Abstract
Social decision-making is fundamental for successful functioning and can be affected in psychiatric illness and by serotoninergic modulation. The Prisoner's Dilemma is the archetypal paradigm to model cooperation and trust. However, the effect of serotonergic enhancement is poorly characterized, and its influence on the effect of variations in opponent behavior unknown. To address this, we conducted a study investigating how the serotonergic enhancer 3,4-methylenedioxy-methamphetamine (MDMA) modulates behavior and its neural correlates during an iterated Prisoner's Dilemma with both trustworthy and untrustworthy opponents. We administered 100 mg MDMA or placebo to 20 male participants in a double-blind, placebo-controlled, crossover study. While being scanned, participants played repeated rounds with opponents who differed in levels of cooperation. On each round, participants chose to compete or cooperate and were asked to rate their trust in the other player. Cooperation with trustworthy, but not untrustworthy, opponents was enhanced following MDMA but not placebo (respectively: odds ratio = 2.01; 95% CI, 1.42–2.84, p < 0.001; odds ratio = 1.37; 95% CI, 0.78–2.30, not significant). Specifically, MDMA enhanced recovery from, but not the impact of, breaches in cooperation. During trial outcome, MDMA increased activation of four clusters incorporating precentral and supramarginal gyri, superior temporal cortex, central operculum/posterior insula, and supplementary motor area. There was a treatment × opponent interaction in right anterior insula and dorsal caudate. Trust ratings did not change across treatment sessions. MDMA increased cooperative behavior when playing trustworthy opponents. Underlying this was a change in brain activity of regions linked to social cognition. Our findings highlight the context-specific nature of MDMA's effect on social decision-making.
SIGNIFICANCE STATEMENT We provide a detailed analysis of the effect of 3,4-methylenedioxy-methamphetamine (MDMA) on cooperative behavior during interpersonal interactions, as well as the neural correlates underlying these effects. We find that, following administration of MDMA, participants behave more cooperatively, but only when interacting with trustworthy partners. While breaches of trustworthy behavior have a similar impact following administration of MDMA compared with placebo, MDMA facilitates a greater recovery from these breaches of trust. Underlying this altered behavior are changes in brain activity during the viewing of opponents' behavior in regions whose involvement in social processing is well established. This work provides new insights into the impact of MDMA on social interactions, emphasizing the important role of the behavior of others toward us.
Keywords: fMRI, MDMA, Prisoner's Dilemma, psychopharmacology, social cognition, social decision-making
Introduction
Social cognitive deficits are recognized as a fundamental difficulty in a range of psychiatric conditions, and current medications do not effectively treat these deficits (Gabay et al., 2015; Kupferberg et al., 2016). These disruptions encompass social decision-making tasks, designed to investigate behavior in strategic social situations, where outcomes depend not only on one's own behavior, but also on that of an interacting partner (McClure et al., 2007; Radke et al., 2013).
The Prisoner's Dilemma (PD) is a prototypical social decision-making game where two players simultaneously choose to cooperate with or compete against each other. Points are allocated based on the combination of their responses. Mutual cooperation is the best combined outcome; however, by cooperating, one risks betrayal. Betraying a cooperator attracts the highest points available and results in the lowest points for the player who is betrayed.
Emerging evidence suggests that manipulation of the serotoninergic system affects social decision-making (Wood et al., 2006; Crockett et al., 2010). However, specific assessment of serotonergic manipulation on trust and cooperation in the PD is lacking, with the exception of Wood et al. (2006) who reported that acute tryptophan depletion reduced cooperative responses compared with a placebo control condition.
Neuroimaging can address whether pharmacological manipulation influences specific cognitive components, such as receiving feedback or making decisions. PD studies have implicated brain regions during feedback, which have been shown to be involved in social processing, including the superior temporal sulcus, temporal-parietal junction, and posterior cingulate gyrus (Rilling et al., 2004; Suzuki et al., 2011). Oxytocin administration altered neural activity when receiving feedback about other players' decisions, with the insula, amygdala, and caudate nucleus being implicated (Rilling et al., 2012; Chen et al., 2017).
The characteristics of PD opponents can have a significant impact on PD behavior, exemplified by Sorgi and Van 't Wout (2016) who showed that depression severity was correlated with cooperation when playing trustworthy opponents, but not untrustworthy opponents. This makes a case for testing the influence of pharmacological manipulations on different opponent types, in particular to test whether effects are limited to trustworthy or untrustworthy opponents.
We performed a functional neuroimaging study investigating the effect of 3,4-methylenedioxy-methamphetamine (MDMA) on PD behavior. MDMA was chosen because it produces profound social and emotional effects and is commonly described in terms of its effects on interpersonal interactions. The neuropharmacological mechanisms of MDMA are mixed. It elicits the release of dopamine (DA), noradrenaline (NA), and serotonin, with the latter believed to be primarily responsible for its social and euphoric effects (Rudnick and Wall, 1992; Kuypers et al., 2014).
Participants played repeated rounds of a newly developed version of the PD with three types of opponents: trustworthy (mostly cooperative), untrustworthy (mostly uncooperative), and a nonsocial control. Thus, we were able to test for differential effects of MDMA on different types of opponent. Additionally, on each round, participants were asked to rate their trust in the other player. The task was performed during functional neuroimaging, allowing us to examine the neural correlates of MDMA-induced changes in behavior.
We also tested other components of social cognition by including an emotion recognition task and empathy task outside of the scanner. Kuypers et al. (2017) show that MDMA increased affective, but not cognitive, empathy. Other research found that MDMA reduces recognition of fearful, angry, and sad facial expressions (Hysek et al., 2014; Kirkpatrick et al., 2014; Schmid et al., 2014). We sought to reproduce these effects and conducted an additional exploratory analysis to determine their relationship to changes in PD behavior under the influence of MDMA.
For the PD, we hypothesized that MDMA would increase cooperation with both types of “human” opponent and increase trust ratings for these opponents. We expected that none of these changes would be seen when playing the nonsocial control opponent. We hypothesized that previously implicated social cognition regions (Wood et al., 2006; Rilling et al., 2004, 2012), including the superior temporal cortex, temporal parietal junction, and the posterior cingulate cortex, would show increased activity when playing the game during the MDMA session compared with the placebo session.
Materials and Methods
Participants.
Twenty-one male participants were recruited from the community. We collected written informed consent, and participants were financially compensated for their time. The study received ethical approval from King's College London's Psychiatry, Nursing and Midwifery Research Ethics Committee (PNM/14/15-32). All participants had at least one previous experience with MDMA.
One participant withdrew from the study after his first visit. This was unrelated to his participation, and unblinding revealed that he received placebo on that visit. Therefore, 20 participants completed the study (mean ± SD age, 24.8 ± 3.7 years; range = 21–37 years).
Participants had no history of psychiatric illness or other neurological disorders, and were asked to refrain from MDMA use for 3 months, and caffeine and alcohol for 24 h, before each study visit. All participants passed a urine-based drugs-of-abuse test before dosing on each study visit. This tested for 10 classes of substance: cocaine, opioids, MDMA, THC, amphetamines, barbiturates, tricyclic antidepressants, methamphetamine, methadone, and benzodiazepines. We did not formally collect data on frequency of previous drug use, but all participants were assessed for substance dependency at screening. Participants were required to not be taking other medications throughout their involvement in the study; and if this was not possible, they were excluded from taking part.
Subjective effects.
At the end of each experimental session, participants were asked to complete the Altered States of Consciousness (ASC) questionnaire. This is an 11-dimension questionnaire and is the gold standard when investigating compounds with psychedelic-like properties (Studerus et al., 2011).
The PD.
A new, computerized, iterated version of the PD was developed by Gilleen and Satkunanathan (2015) which allows measurement of both social decision-making and changes in trust during social interaction. This PD paradigm has shown sensitivity to distinguishing paranoia profiles in high and low schizotypes (Gilleen and Satkunanathan, 2015). Using this paradigm, participants were led to believe that they would log onto a shared network, established in collaboration with two other London-based universities. They were told this network had been designed to allow participants from each site to interact in different tasks. In reality, opponent responses were preprogrammed by the research team.
Participants played an iterated PD with three different types of opponent (for the payoff matrix used, see Fig. 1B). They were told they would play between 8 and 15 rounds with each player but in fact played 15 rounds.
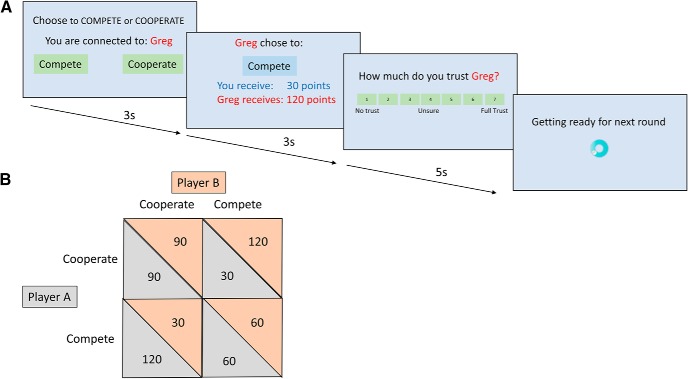
Figure 1.
The Prisoner's Dilemma. A, Paradigm with timings. B, Payoff matrix.
On each round (Fig. 1A), participants were asked to decide whether to compete or cooperate with the other player, then received feedback as to the other player's choice and the distribution of points, then asked to rate their trust in the other player from 1 to 7. The three types of opponent were as follows: (1) trustworthy, cooperated 12 of 15 rounds; (2) untrustworthy, cooperated 3 of 15 rounds; and (3) game server, with this opponent, participants were explicitly told they would be playing with a random response generator. The design of the opponent behavior was fixed across participants to allow us to assess the role of the opponent behavior in terms of trustworthiness. A different design, such as a tit-for-tat strategy, would also allow us to address the impact of MDMA on the PD as a function of opponent behavior but would be less controlled and require larger group sizes. Furthermore, the use of such a response algorithm could result in convergence of outcomes toward complete cooperation or competition, thus making it impossible to address our question on the effect of trustworthiness. A potential advantage of this alternative approach is that trustworthiness may be included as a continuous moderator, but this was beyond the scope of this study.
They played each type of opponent type twice (but were told they had connected to a different “player” for each opponent). Participants were given no prior information about the opponent. Because of the length of the task, it was split into two runs, lasting 9 min 15 s each. In the first run of each session, the order of opponents was as follows: trustworthy-game server-untrustworthy. In the second run of each session, the order of opponents was as follows: untrustworthy-game server-trustworthy.
All participants completed a practice version of the task at the screening visit. Participants were debriefed as to the deception at the end of the study, and belief in the deception was confirmed during this debrief.
Emotion recognition and empathy tasks.
The Affective Bias task was taken from the EMOTICOM cognitive test battery (Bland et al., 2016) and administered ∼195 min after dose. In this task, participants see a face appear on the screen for ∼500 ms and are asked to indicate which emotion the face was expressing from a choice of happy, sad, fear, or anger. For each emotion, there are nine levels of intensity. Control conditions of faces of different ages were also presented. There were 20 presentations of each emotion and 20 control faces.
The Multifaceted Empathy Test (MET) (Dziobek et al., 2008) is a task able to assess cognitive and affective empathy separately, and has been used in MDMA studies previously (Hysek et al., 2014; Kuypers et al., 2014; Schmid et al., 2014). The MET was administered ∼180 min after dose. The MET uses 40 images of people in ecologically valid, naturalistic situations. In 40 trials, participants are asked to identify the emotion the person may be feeling of a choice of 4 (cognitive empathy); and in 40 other trials, they are asked to rate how much they empathize with the person depicted on a scale of 1 to 9 (affective empathy). Cognitive empathy is given a score of 40, and affective empathy is the average rating of 9.
Experimental design and statistical analysis.
This study followed a double-blind, placebo-controlled, crossover, counterbalanced design. Participants attended two experimental study days at least 1 week apart (mean 9.3, SD 5.7, range 7–31 d). This study additionally collected resting-state fMRI, arterial spin labeling, and data on the Ultimatum Game. These are reported previously. MDMA (100 mg) or placebo was administered at 10:15. Blood samples measuring plasma MDMA levels were taken at two time points: 45 min after dose (mean: 91.7 μg/L, SD: 60.2) and 165 min after dose (mean: 188.2 μg/L, SD: 32.8). Samples to measure plasma oxytocin levels were also taken at this time. The PD was completed in two separate runs during functional neuroimaging, beginning 95 min after dose. The timing for the MRI session was chosen because the Tmax of MDMA ranges between 1.5 and 3 h (Kolbrich et al., 2008), and subjective effects peak and remain stable between 1 and 3 h (Harris et al., 2002), meaning functional acquisitions would fall within these time points. The Affective Bias and MET (see below) were completed 3 h after dose.
Two outcome measures were collected from the PD. The first were categorical (compete or cooperate) decisions, and the second were trust ratings of 7. The categorical data were analyzed using repeated-measures logistic regression, implemented with generalized estimating equations using SPSS Statistics for Windows 2012 (IBM). The odds ratio (OR) represents the change in probability of an event (a cooperate decision) occurring with a change in condition (trustworthiness, treatment).
In all models, participant ID was defined as the subject variable so that each participant's responses were nested together. First, a model testing for a main effect of run and the run × treatment interaction was performed. We also assessed the treatment order and effect of time in two separate models. The first looked at treatment order and its interaction with treatment. The second looked at the main effect of visit number and its interaction with time. Next, a model testing the main effects of treatment and opponent (trustworthy, untrustworthy, game server), and their interaction, was analyzed.
Trust ratings were collected on each round, producing a total of 15 ratings for each opponent. To account for uncertainty at the beginning of each game, the mean of the last eight rounds was calculated as the rating for each opponent. For each type of opponent, this was averaged across runs. A repeated-measures ANOVA was performed to assess differences in trust rating across opponent type and experimental session, with post hoc pairwise comparisons performed where appropriate.
The outcome measure for affective bias task was the percentage correct for each emotion (fear, anger, happy, sad) and the control condition. Additionally, one can calculate an “affective bias,” defined by Bland et al. (2016) as the difference between happy and sad emotion accuracy. These outcome measures were compared across experimental sessions using repeated-measures ANOVA, followed by post hoc pairwise comparisons where appropriate.
The MET gives the following outcome measures: a positive valence affective empathy measure, negative valence affective empathy measure, and three measures for cognitive empathy (total, positive affect, negative affect). Following advice from the group who created the task, there is no pooled measure for total affective empathy (I. Dziobek, personal communication). Each of these was assessed with paired-samples t tests to assess differences across experimental sessions.
MRI data acquisition and analysis.
Functional images were acquired with a General Electric MR750 3.0 tesla (T) MR scanner using a 32-channel head coil. A T2*-weighted echo-planar imaging sequence was used, with the following parameters: TR 2000 ms, TE 30 ms, flip angle 75°, slice thickness 3 mm, FOV 247 mm, number of slices 41. The PD had 282 time points per run. We also acquired a T1 structural MPRAGE image with the following parameters: TR 7312 ms, TE 3.02 ms, flip angle 11°, slice thickness 3 mm, 196 sagittal slices, FOV 270 mm.
Data were preprocessed and analyzed using SPM12 (Wellcome Department of Cognitive Neurology, London). Before first-level modeling, fMRI data were reoriented, slice time-corrected, and realigned initially to the first image and then to the mean image. These were then coregistered to the T1 structural file. The structural data were segmented to aid spatial normalization, and a common group-specific template was created using DARTEL registration. The functional files were then normalized to the MNI template using deformation flow fields and structural template created through DARTEL. Finally, functional images were smoothed using an 8 mm FWHM Gaussian kernel.
Both runs of the task were included in a single GLM first-level model. A simple model was defined with each condition (trustworthy, untrustworthy, game server opponents) by trial phase (decision, feedback, trust rating) combination. The decision and feedback phases were defined with durations of 3 s, and the trust rating with 5 s duration. Seven movement parameters (six standard parameters as well as volume-to-volume movement) were included as regressors of no interest. Volumes where the volume-to-volume movement exceeded 1 mm, as well the volume before and after, were also modeled as regressors of no interest.
At the second level, a series of whole-brain analyses were performed. First, we examined the main effects of the task by collapsing data across opponent types and performing a one-sample t test for each phase (decision, feedback, trust rating) of the task in the placebo condition. Three one-way, repeated-measures ANOVAs were performed on the placebo session data to examine differences in activation across opponents for each of the phases.
Next, we performed a series of flexible factorial analyses incorporating opponent type and treatment as within-subject factors, separately for each phase of the task (decision, feedback, trust rating). All maps were thresholded at FWE cluster-corrected p < 0.05 (cluster-defining threshold p = 0.001).
Results
Subjective effects of MDMA
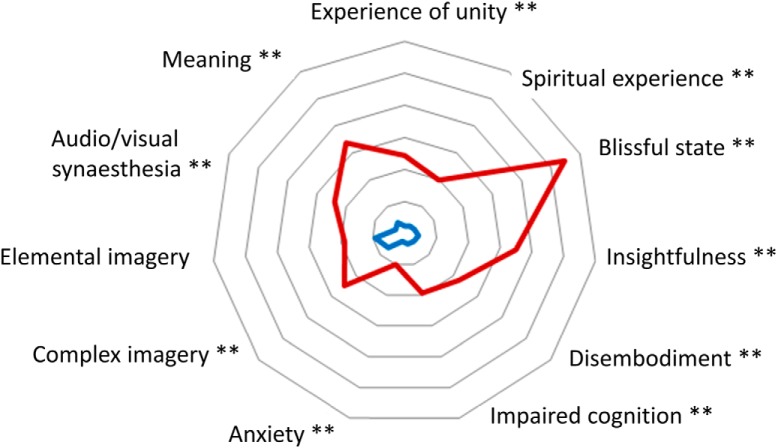
Figure 2 represents the 11 dimensions of the ASC. We found an increase in ratings for all dimensions, except “elemental imagery,” after MDMA compared with placebo.
Figure 2.
Radar graph displaying scores on the 11 dimensions of the ASC questionnaire. Each line in the radar indicates 10% of the total possible score (inner point = 0%; outer ring = 60%). **Bonferroni-corrected p < 0.05. Experience of unity (t(19) = 5.06, p < 0.001); spiritual experience (t(19) = 4.96, p < 0.001); blissful state (t(19) = 7.79, p < 0.001); insightfulness (t(19) = 4.96, p < 0.001); disembodiment (t(19) = 4.15, p < 0.001); impaired cognition (t(19) = 4.02, p < 0.001); anxiety (t(19) = 3.81, p < 0.001); complex imagery (t(19) = 4.54, p < 0.001); elemental imagery (t(19) = 1.53, p = 0.141); audio/visual synesthesia (t(19) = 4.12, p < 0.001); and meaning (t(19) = 5.92, p < 0.001).
Plasma oxytocin
Table 1 displays the plasma oxytocin levels at three time points: 15 min before dose, and 45 min and 165 min after dose. MDMA produced a large increase in plasma levels.
Table 1.
Plasma oxytocin levels measured at three time pointsa
| Time point | Placebo session | MDMA session | t statistic | Bonferroni- corrected p |
|---|---|---|---|---|
| 15 min before dose | 3.81 (4.3) | 4.48 (5.1) | 0.63 | 0.5373 |
| 45 min after dose | 3.00 (3.3) | 7.3 (8.7) | 2.60 | 0.054 |
| 165 min after dose | 3.09 (4.0) | 20.86 (12.9) | 5.66 | <0.001 |
aData are mean (SD) plasma oxytocin level (pg/ml). N = 20, except 45 min after dose due to placebo session levels of 1 participant being below the range of sensitivity at time point.
Emotion recognition
Due to technical problems, we had missing data for 2 participants in the Affective Bias task. Therefore, these analyses were based on N = 18. Summary statistics for this task can be found in Table 2.
Table 2.
Results of the Affective Bias task (Bland et al., 2016) and MET (Dziobek et al., 2008)a
| Placebo (% correct) | MDMA (% correct) | t statistic | p | |
|---|---|---|---|---|
| Affective Bias | ||||
| Nonemotion Control | 85.8 (12.1) | 90.6 (6.2) | 1.64 | 0.120 |
| Happy | 90.6 (9.5) | 91.4 (6.1) | 0.33 | 0.749 |
| Sad | 71.7 (15.4) | 71.7 (21.9) | 0.07 | 0.946 |
| Fear | 79.2 (13.7) | 70.8 (16.9) | −2.21 | 0.041 |
| Anger | 60.3 (13.1) | 53.1 (15.6) | −2.25 | 0.038 |
| MET | ||||
| Cognitive Empathy | ||||
| Total | 73.0 (6.6) | 71.4 (8.0) | −1.00 | 0.330 |
| Positive Valence | 77.9 (6.0) | 74.7 (9.3) | −1.51 | 0.148 |
| Negative Valence | 68.1 (11.3) | 68.1 (10.6) | <0.01 | 1.00 |
| Affective Empathy (rating out of 9) | ||||
| Positive Valence | 5.5 (1.1) | 5.8 (1.1) | 0.91 | 0.377 |
| Negative Valence | 4.4 (1.9) | 4.5 (2.3) | 0.285 | 0.779 |
aData are mean (SD). Post hoc p values are uncorrected for multiple comparisons.
In the Affective Bias task (Bland et al., 2016), MDMA did not alter participants' ability to recognize and make judgments on faces in the nonemotional control condition, compared with placebo (mean difference between scores in the MDMA and placebo condition = −4.8, SD = 12.6, t(17) = −1.64, p = 0.120). A 2 (treatment: placebo, MDMA) × 4 (emotion: happy, sad, fear, anger) repeated-measures ANOVA found no main effect of treatment (F(1,17) = 2.56, p = 0.128, ή2 = 0.13), but both a main effect of emotion (F(3,51) = 29.22, p < 0.001, ή2 = 0.63) and a treatment × emotion interaction (F(1,17) = 3.56, p = 0.029, ή2 = 0.16).
Post hoc comparisons found that participants were more accurate in identifying happy emotions compared with all others (all Bonferroni-corrected p values <0.003); anger was identified less accurately than all other emotions (all Bonferroni-corrected p values <0.001); there was no difference in accuracy in identifying sad compared with fearful faces. The interaction appeared to be driven by reduced accuracy in identifying fear and anger during the MDMA session compared with the placebo.
These results support previous evidence that MDMA reduces negative affect recognition (Bedi et al., 2010; Hysek et al., 2014; Kirkpatrick et al., 2014).
Empathy
Because of computing problems during one session, we had missing data for 1 participant in the MET. Therefore, these analyses were based on N = 19. Summary statistics for this task can be found in Table 2.
Paired-samples t tests showed that there were no differences for any comparison across treatment sessions (all p values > 0.148).
This finding does not replicate previous findings with regard to enhanced empathic processing with MDMA administration, recently confirmed with a pooled analysis of six studies (Kuypers et al., 2017). The mean placebo score for positive valence, explicit affective empathy in our study was 5.5. Interestingly, the equivalent score in the pooled analysis ranged from 4.2 to 5.6. Therefore, the possibility exists that the current sample's high baseline empathic responses made a discernible increase following MDMA administration less likely.
PD behavior
When assessing the effect of completing the task across two runs on each treatment session, no main effect of run was found, but there was a treatment × run interaction (respectively: χ2(1,19) = 0.26, p = 0.614; χ2 = 7.70, p = 0.021), such that there was an increase in the probability of a cooperative response in the second run during the MDMA session compared with the first run (OR = 1.28, 95% CIs 1.01–1.58).
The interaction effect was driven by a small but consistent increase in cooperation on the second run of the task during the MDMA session, which was not seen on the placebo session. The participant-wise mean difference from the first run to the second run on the MDMA session was 0.32 (SD 13.27). During the placebo session, there was a mean difference of −0.63 (SD 10.30). Putting this in context, the mean cooperation rate for the MDMA and placebo sessions (across all opponents) was 50.0% and 42.4%, respectively.
We did not consider this to be of practical significance; and because the effect is restricted to only the MDMA treatment session, all other analyses were combined data across runs.
We then assessed the main effect of visit number and its interaction with treatment, collapsed across opponent type. There was neither a main effect nor interaction (p = 0.453 and p = 0.102, respectively). Additionally, we tested for a main effect of treatment order and its interaction with treatment. Again, there was neither a main effect nor interaction (p = 0.102 and p = 0.453, respectively).
We assessed differences in trust rating across opponent type and experimental session. Table 3 displays these data. A 2 (treatment: placebo, MDMA) × 3 (Trustworthiness: trustworthy, untrustworthy, game server) repeated-measures ANOVA revealed a main effect of trustworthiness (F(2,38) = 25.39, p < 0.001, ή2 = 0.57), but no main effect of treatment, nor an interaction (respectively: F(1,19) = 1.53, p = 0.232, ή2 = 0.07; F(2,38) = 0.08, p = 0.928, ή2 < 0.01).
Table 3.
Mean (SD) trust ratings of each opponent type in each treatment sessiona
| Experimental session |
||
|---|---|---|
| Opponent type | Placebo | MDMA |
| Trustworthy | 4.8 (1.2) | 5.0 (1.4) |
| Untrustworthy | 2.0 (1.2) | 2.2 (1.4) |
| Game server | 2.9 (1.6) | 3.2 (1.5) |
aRating scale 1–7.
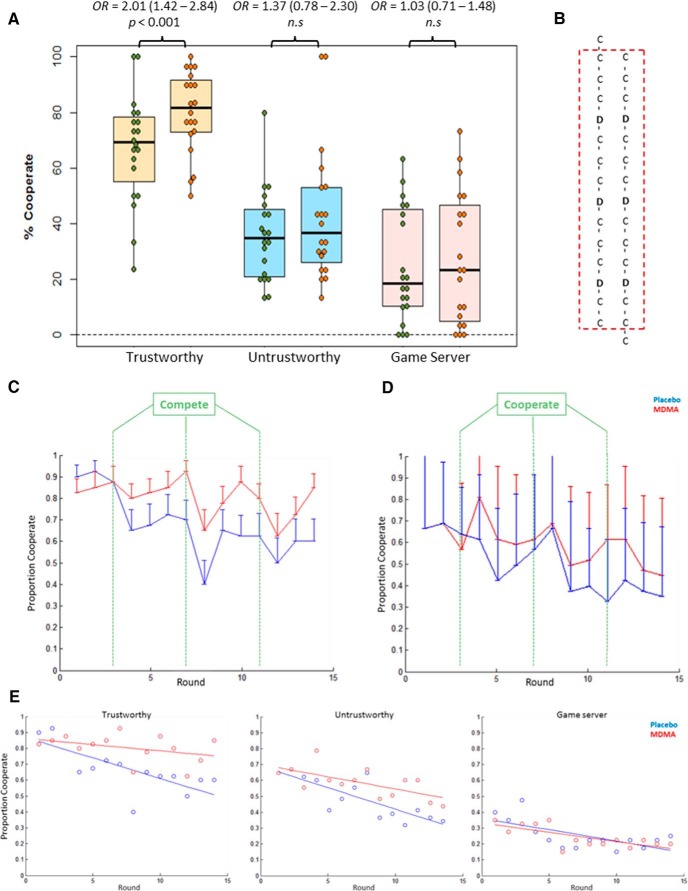
Figure 3A displays the percentage of cooperate decisions when playing each opponent type, across treatment sessions.
Figure 3.
Boxplots: A, PD cooperation rates with each type of opponent. Green dots represent placebo session. Orange dots represent MDMA session. B, The sequence of opponent responses. Here we show the lining up of decisions across runs: to account for the jitter in opponent responses, the last trial of the first run and first trial of the final run were removed. “C,” Opponents were congruous with their trustworthiness; “D,” opponents deviated from this. C, Proportion of cooperate decisions on each round, averaged across participants, for the trustworthy opponent. D, Proportion of cooperate decisions on each round, averaged across participants for the untrustworthy opponent. E, Proportion of cooperate decisions on each round plotted as scatterplots with the line of best fit for each session; for the trustworthy opponent, there is a steady decline in cooperative decisions during the placebo session, which does not occur during the MDMA session (generalized estimating equation round × experimental session interaction: χ2(1,19) = 16.79, OR = 1.08; 95% CI, 1.04–1.13, p < 0.001). The parameter estimate for a quadratic fit of trial number was not statistically significant. Error bars indicate SE.
There was a statistically significant main effect of treatment, trustworthiness, and its interaction (all p values <0.005). MDMA increased the probability of a cooperative decision when playing a trustworthy player (χ2(1,19) = 15.33, OR = 2.01; 95% CI, 1.42–2.84, p < 0.001), but not when playing an untrustworthy player (OR = 1.37; 95% CI, 0.78–2.30) or the game server (OR = 1.03; 95% CI, 0.71–1.48). To explore this effect further, the mean proportion of cooperative decisions were plotted on a round × round basis for each opponent type (Fig. 3).
For the untrustworthy opponent, Figure 3D shows a steady decline in cooperation over the course of the game, regardless of experimental session. Visual examination of Figure 3C shows a difference across sessions for the trustworthy opponent: following the first decision to compete by these opponents, a greater proportion of participants continued to cooperate more in the MDMA session than the placebo session. It seems the overall steady decline in cooperation differs across sessions, as visualized in Figure 3E. This figure plots the data as scatterplots and includes a line of best fit.
To test this formally, we performed an exploratory analysis on the round × round data, defining a new model in the generalized estimating equation framework. We included the round number as a covariate and modeled the main effect of round as well as treatment, opponent type, and their three-way interaction. We found no main effect of treatment (χ2(1,19) = 0.03, p = 0.853) but did find a main effect of opponent type (χ2(1,19) = 69,72 p < 0.001), round (χ2(1,19) = 44.87, p < 0.001), and a three-way interaction (χ2(1,19) = 11.30, p = 0.003). Post hoc tests revealed that, for all three opponent types, there was a statistically significant main effect of round such that there was a decreased probability of a cooperate decision as the game progressed (trustworthy: χ2(1,19) = 15.06, OR = 0.90; 95% CI, 0.87–0.93, p < 0.001; untrustworthy: χ2(1,19) = 48.04, OR = 0.84; 95% CI, 0.79–0.89, p < 0.001; game server: χ2(1,19) = 8.41, OR = 0.94; 95% CI, 0.90–0.97, p = 0.004). Only for the trustworthy opponent was there a round × treatment interaction (χ2(1,19) = 16.79, OR = 1.08; 95% CI, 1.043–1.13, p < 0.001): the overall level of cooperation is maintained on MDMA whereas it declined with placebo (Fig. 3E).
Sustained maintenance of overall cooperation with a trustworthy player on MDMA compared with placebo could be due to three scenarios. First, MDMA may reduce the psychological impact of an opponent's compete decision. Second, MDMA may cause a greater recovery of cooperation following a compete decision. Third, both of these could be true. We tested these statistically by defining an “impact” and “recovery” phase for each compete decision by a trustworthy opponent. The “impact” refers to the change in cooperation in the trial immediately following the opponents' compete decisions. The “recovery” refers to the change in cooperation over the next four decisions (i.e., to the next compete decision). Separate generalized estimating equation models were conducted for each of these stages.
There was a main effect of impact, such that there was a reduced probability that participants cooperated following a trustworthy opponent's compete decision (χ2(1,19) = 10.44, OR = 0.40; 95% CI, 0.23–0.70, p = 0.001). However, there was no impact × treatment interaction (χ2(1,19) = 0.21, p = 0.647). There was a main effect of recovery (χ2(1,19) = 3.87, OR = 1.19; 95% CI, 1.00–1.43, p = 0.049), as well as a recovery × treatment interaction (χ2(1,19) = 4.51, OR = 1.27; 95% CI, 1.02–1.59, p = 0.034): when playing a trustworthy opponent, there was an increase in probability of a cooperate decision during the recovery phase, and this was greater during the MDMA session than placebo session. For the untrustworthy opponents, there was a main effect of both impact (in this case, of a cooperative decision) and recovery (respectively: χ2(1,19) = 8.45, OR = 1.89; 95% CI, 1.23–2.91, p = 0.004; χ2(1,19) = 22.85, OR = 0.684; 95% CI, 0.59–0.80, p < 0.001). Neither analysis showed an interaction (p values >0.54), suggesting that MDMA did not differentially affect participants' reactions to incongruous cooperative decisions.
The above exploratory analyses suggest that the effect found in the planned analysis can be explained by MDMA enhancing the recovery of cooperation following the negative impact of a compete decision from a trustworthy opponent.
Exploratory analysis of behavior data
An exploratory analysis was performed to investigate the relationship between changes in cooperation with trustworthy opponents and the other behavioral effects of MDMA. We performed a forward selection stepwise regression analysis with change in PD cooperation with trustworthy opponents as the outcome variable. Using the stepAIC R package (Venables and Ripley, 2002), we entered change in each subscale of the ASC, each outcome measure of the MET, and change in accuracy of each emotion in the Affective Bias task, as potential predictor variables. The method uses the Akaike Information Criterion to find the best model to explain the outcome variable. Due to the Affective Bias task and MET not having complete datasets, this analysis was based on N = 17.
Table 4 shows that change in PD behavior can be explained by a broad range of MDMA effects, including subjective effects, emotion processing, and empathic processing. We performed a leave-one-out cross-validation analysis to assess the quality of the model output, and found a high correlation between predicted and observed changes in PD cooperation (Pearson's r(15) = 0.84, p < 0.001). The results suggest that the changes in cooperation can be explained by a broad range of MDMA's other behavioral and subjective effects.
Table 4.
Model output for the forward selection stepwise regression
| Estimate | SE | t | p | |
|---|---|---|---|---|
| Intercept | 63.08 | 8.35 | 7.55 | <0.001 |
| MET Cognitive | 147.47 | 22.88 | 6.45 | <0.001 |
| Affective Bias Anger | 1.91 | 0.27 | 7.13 | <0.001 |
| ASC Anxiety | −2.51 | 0.32 | −7.95 | <0.001 |
| Affective Bias Sad | −1.04 | 0.20 | −5.13 | <0.001 |
| ASC Elemental Imagery | 0.47 | 0.11 | 4.17 | 0.003 |
| ASC Impaired Cognition | 0.97 | 0.23 | 4.23 | 0.003 |
| ASC Bliss | −0.31 | 0.09 | −3.32 | 0.011 |
| ASC Complex Imagery | −0.73 | 0.28 | −2.58 | 0.034 |
| Model statistics | Adjusted R2 | F | df | p |
| 0.88 | 15.87 | 8.8 | <0.001 |
Functional neuroimaging
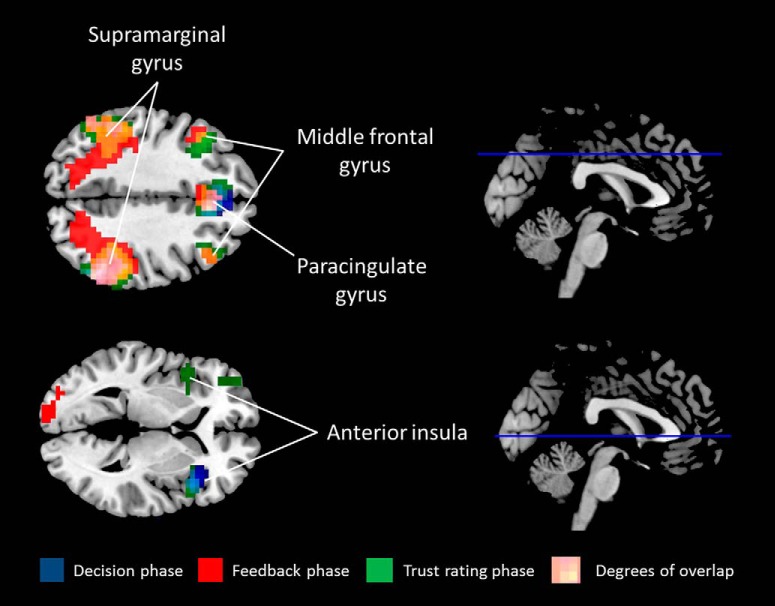
Main effect of task
To assess the main task effects, we performed a one-sample t test for each trial phase (participant decision, feedback, trust rating) in the placebo session, collapsing across opponent types (Table 5; Fig. 4). There were overlapping clusters across phases in the following regions (FWE cluster-corrected p < 0.05): (1) bilateral paracingulate gyrus, extending bilaterally into the superior frontal gyrus and middle frontal gyrus; (2) bilateral supramarginal gyrus; and (3) right inferior frontal gyrus. A cluster was activated in the right anterior insula/frontal operculum during the decision phase. There was an overlapping, smaller cluster in this region during the trust rating phase.
Table 5.
fMRI activations during the different phases of the PD in the placebo condition, collapsed across opponent typesa
| Region | FWE cluster-corrected p | Cluster size | MNI |
z | Region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Decision phase | |||||||
| Right superior frontal gyrus | <0.001 | 245 | 4 | 41 | 36 | 5.49 | Superior frontal gyrus |
| 8 | 26 | 49 | 5.23 | ||||
| 8 | 30 | 36 | 5.05 | Paracingulate gyrus | |||
| Right frontal operculum | 0.004 | 116 | 41 | 19 | 7 | 5.33 | Right frontal operculum |
| 31 | 22 | −7 | 4.62 | Anterior insula | |||
| Right angular gyrus | <0.001 | 221 | 52 | −52 | 40 | 5.21 | Angular gyrus |
| 49 | −34 | 46 | 4.93 | Supramarginal gyrus | |||
| Left supramarginal gyrus | 0.011 | 91 | −49 | −38 | 46 | 4.14 | Supramarginal gyrus |
| −49 | −49 | 49 | 4.09 | ||||
| −52 | −45 | 43 | 4.04 | ||||
| Feedback phase | |||||||
| Right supramarginal gyrus | <0.001 | 717 | 52 | −45 | 43 | 5.72 | Supramarginal gyrus |
| 49 | −45 | 53 | 5.45 | ||||
| 26 | −64 | 46 | 5.27 | Lateral occipital cortex | |||
| Left lateral occipital cortex | <0.001 | 1228 | −19 | −64 | 53 | 5.59 | Lateral occipital cortex |
| −34 | −45 | 43 | 5.58 | Superior parietal lobule | |||
| −11 | −101 | 3 | 5.30 | Occipital pole | |||
| Right inferior frontal gyrus | 0.014 | 96 | 52 | 11 | 20 | 4.78 | Inferior frontal gyrus |
| Left precentral gyrus | 0.004 | 132 | −52 | 8 | 33 | 4.33 | Precentral gyrus |
| −52 | 22 | 30 | 4.10 | Middle frontal gyrus | |||
| Trust rating phase | |||||||
| Right superior frontal gyrus | <0.001 | 511 | 15 | 22 | 59 | 5.71 | Superior frontal gyrus |
| 8 | 26 | 40 | 5.45 | Paracingulate gyrus | |||
| −11 | 38 | 33 | 4.37 | Superior frontal gyrus | |||
| Right angular gyrus | <0.001 | 365 | 52 | −49 | 46 | 5.52 | Angular gyrus |
| 56 | −56 | 40 | 5.33 | ||||
| 52 | −34 | 46 | 5.27 | Supramarginal gyrus | |||
| Left supramarginal gyrus | <0.001 | 412 | −49 | −38 | 46 | 5.00 | Supramarginal gyrus |
| −52 | −60 | 36 | 4.95 | Lateral occipital cortex | |||
| −41 | −52 | 56 | 4.76 | Superior parietal lobule | |||
| Right lateral occipital cortex | 0.009 | 92 | 15 | −71 | 53 | 4.91 | Lateral occipital cortex |
| 11 | −64 | 49 | 4.75 | Precuneous cortex | |||
| Left middle frontal gyrus | 0.007 | 96 | −41 | 26 | 36 | 4.76 | Middle frontal gyrus |
| Left inferior frontal gyrus | 0.014 | 82 | −49 | 15 | −3 | 4.17 | Inferior frontal gyrus |
| −30 | 15 | 7 | 3.52 | Anterior insula | |||
| Right frontal operculum cortex | 0.020 | 75 | 45 | 19 | 3 | 4.15 | Frontal operculum cortex |
| 56 | 19 | 17 | 3.55 | ||||
| Left frontal pole | 0.050. | 75 | −41 | 45 | −3 | 4.11 | Frontal pole |
aRegions identified by the Harvard-Oxford probabilistic atlas (Desikan et al., 2006).
Figure 4.
Main effects of each phase of the task in the placebo session. FWE cluster-corrected p < 0.05.
The bilateral supramarginal clusters activated during the feedback phase extended into the lateral occipital lobe and precentral gyrus. Furthermore, this phase had larger superior frontal clusters than the other two phases. Finally, a cluster in the left anterior insula/frontal operculum region was activated during the trust rating phase, not seen in the other phases of the task.
These regions overlap with those reported by Rilling et al. (2004), indicating that the task design is producing results in line with that which would be expected. No effects were found (threshold of FWE-corrected p < 0.05) in three one-way, within-subject ANOVAs, looking at the effect of opponent type (trustworthy, untrustworthy, game server) phase during each phase of the trial (decision, feedback, trust rating).
Treatment and opponent effects
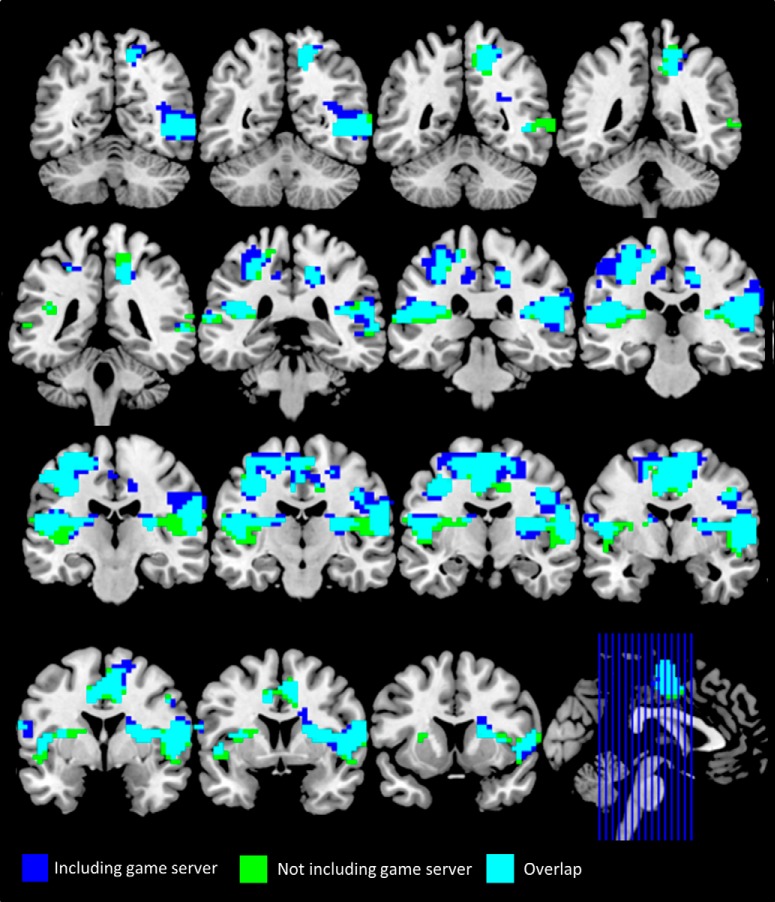
No significant clusters were found with a flexible factorial design examining the effect of treatment and opponent type (trustworthy, untrustworthy, game server) during the decision or trust-rating phase. Two models were run, with and without the game server. Table 6 presents the findings from both analyses, with Figure 5 displaying the overlap map. As the results overlap and we were primarily interested in different types of opponent behavior, the model excluding the game server is reported hereafter. During the feedback phase, there was a main effect of treatment, such that four clusters showed greater activation on MDMA compared with placebo: (1) left central operculum, extending to the parietal operculum; (2) right precentral gyrus, extending to the parietal operculum and anterior insula; (3) supplementary motor area, extending to the midcingulate cortex (MCC); and (4) right precuneus.
Table 6.
fMRI activations for the PD, treatment contrastsa
| Region | FWE cluster-corrected p | Cluster size | MNI |
z | Region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Feedback phase with the trustworthy opponent, flexible factorial including all opponent types | |||||||
| Main effect of treatment (MDMA > Placebo) | |||||||
| Precentral gyrus | <0.001 | 875 | 56 | 4 | 10 | 4.91 | Precentral gyrus |
| 60 | −22 | 23 | 4.36 | Supramarginal gyrus | |||
| 41 | −8 | 33 | 4.36 | Precentral gyrus | |||
| Supplementary motor area | <0.001 | 1008 | −4 | −8 | 49 | 4.77 | Supplementary motor area |
| 4 | −8 | 49 | 4.73 | Supplementary motor area | |||
| −22 | −15 | 59 | 4.42 | Precentral gyrus | |||
| Middle temporal gyrus | 0.001 | 275 | 49 | −60 | 0 | 4.68 | Middle temporal gyrus |
| 52 | −71 | 10 | 4.18 | Lateral occipital cortex | |||
| 38 | −60 | 17 | 3.79 | Lateral occipital cortex | |||
| Central opercular cortex | <0.001 | 369 | −49 | −19 | 17 | 4.64 | Central opercular cortex |
| −41 | −34 | 20 | 4.17 | Parietal operculum | |||
| −64 | −34 | 13 | 3.84 | Superior temporal gyrus, posterior | |||
| Feedback phase with the trustworthy opponent, flexible factorial excluding game server | |||||||
| Main effect of treatment (MDMA > Placebo) | |||||||
| Central operculum cortex | <0.001 | 469 | −45 | −19 | 14 | 4.92 | Central operculum cortex |
| −41 | −38 | 20 | 4.63 | Parietal operculum | |||
| −45 | −15 | 3 | 4.01 | Heschl's gyrus | |||
| Precentral gyrus | <0.001 | 957 | 60 | 8 | 10 | 4.83 | Precentral gyrus |
| 45 | −26 | 17 | 4.62 | Parietal operculum | |||
| 30 | 4 | 10 | 4.49 | Anterior insula | |||
| Supplementary motor area | <0.001 | 631 | 0 | −8 | 59 | 4.69 | Supplementary motor area |
| −8 | −4 | 43 | 4.56 | Midcingulate | |||
| −38 | −19 | 43 | 4.39 | Postcentral gyrus | |||
| 0.006 | 159 | 15 | −52 | 49 | 4.64 | Precuneus | |
| 15 | −30 | 43 | 3.95 | Precentral gyrus | |||
| Treatment × opponent interaction | |||||||
| Anterior insula | <0.001 | 293 | 34 | 15 | −10 | 4.65 | Anterior insula |
| 34 | 26 | −10 | 4.53 | Orbital frontal cortex | |||
| 19 | 8 | −7 | 4.13 | Putamen | |||
| Trust rating phase with the trustworthy opponent, flexible factorial excluding game server | |||||||
| Treatment × opponent interaction | |||||||
| Dorsal caudate | 0.023 | 136 | 8 | 8 | 13 | 4.57 | Dorsal caudate |
| −15 | 15 | 13 | 4.04 | Dorsal caudate | |||
| −8 | 4 | 13 | 4.36 | Dorsal caudate | |||
aRegions identified by the Harvard-Oxford probabilistic atlas (Desikan et al., 2006).
Figure 5.
Significant clusters from the main effect of treatment contrast, with and without including the game server in the model. FWE cluster-corrected p < 0.05.
To explore these effects further, we extracted the first-level contrast estimates. Due to the large size of the clusters resulting from the main effect of treatment contrast, we defined spherical ROIs centered on the peak coordinate of each of these clusters (radius = 8 mm). For the two interaction effects, we extracted the contrast estimates from the whole clusters. We performed four post hoc paired t tests on each cluster.
The main effect of drug in the left central operculum and right precentral/supramarginal clusters was largely driven by increased activation on MDMA compared with placebo when receiving feedback from trustworthy opponents (respectively: t(19) = −5.33; Bonferroni-corrected p < 0.001; t(19) = −4.37; Bonferroni-corrected p = 0.001). No other comparison survived correction for multiple comparisons in these clusters. Post hoc comparisons from the other two clusters of the main effect of treatment contrast showed increased activation during the MDMA session for both trustworthy and untrustworthy opponents.
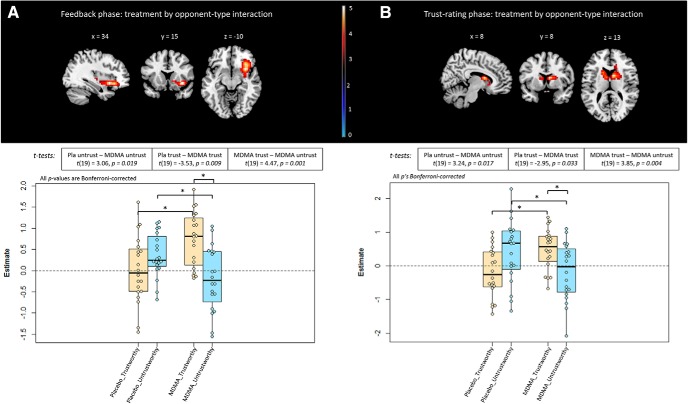
While there was no main effect of opponent type during the feedback phase, there was a significant opponent × treatment interaction effect in one cluster incorporating the right anterior insula, orbital frontal cortex, and putamen (Table 6; Fig. 6A). During the trust rating phase, there was no main effect of treatment, nor opponent type, but there was a treatment × opponent interaction in a single cluster in bilateral dorsal caudate (Table 6; Fig. 6B).
Figure 6.
Interaction effects for the (A) feedback and (B) trust rating phases. FWE cluster-corrected p < 0.05. Boxplots represent cluster-wise, extracted first-level contrast estimates with post hoc pairwise comparisons. * Indicates statistical significance at Bonferroni-corrected p < 0.05.
We extracted the first-level contrast estimates from the clusters showing interaction effects. Figure 6 shows that both the feedback phase and trust rating phase interaction effects were driven by changes in activation for both opponents on MDMA compared with placebo, in opposite directions: an increase in activation for trustworthy opponents, and a decrease in activation for untrustworthy opponents.
Finally, we entered the residuals of the first-level model into a second-level paired t test contrasting MDMA and placebo conditions to test for differences in task-unrelated variations in brain activity. This analysis returned no significant differences between treatment conditions.
Exploratory analysis examining the relationship between changes in neural activity and serotonin receptor densities
Given MDMA's potent serotonergic activity (de la Torre et al., 2004), we performed an exploratory analysis to investigate the relationship between the neuroimaging results detailed above and the receptor densities of the three serotonin receptors for which MDMA has high affinity (1A, 2A, transporter). We calculated the mean receptor density of each receptor in each of the 85 regions of the Deskin-Killiany ROI atlas (Desikan et al., 2006), using high-resolution receptor density maps from Beliveau et al. (2017). We performed correlation analyses with the second-level contrast maps from the analyses showing significant effects of MDMA: the main effect of treatment during feedback, the treatment × opponent type interaction during feedback, and the treatment × opponent type interaction during the trust rating phase. We corrected for multiple comparisons at the level of each receptor.
We found a significant correlation between contrast estimates and 2A receptor density for the main effect of treatment contrast (r = 0.28; Bonferroni-corrected p = 0.027). For the interaction effect during feedback, we found a significant correlation between contrast estimates and 1A receptor density (r = −0.28; Bonferroni-corrected p = 0.030) and 2A receptor density (r = 0.27; Bonferroni-corrected p = 0.039). For the interaction effect in the trust rating phase, we found a significant interaction between contrast estimates and 1A receptor density (r = −0.33; Bonferroni-corrected p = 0.006).
Discussion
The present findings provide evidence for context-specific modulation of cooperative behavior by MDMA. The effect of MDMA during an iterated PD was to increase cooperation with trustworthy opponents but not untrustworthy or nonsocial control opponents. This was accompanied by increased cortical activation when receiving feedback of the trustworthy players' decisions during the MDMA session, in brain regions previously associated with social interactions.
Counter to our hypothesis, mean trust ratings did not change with MDMA administration. This suggests that participants' changes in cooperative behavior were not due to an altered conceptualization of trustworthiness per se. Rather, there was a greater recovery of cooperation following the initial negative impact of compete decisions by the trustworthy opponents. In contrast, when the other player consistently displayed uncooperative behavior, participants reacted similarly on MDMA and placebo, by increasingly protecting themselves from betrayal over the course of the game. An exploratory analysis showed that changes in PD cooperation were associated with changes in a range of variables related to the overall MDMA effect.
The context-specific nature of MDMA-induced changes in behavior was reflected in the fMRI data, where we found several interactions at the different phases of the social interaction. First, we observed an interaction between treatment and opponent type in a cluster incorporating the right insula, orbital frontal cortex, and putamen. Exploring the signal changes driving this interaction showed that, when receiving feedback on MDMA compared with placebo, these areas had higher activation during the interaction with trustworthy opponents, but lower activation when interacting with untrustworthy opponents. This fits well with the proposed role of anterior insula in integrating uncertainty, appraisal, and personal preferences proposed by Singer et al. (2009). In turn, it has been suggested that the role of anterior insula in the appraisal of outcomes may be linked to its tracking of expected and experienced risk in the context of reward processing (Sescousse et al., 2013). Below we argue that the neural changes revealed as the main effect of MDMA when receiving feedback may represent differential appraisal of the opponents' actions. The interaction effect seen in this cluster suggests that what drives the MDMA-induced changes in cooperation with trustworthy opponents could be the specific alteration in how these appraisals are integrated with expected reward.
In the trust rating phase, there was also an interaction between treatment and opponent type, such that the dorsal caudate had increased activity on MDMA compared with placebo when interacting with trustworthy opponents. Previous research in social decision-making has interpreted caudate activation as reward signals when receiving feedback of mutual cooperation (Rilling et al., 2002; King-Casas et al., 2005) and when given the opportunity to punish norm violators (de Quervain, 2004). Furthermore, in the context of a Trust Game, where participants make decisions on whether to trust others with a monetary investment, the dorsal caudate has been associated with developing a model of partner reputation through signaling an “intention to trust” (King-Casas et al., 2005). The finding that this region is activated more on MDMA when considering trust in trustworthy opponents, but not with untrustworthy opponents, could represent greater potential subjective reward from this evaluation, leading to stronger reputation building and greater future cooperation.
During the feedback phase, there was a main effect of treatment with MDMA increasing activity in four clusters. One of these clusters incorporated a large amount of the left superior temporal cortex and posterior insula/central operculum. Both areas have previously been implicated in the processing of social interactions. The superior temporal cortex is frequently implicated in theory of mind, mentalizing and attribution of intent to others (Rilling et al., 2004; Kestemont et al., 2015). The posterior insula/central operculum has been implicated when reappraising the intentionality of unfair offers in the Ultimatum Game, a task where people have the opportunity punish violators of social norms (Güth et al., 1982; Güroğlu et al., 2011; Grecucci et al., 2013).
Neuroimaging studies have used computational modeling to investigate how these processes relate to interactive contexts, such as the PD (Hampton et al., 2008; Haruno and Kawato, 2009; Bault et al., 2015). These models, while not applied directly to the PD, provide candidate processes, which may be modulated by MDMA. The concept of social tie, introduced by van Dijk and van Winden (1997), represents the history of other players' behavior. The greater the social tie, the greater the interacting partner's outcome affects one's own utility. Bault et al. (2015) found that the brain regions encoding particular parameters of this model showed a remarkable overlap with the superior temporal and posterior insula changes seen during feedback on MDMA in the present study. Reappraisal of the other players' behavior and alterations in social tie may be one mechanism underlying the MDMA effects seen in present study.
In a task similar to the PD, Hampton et al. (2008) investigated a model of how one's own strategy influences that of other players in a task similar to the PD. They found that posterior temporal cortex represented differences between the expected and actual influence of one's own behavior. Furthermore, they reported that a region overlapping the MCC cluster in the current analysis encoded the expected reward from the other player's decision. The MCC cluster here spans anterior MCC and posterior regions. Vogt (2016) argues that anterior MCC, with high expression of DA D1 receptors, plays a role in value-based action selection. Furthermore, this region plays a key role in feedback-mediated decision-making. King-Cases et al. (2005) showed MCC activity correlated with participants' “intention to trust” during a trust game. Together, we can infer that the MCC change in the current analysis may represent alterations to the incorporation of feedback in planning of the next decision, and its associated action.
Pharmacological mechanisms underlying the effect of MDMA
MDMA increases the synaptic availability of serotonin, DA, and NA (de la Torre et al., 2004). The magnitude of the 5-HT effect is far larger than those of DA and NA (Carhart-Harris and Nutt, 2017), and MDMA increases oxytocin levels. While we cannot parse the effects of MDMA across these mechanisms within this study, it would be prudent to consider the possibility that selective MDMA-induced effects, particularly via changes in NA or DA, may be responsible for the changes reported here.
It is not possible to draw firm conclusions as to the precise mechanisms of any 5-HT effect underlying the results reported here. However, we conducted an exploratory analysis using high-resolution atlases of serotonin receptor densities (Beliveau et al., 2017), which show significant relationships between the signal change in our neuroimaging data for the main effects of MDMA during feedback and 5-HT2A receptor densities (r = 0.28) and the interaction effects of MDMA and opponent during feedback and the 5-HT2A (r = 0.27) and 5-HT1A (r = −0.28) densities. This is preliminary evidence that the results reported here are likely driven by MDMA's 5-HT activity. Equivalent density maps would be required for DA and NA receptors to better establish this relationship.
As has been found in previous studies (Hysek et al., 2014; Kirkpatrick et al., 2014), MDMA administration caused a large increase in plasma oxytocin levels. This is another potential mediator of the findings presented in this manuscript, particularly when considering its impact on ingroup and outgroup relations (De Dreu and Kret, 2016; Daughters et al., 2017). While group membership was not manipulated in the current study, it remains an interesting avenue for future research.
Limitations and implications
A limitation of the current study is the use of an inactive placebo. Given the clear subjective effects of MDMA, participants became aware that they had been given the active compound. This is a recognized challenge among researchers investigating compounds with potent subjective effects, such as psychedelic and dissociative drugs.
The current study recruited only male participants. Psychopharmacological studies are particularly sensitive to differences in hormonal levels across participants. As such, single-sex studies have more power to detect a given effect. It should be noted, however, that gender differences have been reported in the subjective effects of MDMA (Allott and Redman, 2007). As such, it is possible that the effects reported here may not be generalizable across genders.
Any within-participant design does hold the potential for task learning effects as well as order effects. However, the counterbalanced, crossover design of this study will have minimized these potential limitations, and our analysis of visit and order effects did not yield significant results.
Without blocking the 5-HT2A and other candidate target receptors, it is not possible to causally attribute the effects of MDMA to specific receptor subtypes. Alternatively, to establish whether MDMA's dopaminergic modulation was responsible for the results seen here, one could include a pretreatment with a DA antagonist, such as haloperidol.
The behavioral changes seen in the PD could be interpreted as being a result of MDMA altering how participants process reward. However, given the context-specific nature of the changes, with changes in behavior limited to trustworthy opponents, we do not believe this to be the case. Furthermore, Gabay et al. (2018) reported results of a reward sensitivity task in these same participants, showing no changes with MDMA administration.
In conclusion, the work presented here provides evidence that MDMA alters cooperative behavior in a context-specific manner. As well as behavioral changes in the PD, we show alterations in social brain regions when receiving feedback of interacting partner's decisions under MDMA (compared with placebo), when interacting with a generally trustworthy opponent. Our work provides testable hypotheses of the mechanisms underlying this change in behavior: that MDMA causes a change in social tie and appraisal of others' actions, leading to greater recovery of cooperation with trustworthy partners following a breach in trust. Crucially, there is no global change in behavior due to MDMA effects being highly specific to trustworthy interactions as it does not alter behavior when playing the game with untrustworthy opponents. With MDMA showing strong potential as an adjunct to psychotherapy in the treatment of post-traumatic stress disorder (Mithoefer et al., 2011), these results represent an important and timely step in teasing apart its well-reported social and emotional effects.
Footnotes
A.S.G. was supported by an IoPPN-MRC Excellence award. M.J.K. was supported by Medical Research Council Fellowship Grant MR/J008915/1. M.A.M. and M.J.K. were supported by the National Institute for Health Research Biomedical Research Centre at South London and the Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care. J.G. was supported by the British Academy. We thank the study participants for time and commitment to the study; Dr. Ottavia Dipasquale for assistance with the receptor density analysis; and South London & Maudsley NHS Trust Pharmacy for managing the supply and access to MDMA.
The authors declare no competing financial interests.
References
- Allott K, Redman J (2007) Are there sex differences associated with the effects of ecstasy/3,4-methylenedioxymethamphetamine (MDMA)? Neurosci Biobehav Rev 31:327–347. 10.1016/j.neubiorev.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Bault N, Pelloux B, Fahrenfort JJ, Ridderinkhof KR, van Winden F (2015) Neural dynamics of social tie formation in economic decision-making. Soc Cogn Affect Neurosci 10:877–884. 10.1093/scan/nsu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H (2010) Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological Psychiatry 68:1134–1140. 10.1016/j.biopsych.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, Svarer C, Greve DN, Knudsen GM (2017) A high-resolution in vivo atlas of the human brain's serotonin system. J Neurosci 37:120–128. 10.1523/JNEUROSCI.2830-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland AR, Roiser JP, Mehta MA, Schei T, Boland H, Campbell-Meiklejohn DK, Emsley RA, Munafo MR, Penton-Voak IS, Seara-Cardoso A, Viding E, Voon V, Sahakian BJ, Robbins TW, Elliott R (2016) EMOTICOM: a neuropsychological test battery to evaluate emotion, motivation, impulsivity, and social cognition. Front Behav Neurosci 10:25. 10.3389/fnbeh.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ (2017) Serotonin and brain function: a tale of two receptors. J Psychopharmacol 31:1091–1120. 10.1177/0269881117725915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gautam P, Haroon E, Rilling JK (2017) Within vs. between-subject effects of intranasal oxytocin on the neural response to cooperative and non-cooperative social interactions. Psychoneuroendocrinology 78:22–30. 10.1016/j.psyneuen.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW (2010) Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci U S A 107:17433–17438. 10.1073/pnas.1009396107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters K, Manstead AS, Ten Velden FS, De Dreu CK (2017) Oxytocin modulates third-party sanctioning of selfish and generous behavior within and between groups. Psychoneuroendocrinology 77:18–24. 10.1016/j.psyneuen.2016.11.039 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J (2004) Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26:137–144. 10.1097/00007691-200404000-00009 [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Kret ME (2016) Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol Psychiatry 79:165–173. 10.1016/j.biopsych.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A (2008) Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the multifaceted empathy test (MET). J Autism Dev Disord 38:464–473. 10.1007/s10803-007-0486-x [DOI] [PubMed] [Google Scholar]
- Gabay AS, Kempton MJ, Mehta MA (2015) Facial affect processing deficits in schizophrenia: a meta-analysis of antipsychotic treatment effects. J Psychopharmacol 29:224–229. 10.1177/0269881114560184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay AS, Carhart-Harris RL, Mazibuko N, Kempton MJ, Morrison PD, Nutt DJ, Mehta MA (2018) Psilocybin and MDMA reduce costly punishment in the Ultimatum Game. Sci Rep 8:8236. 10.1038/s41598-018-26656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleen J, Satkunanathan S (2015) High schizotypes show selectively reduced trust of malevolent but not benevolent opponents during social interaction compared to low schizotypes. Schizophr Bull 41:S45–S46. [Google Scholar]
- Grecucci A, Giorgetta C, van 't Wout M, Bonini N, Sanfey AG (2013) Reappraising the ultimatum: an fMRI study of emotion regulation and decision making. Cereb Cortex 23:399–410. 10.1093/cercor/bhs028 [DOI] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, van Dijk E, Rombouts SA, Crone EA (2011) Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. Neuroimage 57:634–641. 10.1016/j.neuroimage.2011.04.032 [DOI] [PubMed] [Google Scholar]
- Güth W, Schmittberger R, Schwarze B (1982) An experimental analysis of ultimatum bargaining. J Econ Behav Organ 3:367–388. 10.1016/0167-2681(82)90011-7 [DOI] [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP (2008) Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci U S A 105:6741–6746. 10.1073/pnas.0711099105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT (2002) Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Pharmacology 162:396–405. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kawato M (2009) Activity in the superior temporal sulcus highlights learning competence in an interaction game. J Neurosci 29:4542–4547. 10.1523/JNEUROSCI.2707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME (2014) MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci 9:1645–1652. 10.1093/scan/nst161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestemont J, Ma N, Baetens K, Clément N, Van Overwalle F, Vandekerckhove M (2015) Neural correlates of attributing causes to the self, another person and the situation. Soc Cogn Affect Neurosci 10:114–121. 10.1093/scan/nsu030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR (2005) Getting to know you: reputation and trust in a two-person economic exchange. Science 308:78–83. 10.1126/science.1108062 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H (2014) Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology 39:1654–1663. 10.1038/npp.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis M (2008) Plasma Pharmacokinetics of 3,4-Methylenedioxymethamphetamine After Controlled Oral Administration to Young Adults. Ther Drug Monit 30:320–332. 10.1097/FTD.0b013e3181684fa0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, Hasler G (2016) Social functioning in major depressive disorder. Neurosci Biobehav Rev 69:313–332. 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Kuypers KP, de la Torre R, Farre M, Yubero-Lahoz S, Dziobek I, Van den Bos W, Ramaekers JG (2014) No evidence that MDMA-induced enhancement of emotional empathy is related to peripheral oxytocin levels or 5-HT1a receptor activation. PLoS One 9:e100719. 10.1371/journal.pone.0100719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers KP, Dolder PC, Ramaekers JG, Liechti ME (2017) Multifaceted empathy of healthy volunteers after single doses of MDMA: a pooled sample of placebo-controlled studies. J Psychopharmacol 31:589–598. 10.1177/0269881117699617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Parrish JM, Nelson EE, Easter J, Thorne JF, Rilling JK, Ernst M, Pine DS (2007) Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol 35:567–577. 10.1007/s10802-007-9113-8 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R (2011) The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 25:439–452. 10.1177/0269881110378371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Schäfer IC, Müller BW, de Bruijn ER (2013) Do different fairness contexts and facial emotions motivate ‘irrational’ social decision-making in major depression? An exploratory patient study. Psychiatry Res 210:438–443. 10.1016/j.psychres.2013.07.017 [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C (2002) A neural basis for social cooperation. Neuron 35:395–405. 10.1016/S0896-6273(02)00755-9 [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD (2004) The neural correlates of theory of mind within interpersonal interactions. Neuroimage 22:1694–1703. 10.1016/j.neuroimage.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G (2012) Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37:447–461. 10.1016/j.psyneuen.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Wall SC (1992) The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A 89:1817–1821. 10.1073/pnas.89.5.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME (2014) Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol 28:847–856. 10.1177/0269881114542454 [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher JC (2013) Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev 37:681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009) A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Sorgi KM, van 't Wout M (2016) The influence of cooperation and defection on social decision making in depression: a study of the iterated Prisoner's dilemma game. Psychiatry Res 246:512–519. 10.1016/j.psychres.2016.10.025 [DOI] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol 25:1434–1452. 10.1177/0269881110382466 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Niki K, Fujisaki S, Akiyama E (2011) Neural basis of conditional cooperation. Soc Cogn Affect Neurosci 6:338–347. 10.1093/scan/nsq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk F, van Winden F (1997) Dynamics of social ties and local public good provision. J Public Econ 64:323–341. 10.1016/S0047-2727(96)01620-9 [DOI] [Google Scholar]
- Venables WN, Ripley BD (2002) Modern applied statistics with R, Ed 4 New York: Springer. [Google Scholar]
- Vogt BA. (2016) Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat 74:28–46. 10.1016/j.jchemneu.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Wood RM, Rilling JK, Sanfey AG, Bhagwagar Z, Rogers RD (2006) Effects of tryptophan depletion on the performance of an iterated Prisoner's dilemma game in healthy adults. Neuropsychopharmacology 31:1075–1084. 10.1038/sj.npp.1300932 [DOI] [PubMed] [Google Scholar]