients over eight years who underwent EUS‐FNA after CT or MRI had revealed mediastinal lesions, which were then further confirmed by EUS as cystic lesions at our endoscopy center. Sonographic diagnosis of cystic lesions was made using hypoechoic or anechoic images with relatively thin and smooth outer walls. The vascular distribution of the lesions, the presence of enlarged lymph nodes, and the attachment of lesions and the surrounding tissues were also examined. FNA was subsequently performed and cystic fluid was aspirated separately for cytological examination and cystic fluid biochemical analysis. The cytological findings of ciliated columnar epithelial cells, cartilage/bronchial glands in their wall, alimentary (squamous or enteric) epithelium, cellular debris, hemosiderin‐laden macrophages, and goblet cells were used to characterize the mediastinal cyst. Malignancy was suspected when the cells showed evidence of giant nucleus, obvious nucleoli, and karyokinesis. A final diagnosis was established via: (i) FNA cytological findings; (ii) pathological confirmation after thoracotomy; and (iii) long‐term follow‐up of at least nine months without imaging manifestation and changes in clinical symptoms.
An anesthetist applied intravenous propofol for sedation according to the principles of “monitored anesthesia care.” Patients uniformly took the left lateral decubitus position during the procedure. All patients received oxygen during the procedure and blood pressure and heart rate were monitored. EUS examination was initially performed using a radial echoendoscope (Olympus GF‐UM130; Olympus Inc., Melville, NY, USA). Once the lesion was identified and characterized as a mediastinal cystic lesion, EUS‐FNA was performed using a curvilinear echoendoscope (UC‐30P; Olympus). Color and Doppler sonography were performed to identify a needle insertion site free of significant blood vessels. All FNAs were carried out with a 22‐gauge needle (Echotip; Wilson‐Cook Medical Inc., Winston‐Salem, NC, USA). The needle was advanced from the catheter sheath under US guidance to the cystic lesion. The stylet was subsequently removed, and a syringe was replaced to collect the fluid. Aspiration was performed by moving the needle back and forth within the target lesion to generate negative pressure.
Aspirate drops were placed on glass slides and smeared, air‐dried, and stained by Diff‐Quik stain. If the sample was sufficient, alcohol fixing was also performed. The smears were then immediately reviewed by a cytopathologist. A sample of the cyst fluid was sent to the clinical lab to detect glucose, adenosine deaminase, carcinoembryonic antigen (CEA), carbohydrate antigen‐199, and LDH levels.
Patients were routinely assessed for potential complications, including chest pain, fever, or other symptoms that can result from the procedure. Patients were telephoned 30 days after discharge. All patients were prescribed one dose (400 mg) of ciprofloxacin intravenously before EUS‐FNA. Patients were also administered 500 mg ciprofloxacin, orally, twice a day for five days after the procedure.
Results
Thirty‐seven patients (22 men, 15 women) with mediastinal cystic lesions were assessed by EUS‐FNA (Fig 1a,b). The median age was 49 years (46.61 ± 17.61 mean ± standard deviation [SD]). Nineteen were diagnosed with benign cysts, 14 with benign or malignant tumors, 2 with tuberculosis, 1 with an abscess, and 1 with a pancreatic pseudocyst. All clinical diagnoses were based on EUS‐FNA histology findings, surgical pathology findings, and long‐term follow‐up. Prophylaxis antibiotics were administered to all patients, and no infection or bleeding at the needle aspiration site was observed during the follow‐up period. Fourteen (37.84%) patients underwent surgical resection after EUS‐FNA. All FNA cytological results agreed with surgical pathological findings. One patient with a benign mediastinal cyst died of unknown causes and four patients with malignant lesions died. Two patients were lost to follow‐up (6.06%).
Figure 1.
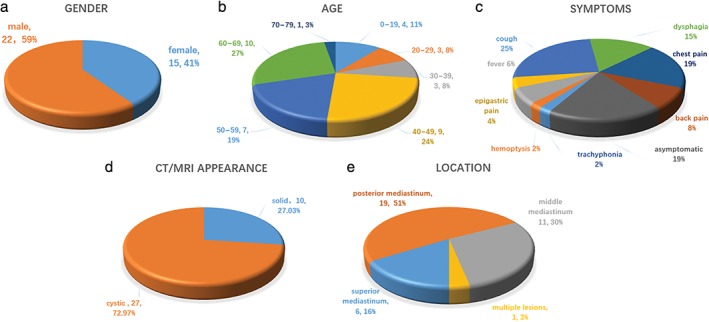
Suspected mediastinal lesions: summary of patient characteristics: (a) gender, (b) age, (c) symptoms, (d) computed tomography/magnetic resonance imaging (CT/MRI) appearance, and (e) location.
Most of the patients exhibited symptoms (Fig 1c), including trachyphonia in 1 (2.70%), hemoptysis in 1 (2.70%), fever in 3 (8.11%), epigastric pain in 2 (5.41%), cough in 12 (32.43%), dysphagia in 7 (18.92%), chest pain in 9 (27.27%) and back pain in 4 (12.12%). The remaining nine patients presented no symptoms (24.32%). All patients underwent either CT or MRI assays; however, CT or MRI mistakenly diagnosed 10 cases with solid masses (27.03%) prior to EUS (Fig 1d). The mean size of the lesions was 30.31 mm (SD = 15.63) in the short axis and 40.77 mm (SD = 20.04) in the long axis, ranging from 7 × 12.8 mm to 73 × 88 mm. Nineteen of the 37 (51.35%) lesions were located in the posterior mediastinum, 11 in the middle, and 6 in the superior mediastinum; one patient had multiple lesions (Fig 1e). Nine of the 37 (24.32%) lesions were adjacent to the bronchial tree and 16 (43.24%) were adherent to the esophagus with no communication to the lumen detected; one patient had two consequent cysts.
The cystic lesions appeared distinct on EUS (Table 1). Seventeen cases (44.73%) aspirated with clear cystic fluid presented anechoic images without apparent debris, including 14 (82.35%) benign mediastinal cysts (Fig 2a depicts an anechoic cyst), 2 cystic sarcomas, and 1 pancreatic pseudocyst. Eighteen cases (47.63%) aspirated with thick proteinaceous gel‐like fluid or without liquid showed either hypoechoic or hyperechoic images with dense debris. Among them, six were diagnosed as mediastinal cysts (Fig 2b depicts a hypoechoic cyst), nine as cyst‐like tumors, one as a mediastinal abscess, and two as tuberculosis. Four hypoechoic cystic tumors presented abundant blood supply signals based on Doppler analysis and additionally, enlarged lymph nodes surrounded the tumors in four cases. Moreover, three (7.89%) space‐occupying lesions presented cyst‐and‐solid appearance, including one bronchogenic cyst, one synovial sarcoma (Fig 2c depicts a lesion with cyst‐and‐solid appearance), and a liposarcoma (Table 2). Ciliated columnar cells were detected in 4 out of 19 patients diagnosed with mediastinal cysts (Fig 3a), and hemosiderin‐laden macrophages were observed in the FNA cytological examinations of three patients. Suspicious tumor cells with obvious nucleoli were found in the FNA smears of 8 of the 14 malignant tumor patients (Fig 3b,c).
Table 1.
Suspected hypoechoic and anechoic mediastinal lesions: EUS and EUS‐guided FNA diagnosis, cyst fluid chemical analysis, final diagnosis, and management
| EUS | Cyst fluid | Management | Final diagnosis | ||||
|---|---|---|---|---|---|---|---|
| Cyst characteristics | Size (mm) | FNA | CEA | CA19‐9 | LDH | ||
| Hypoechoic | 22 × 28 | Mucin, inflammatory cells | 349.36 | > 1200 | NA | Surgery | Bronchogenic cyst |
| Hypoechoic | 47 × 37 | Lymphocyte, basal‐like cell cluster | 7.54 | 7.91 | NA | Surgery | Thymoma |
| Anechoic | 28 × 25 | Squamous cells | 0.62 | NA | 12 | Observation | Bronchogenic cyst |
| Hypoechoic | 55 × 68 | Erythrocyte, neutrophil, mucin, tumor cells | 85.01 | NA | 15 396 | Surgery | Carcinoma of the lung |
| Anechoic | 50 × 30 | Mucin, lymphocyte | 2.41 | 2.14 | NA | Observation | Bronchogenic cyst |
| Hypoechoic | 88 × 73 | Squamous cells, cholesterol crystal | 15.29 | 229.22 | 164 | Surgery | Displaced parathyroid adenoma |
| Anechoic | 74 × 39 | Inflammatory and squamous cells, mucin | 3.89 | 16.0 | < 10 | Observation | Bronchogenic cyst |
| Hypoechoic | 69 × 53 | Mesothelium, inflammatory, and tumor cells | 4.22 | NA | 462 | Surgery | Carcinoma |
| Anechoic | 47.1 × 46.1 | Ciliated columnar and hemosiderin cells | < 0.5 | 2.98 | 185 | Surgery | Bronchogenic cyst |
| Anechoic | 48.5 × 39.6 | Ciliated columnar, inflammatory, and squamous cells | 40.99 | 18.85 | NA | Surgery | Bronchogenic cyst |
| Cystic and solid | 43 × 30 | Inflammatory and epithelial cells | 40.99 | 18.85 | NA | Surgery | Bronchogenic cyst |
| Anechoic | 40.8 × 39.9 | Squamous cells, phagocytes | 11.84 | NA | NA | Surgery | Bronchogenic cyst |
| Cystic and solid | 61.7 × 41.9 | Inflammatory and squamous cells, fibrocytes, mucin | < 0.5 | NA | 122 | Surgery | Synovial sarcoma |
| Anechoic | 68 × 40 | Ciliated columnar and hemosiderin cells, mucin | 33.01 | NA | 184 | Surgery | Bronchogenic cyst |
| Anechoic | 30.2 × 28.5 | Epithelial cells | 16.73 | NA | 40 | Surgery | Bronchogenic cyst |
| Anechoic | 18 × 15 | Mesothelial cells, lymphocytes | 0.71 | NA | 409 | Observation | Sarcoma |
| Anechoic | 48.5 × 39.6 | Necrosis, fibrosis | 3.88 | NA | 779 | Surgery | Pancreatic pseudocyst |
| Anechoic | 23 × 12 | Squamous andinflammatory cells | 20.71 | NA | 17 | Observation | Bronchogenic cyst |
| Hypoechoic | 14.8 × 11.8 | Neutrophils, tumor cells | 50.58 | NA | 542 | Observation | Carcinoma |
| Hypoechoic | 13.8 × 10.1 | Erythrocytes, epithelial and ciliated columnar cells | 140.42 | NA | 61 | Observation | Bronchogenic cells |
| Anechoic | 60 × 40 | Hemosiderin and epithelial cells, mucin | 4.77 | NA | 21 | Surgery | Bronchogenic cells |
CA, carbohydrate antigen; CEA, carcinoembryonic antigen; EUS, endoscopic ultrasound; FNA, fine needle aspiration; LDH, lactate dehydrogenase; NA, not applicable.
Figure 2.
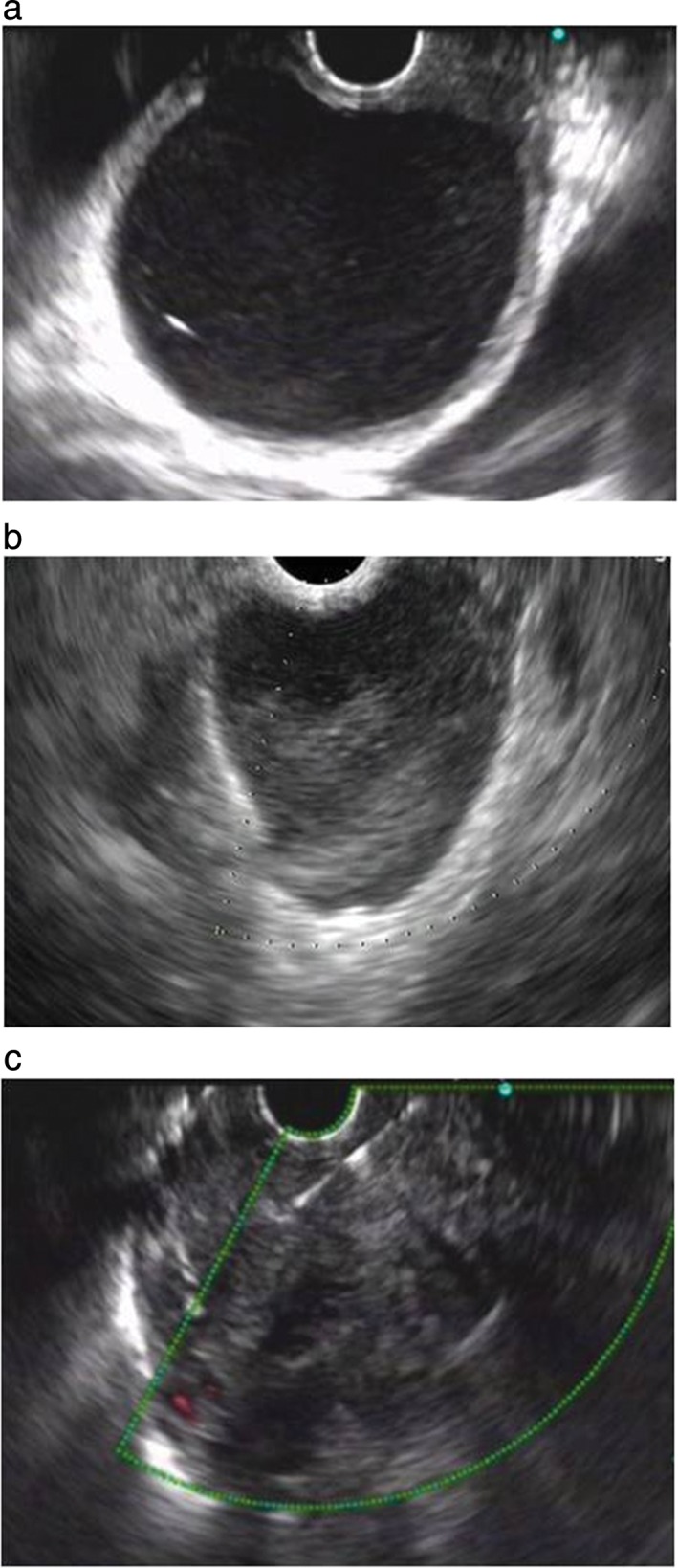
(a) Endoscopic ultrasound (EUS) images (7.5 MHz) of (a) a 4.0 × 4.9 cm anechoic mediastinal duplication cyst, (b) a 2.8 cm × 2.2 cm hypoechoic mediastinal duplication cyst, and (c) a 6.2 × 4.2 cm synovial sarcoma with cyst‐and‐solid appearance.
Table 2.
Main analytical characteristics associated with cystic fluid
| Benign lesions | Malignant lesions | P | |
|---|---|---|---|
| CEA | 16.73 (0–1010.05) | 4.22 (0–7.54) | 0.278 |
| LDH | 40 (10–185) | 462 (122–15 396) | 0.006 |
Data are presented as medians (range). CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase.
Figure 3.
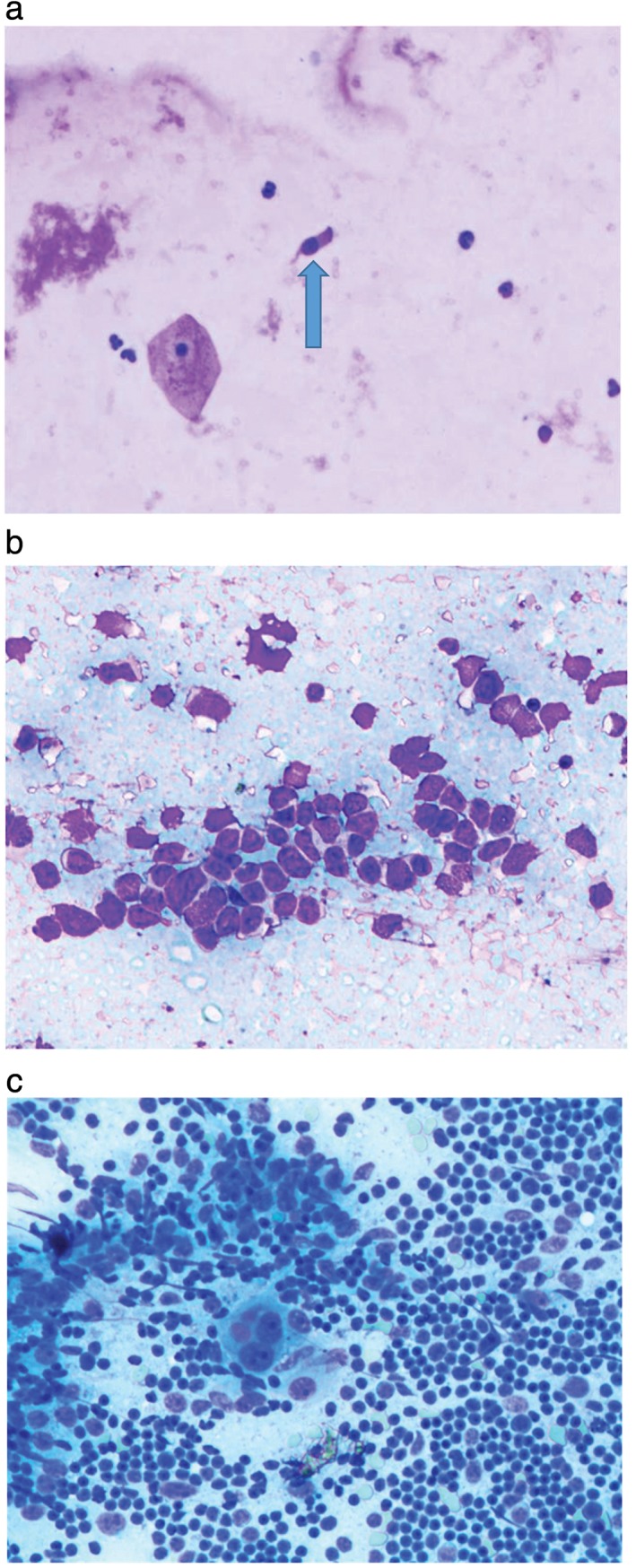
View of a deposit from the cyst fluid showing (a) detached ciliated columnar cells, (b) tumor cells, and (c) proliferative active lymphoblasts of a cystic thymoma.
Twenty‐one patients underwent cyst fluid CEA detection. In 13 patients with a final diagnosis of benign foregut mediastinal cysts, the mean of the CEA value was 125.75 (SD = 282.85), while the mean of the 5 patients diagnosed with malignancies was 12.61 (SD = 21.44). The Kruskal–Wallis Test revealed no statistical difference between the groups (P > 0.05).
Fourteen patients underwent cyst fluid LDH detection: nine diagnosed with benign mediastinal cyst and five with malignant mediastinal lesions. The Kruskal–Wallis Test revealed a statistical difference between these groups (P < 0.05) (Table 2).
Discussion
The diagnosis of mediastinal cystic lesions is difficult as benign mediastinal cysts can mimic the manifestations of cancer in CT or MRI evaluation.7, 8, 16 Our study aimed to combine EUS imaging analysis with biochemical examination of aspirated cystic fluid to diagnose mediastinal lesions.
The EUS approach indeed showed an advantage for examining cystic lesions, differentiating 10 cystic lesions mistakenly diagnosed as soft tissue masses on CT or MRI. The misdiagnosis rate with EUS was relatively low compared to previous reports.
The esophagus is a natural acoustic media for EUS to image the esophagus wall and mediastinum. Compared to CT and MRI, EUS is a more accurate method to differentiate cystic and solid masses.17 EUS can detect intracystic hyperechoic contents and determine the cyst nature. EBUS‐FNA and EUS‐FNA are both less invasive methods than thoracoscope detection for confirming suspected malignant lesions and staging metastatic lymph nodes in mediastinal tumors. It is commonly believed that EBUS‐FNA significantly improves anterior mediastinum sampling (American Joint Committee on Cancer stations 7, 2, 3, and 4) and EUS‐FNA is more suitable in the subcarinal and posterior mediastinum (stations 7, 6, and 5).18, 19, 20, 21 A combination of EBUS‐FNA and EUS‐FNA is an accurate complimentary method in patients with suspected mediastinal malignant lesions and a replacement for mediastinoscopy in diagnosing and staging indications.22 Meta‐analysis showed that the sensitivity of EUS‐FNA in positron emission tomography‐positive EUS suited areas (subaortic, paraesophageal, and pulmonary ligament locations), EBUS suited areas (paratracheal), bronchoscopy suited areas, and positron emission tomography‐negative areas was 75%, 69%, 74%, and 67% respectively, and the sensitivity of EBUS‐FNA was 70%, 76%, 68%, and 50% respectively.19, 20, 23 Although EUS and EBUS have different predominant exploratory regions, EBUS + EUS manifested higher accuracy than any of the abovementioned procedures in the same areas, with sensitivity of 100%, 100%, 100% and 75%.20 However, these conclusions were drawn from solid tumor tissues, where the cytological diagnostic rate of cystic lesions is relatively lower than in solid tissues. Several clinical trials have reported the use of EBUS‐FNA to treat bronchogenic cysts by draining cyst fluid.24, 25 Nevertheless, this procedure remains controversial because simple aspiration without obliterating the lining could lead to recurrence.
Traditionally, we rely on FNA cytology to make a pathological diagnosis. The cytological findings of ciliated columnar epithelial cells, cartilage/bronchial glands in their wall, alimentary (squamous or enteric) epithelium, cellular debris, hemosiderin‐laden macrophages, and goblet cells help to determine a diagnosis of mediastinal cyst.9, 10 However in practice, while EUS‐FNA yields sufficient samples to differentiate whether a lesion is benign or malignant, such samples are inadequate to determine specific cells to make a definitive diagnosis. As reported, only 1 in 4 patients finally diagnosed with hypoechoic mediastinal cysts presented with ciliated columnar cells, even by electron microscopy.8 Histiocytes and epithelial cells can only be considered assistant indicators; FNA is the most common modality used to exclude malignant lesions.
Thus, we have attempted to find other valuable variables to differentiate benign and malignant cystic lesions. EUS characteristics are crucial to a final diagnosis. Mediastinal duplication cysts, which are mainly located adjacent to the esophagus, usually appear round or oval, anechoic or hypoechoic, with distinct borders and multiple muscle layers, rarely infiltrating nearby structures. On the inside, a cyst may contain hyperechoic content debris and anechoic cyst fluid.4 Diagnosis via mediastinal tuberculosis lymphadenopathy is rather difficult and such a diagnosis is primarily based on cytological findings of caseating granulomas. Firstly, we should observe whether the pulmonary parenchyma is involved, and the adenopathy center usually presents as low‐density with an enhanced rim on EUS.12 EUS‐FNA is the standard modality for staging mediastinal malignancy as it can reach the lower mediastinum and aortopulmonary window, which EBUS cannot.19 Mediastinal cystic malignancy manifestations on EUS are mainly hypoechoic, relatively large, and inhomogeneous. Characteristics that help to differentiate malignant cysts are hypervascular signals of the lesion, enlarged peripheral lymph nodes, and infiltration to the adjacent structures. When a tumor invades the esophagus, necrosis and ulceration may be observed.
Humoral fluid biochemical analysis is routinely applied in a clinical setting. Tumor markers and LDH are the important parameters to differentiate benign and malignant lesions, particularly when detected in pleural effusion, ascetic fluid, and pancreatic cystic fluid.14, 15, 26 However, we did not consider mediastinal cyst fluid biochemical examinations in this study. Previous studies have shown that serum carbohydrate antigen 19‐9 and CEA levels are only elevated in patients with benign mediastinal cysts; often an elevated CEA level indicates that the occupying lesions are cystic.27, 28 Consistent with previous studies, our results indicated no statistical differences in CEA values between benign and malignant cysts. LDH is used as a prognostic and a potential protumor factor; when pleural and ascetic fluid LDH is > 500, malignancy needs to be taken into consideration.15, 29 In our study, the average LDH value in the malignancy group was significantly higher than in the benign group, which indicates that LDH may help to differentiate mediastinal benign and malignant lesions and guide clinical work. Surgery is still the mainstream method for a duplication cyst; however, whether an asymptomatic cyst requires surgery remains controversial. Mediastinal cyst rupture could lead to mediastinitis, and carcinoma arising from a benign duplication cyst has been reported.30, 31
The main limitation to this study was the small numbers of patients included, leading to a lack of power of the results. EUS‐FNA is also relatively inadaptable in the anterior mediastinum.
We suggest that ablation via EUS‐FNA could be a new method to manage benign cysts. Further randomized controlled trials with a larger sample and comparison with EBUS‐FNA are required to confirm our results.
Disclosure
No authors report any conflict of interest.
References
- 1. Ödev K, Arıbaş BK, Nayman A, Arıbaş OK, Altınok T, Küçükapan A. Imaging of cystic and cyst‐like lesions of the mediastinum with pathologic correlation. J Clin Imaging Sci 2012; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eloubeidi MA, Cohn M, Cerfolio RJ et al Endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis of foregut duplication cysts: The value of demonstrating detached ciliary tufts in cyst fluid. Cancer 2004; 102: 253–8. [DOI] [PubMed] [Google Scholar]
- 3. Wildi SM, Hoda RS, Fickling W et al Diagnosis of benign cysts of the mediastinum. Gastrointest Endosc 2003; 58: 362–8. [PubMed] [Google Scholar]
- 4. Liu R, Adler DG. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound 2014; 3: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nobuhara KK, Gorski YC, La Quaglia MP, Shamberger RC. Bronchogenic cysts and esophageal duplications: Common origins and treatment. J Pediatr Surg 1997; 32: 1408–13. [DOI] [PubMed] [Google Scholar]
- 6. Hall DA, Pu RT, Pang Y. Diagnosis of foregut and tailgut cysts by endosonographically guided fine‐needle aspiration. Diagn Cytopathol 2007; 35: 43–6. [DOI] [PubMed] [Google Scholar]
- 7. Jeung MY, Gasser B, Gangi A et al Imaging of cystic masses of the mediastinum. Radiographics 2002; 22: 79–93. [DOI] [PubMed] [Google Scholar]
- 8. Fazel A, Moezardalan K, Varadarajulu S, Dragonov P, Eloubeidi MA. The utility and safety of EUS‐guided FNA in the evaluation of duplication cysts. (Published erratum appears in Gastrointest Endosc 2005; 62: 996.). Gastrointest Endosc 2005; 62: 575–80. [DOI] [PubMed] [Google Scholar]
- 9. Mcadams HP, Kirejczyk WM, Rosado‐De‐Christenson ML, Matsumoto S. Bronchogenic cyst: Imaging features with clinical and histopathologic Correlation1. Radiology 2000; 217: 441–6. [DOI] [PubMed] [Google Scholar]
- 10. Ponder TB, Collins BT. Fine needle aspiration biopsy of gastric duplication cysts with endoscopic ultrasound guidance. Acta Cytol 2003; 47: 571–4. [DOI] [PubMed] [Google Scholar]
- 11. Herth FJF, Rabe KF, Gasparini S, Annema JT. Transbronchial and transoesophageal (ultrasound‐guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J 2006; 28: 1264–75. [DOI] [PubMed] [Google Scholar]
- 12. Puri R, Vilmann P, Sud R et al Endoscopic ultrasound‐guided fine‐needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy 2010; 42: 462–7. [DOI] [PubMed] [Google Scholar]
- 13. Annema JT, Veseliç M, Versteegh MIM, Rabe KF. Mediastinitis caused by EUS‐FNA of a bronchogenic cyst. Endoscopy 2003; 35: 791–3. [DOI] [PubMed] [Google Scholar]
- 14. Pitman MB, Brugge WR, Warshaw AL. The value of cyst fluid analysis in the pre‐operative evaluation of pancreatic cysts. J Gastrointest Oncol 2011; 2: 195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Guo M, Fan J et al Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta‐analysis. Cancer Biomark 2016; 16: 415–23. [DOI] [PubMed] [Google Scholar]
- 16. Murayama S, Murakami J, Watanabe H et al Signal intensity characteristics of mediastinal cystic masses on T1‐weighted MRI. J Comput Assist Tomogr 1995; 19: 188–91. [DOI] [PubMed] [Google Scholar]
- 17. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography‐guided fine‐needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology 1997; 112: 1087–95. [DOI] [PubMed] [Google Scholar]
- 18. Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound‐guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta‐analysis. Eur J Cancer 2009; 45: 1389–96. [DOI] [PubMed] [Google Scholar]
- 19. Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) and endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) biopsy: A combined approach in the evaluation of mediastinal lesions. Endoscopy 2005; 37: 833–9. [DOI] [PubMed] [Google Scholar]
- 20. Wallace MB, Pascual JMS, Raimondo M et al Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008; 299: 540–6. [DOI] [PubMed] [Google Scholar]
- 21. De Leyn P, Dooms C, Kuzdzal J et al Revised ESTS guidelines for preoperative mediastinal lymph node staging for non‐small‐cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 787–98. [DOI] [PubMed] [Google Scholar]
- 22. Cameron SEH, Andrade RS, Pambuccian SE. Endobronchial ultrasound‐guided transbronchial needle aspiration cytology: A state of the art review. Am J Clin Pathol 2010; 21: 6–26. [DOI] [PubMed] [Google Scholar]
- 23. Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound‐guided fine‐needle aspiration for non‐small cell lung cancer staging : A systematic review and metaanalysis. Chest 2007; 131: 539–48. [DOI] [PubMed] [Google Scholar]
- 24. Twehues A, Islam S. Cystic lesions of the thorax: Role of endobronchial ultrasound‐guided transbronchial needle aspiration. J Bronchology Interv Pulmonol 2011; 18: 265–8. [DOI] [PubMed] [Google Scholar]
- 25. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst's recurrence treated with EBUS‐FNA with a long‐term follow‐up. Eur J Cardiothorac Surg 2006; 29: 627–9. [DOI] [PubMed] [Google Scholar]
- 26. Herrera LS, Fernándezfabrellas E, Juan SG et al Predicting malignant and paramalignant pleural effusions by combining clinical, radiological and pleural fluid analytical parameters. Lung 2017; 195: (Suppl 2): 1–8. [DOI] [PubMed] [Google Scholar]
- 27. Uyama T, Monden Y, Sumitomo M, Miura K, Kimura S. CEA and CA 19‐9 in benign pulmonary or mediastinal cystic lesions. J Surg Oncol 1989; 41: 103–8. [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki A, Miyamoto H, Anami Y et al [A case of mediastinal esophago‐bronchogenic cyst associated with high serum level of CA 19‐9]. Kyobu Geka the Japanese Journal of Thoracic Surgery 2001; 54: 805–8. [PubMed] [Google Scholar]
- 29. Valdés L, Sanjosé E, Ferreiro L et al Predicting malignant and tuberculous pleural effusions through demographics and pleural fluid analysis of patients. Clin Respir J 2015; 9: 203–13. [DOI] [PubMed] [Google Scholar]
- 30. Lozano FM, Martínez BG, More SL, Rodríguez AV. Carcinoma arising in a calcified bronchogenic cyst. Respiration 1981; 42: 135–7. [DOI] [PubMed] [Google Scholar]
- 31. Horne G, Ming‐Lum C, Kirkpatrick AW, Parker RL. High‐grade neuroendocrine carcinoma arising in a gastric duplication cyst: A case report with literature review. Int J Surg Pathol 2007; 15: 187–91. [DOI] [PubMed] [Google Scholar]


