Abstract
Introduction: The effect of local ablative therapy (LAT) for oligoprogressive epidermal growth factor receptor (EGFR) mutation non-small cell lung cancer (NSCLC) remains undetermined. This study aimed to investigate the survival benefit of addition of LAT to EGFR-TKIs in EGFR-mutant NSCLC patients with oligoprogression during TKI therapy.
Materials and Methods: Patients with stage IIIB/IV EGFR mutant NSCLC who had oligoprogressive disease during the first-line EGFR-TKI therapy from March 2011 to February 2016 were identified. The primary research point were progression-free survival1 (PFS1), defined as time of initiation of TKI therapy to Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 defined progress disease (PD) or death and PFS2, defined as time of initiation of TKI therapy to off-TKI PD. The second research piont inclued overal survival (OS) and safety.
Results: A total of 206 patients were included. The median follow-up time was 42 months (20.0-69.6 months). The median PFS1, median PFS2 and median OS for the related cohort were 10.7 months (95% CI, 10.1-13.3 months), 18.3 months (95% CI, 17.4-19.2 months) and 37.4 months (95% CI, 35.9-38.9 months) respectively. Survival rates of 1 year, 2 years and 3 years were 94.1%, 78.9%, and 54.7%, respectively. Multivariate analysis revealed that female, EGFR exon 19 mutation, one metastatic lesion, partial or complete response to prior EGFR TKIs therapy were the independent prognostic factors. No unexpected toxicities were observed.
Conclusion: The current study suggested that the addition of LAT to EGFR-TKI could provide satisfactory survival benefit for EGFR-mutant NSCLC patients with oligoprogression during first-line EGFR-TKI treatment.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1-2 Non-small cell lung cancer (NSCLC) accounts for more than 80% of all cases. Epidermal growth factor receptor (EGFR) plays a critical role in cancer cells proliferation and survival. EGFR activating mutations occur more frequently in Asian patients compared with Caucasian patients.3-5 Landmark clinical trials have demonstrated the superior progression-free survival (PFS) and quality-of-life of EGFR tyrosine kinase inhibitors (TKIs) over the standard platinum-based doublet chemotherapy in NSCLC patients with EGFR activating mutations.6-12 Several EGFR TKIs (gefitinib, erlotinib, icotinib and afatinib) have been the standard first-line therapy in patients with EGFR-mutant NSCLC. However, acquired resistance would occur in patients who initially responded to EGFR TKI, after median PFS of 10-14 months.6-12 Radiological progression does not always imply that all metastatic sites share the same cause of resistance. Some tumor sites might still be sensitive to EGFR TKI beyond Response Evaluation Criteria in Solid Tumors (RECIST) disease progression. The potential tumor heterogeneity suggests that continuation of EGFR TKIs may provide additional benefit in patients with slow progression.13-15
The oligoprogressive disease represents an indolent status of patients with advanced disease who are receiving active systemic therapy but with limited number of metastatic sites showing disease progression. Local treatment to all oligoprogressive lesions is thought to eradicate the de-differentiated clones and restore sensitivity of the metastatic disease.16-18 For patients with oligoprogressive disease harboring EGFR mutation and treated with EGFR TKI, whether the addition of local ablative therapy (LAT) could extend the survival benefit in oligoprogressive disease remains undetermined. To address this issue, we performed this study to investigate the outcomes of EGFR-mutant NSCLC patients treated with continual EGFR TKI and LAT after oligoprogression.
Materials and Methods
Patients cohort
We conducted a retrospective study on patients with stage IIIB/IV EGFR-mutant NSCLC who had oligoprogressive disease during the first-line EGFR-TKI therapy from March 2011 to February 2016 at Shanghai Pulmonary Hospital. Recruited patients met the following criteria: they had diagnosis of pathologically confirmed NSCLC with confirmed activating EGFR mutation (exon 19 deletion or exon 21 L858R mutation), with stage IIIB/IV disease according to the 7th edition of the American Joint Committee on Cancer staging system, were 18 years or older, with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 2 or less, had received EGFR-TKI as first-line therapy, with five or fewer metastases, not including the primary tumor during the first-line EGFR-TKI therapy. The oligoprogressive lesions were radically treated with LATs. Oligometastatic disease was defined as the presence of ≤ 5 lesions in 1 to multiples organs during EGFR TKI therapy. Never-smoker was defined as a person who had smoked fewer than 100 cigarettes during his/her lifetime. Patients received continuous EGFR-TKI therapy until symptomatic extracranial progression, rapid progression, worsening ECOG PS or life-threatening complications as examples of those who may not be suitable for continued TKI therapy or clinicians believed that patients could not benefit from continuation of TKI therapy. The treatment response was evaluated 6-8 weeks after the initiation of therapy and then every 2 months according to RECIST 1.1. The EGFR-TKIs used in this study included gefitinib (250 mg, once a day), erlotinib (150 mg, once a day), and icotinib (125 mg, three times a day). EGFR mutations were tested by an amplification refractory mutation system as described in our previous studies (Amoy Diagnostics Co., Ltd., Xiamen, China).19 All mutational analyses were performed at the Thoracic Cancer Institute, Tongji University Medical School, Shanghai, People's Republic of China.
Baseline characteristics were recorded from electronic records by retrospective collection, including age at diagnosis (taken at date of diagnostic biopsy), sex, smoking status, ECOG PS, tumor histology prior therapy, treatment, oligometastatic disease and number of oligometastatic sites. Routine surveillance imaging includes chest computed tomography (CT), abdomen CT and brain magnetic resonance imaging. Patients underwent bone scan when suspected for bone metastasis, and positron emission tomography scan when suspected for systemic progression.
Statistical analysis
Descriptive statistics were used to summarise patient characteristics by treatment group. PFS1 was calculated from time of initiation of TKI therapy to first RECIST 1.1 defined progress disease (PD). PFS2 was calculated from time of initiation of TKI therapy to off-TKI PD. Overal survival (OS) was calculated from the date of lung cancer diagnosis to death from any cause or was censored at the last follow-up date. Kaplan-Meier curve and 2-sided log-rank test were used for univariate survival analyses. The Cox proportional hazards model was used for uni- and multivariate survival analyses to calculate the hazard ratio (HR) and corresponding 95% confidence intervals. Subgroup analysis was also carried out with age, gender, ECOG PS, hitology, Disease stage, metastases number, mutation type, EGFR TKIs, reponse to EGFR TKIs in 6 months and oligoprogressive symptom as the stratification factors. P values were 2-sided and considered significant if < 0.05. All statistical analyses were performed using the SPSS statistical software, version 22.0 (IBM Corp., Armonk, NY).
Results
Patient Characteristics
A total of 206 patients with oligoprogressive diseases harboring EGFR mutations were included, who were treated with first-line EGFR-TKI within the study period. The patient characteristics were presented in Table 1. The majority of patients (174/206, 84.5%) had histology of adenocarcinoma and the median age was 58 years. Briefly, 58.3% of patients were female, 74.3% had ECOG PS 0 or 1, 81.1% had stage IV disease, 60.7% were never smokers, 38.3% had only one site metastases, 56.8% had exon 21 L858R mutation and 68.0% had partial or complete response.
Table 1.
Baseline patient characteristics.
| Characteristic | Patients (n) | (%) |
|---|---|---|
| Median age, y (range) | 58 (28~83) | |
| <65 | 143 | 69.4 |
| ≥65 | 63 | 30.6 |
| Gender | ||
| Male | 97 | 41.7 |
| Female | 109 | 58.3 |
| ECOG performance status | ||
| 0~1 | 153 | 74.3 |
| 2 | 53 | 25.7 |
| Histology | ||
| Adenocarcinoma | 174 | 84.5 |
| Squamous cell | 2 | 0.9 |
| Large cell | 7 | 3.4 |
| Adeno-squamous | 14 | 6.8 |
| NOS | 9 | 4.4 |
| Disease stage | ||
| IIIB | 39 | 18.9 |
| IV | 167 | 81.1 |
| Smoking status | ||
| Non-smoker | 125 | 60.7 |
| Present or former smoker | 81 | 39.3 |
| Metastases number | ||
| 1 | 79 | 38.3 |
| 2 | 25 | 12.1 |
| 3 | 31 | 15.1 |
| 4 | 42 | 20.4 |
| 5 | 29 | 14.1 |
| EGFR mutation | ||
| Exon 19 deletion | 89 | 43.2 |
| Exon 21 L858R | 117 | 56.8 |
| EGFR TKIs | ||
| Gefitinib | 107 | 51.9 |
| Erlotinib | 47 | 22.8 |
| Icotinib | 52 | 25.3 |
| Response to first-line EGFR TKIs | ||
| Partial or complete response | 140 | 68.0 |
| Stable disease | 41 | 19.9 |
| Disease progression | 25 | 12.1 |
| Oligoprogressive symptom | ||
| Symptomatic | 71 | 34.5 |
| Asymptomatic | 135 | 65.5 |
| Second- or further-line treatment | ||
| Chemotherapy | 164 | 79.6 |
| Osimertinib±chemotherapy | 11 | 5.4 |
| CTHM | 31 | 15.0 |
| Metastasis location | ||
| Brain | 124 | 60.2 |
| Bone | 86 | 41.7 |
| Adrenal | 35 | 17.0 |
| Lung | 40 | 19.4 |
| Liver | 18 | 8.7 |
| Chest wall | 10 | 4.9 |
| Neck lymph nodes | 9 | 4.4 |
| Intestine | 1 | 0.5 |
| LAT for oligometastasis | ||
| Brain | 124 | |
| Whole-brain irradiation | 23 | 18.5 |
| SRS | 89 | 71.8 |
| Surgery + whole-brain irradiation | 12 | 9.7 |
| Bone | 86 | |
| Surgery + EBRT (30 Gy) | 4 | 4.7 |
| EBRT (30-40 Gy) | 82 | 95.3 |
| Adrenal | 35 | |
| Surgery | 10 | 28.6 |
| SBRT | 16 | 45.7 |
| EBRT (45-50 Gy) | 9 | 25.7 |
| Lung | 40 | |
| SBRT | 26 | 65.0 |
| EBRT (55-63 Gy) Surgery |
11 3 |
27.5 7.5 |
| Liver | 18 | |
| Radiofrequency ablation | 16 | 88.9 |
| Surgery | 2 | 11.1 |
| Chest wall | 10 | |
| EBRT (45-55Gy) | 10 | 100.0 |
| Neck lymph nodes | 9 | |
| EBRT (55-63Gy) | 9 | 100.0 |
| Intestine | 1 | |
| Surgery | 1 | 100.0 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
LAT, local ablative therapy; SBRT, stereotactic body radiation therapy; SRS, stereotactic radiosurgery; EBRT, external beam radiotherapy; RFA, radio frequency ablation; CTHM, Chinese traditional herbal medicine.
124 (60.2%) cases of Oligoprogressive diseases were identified in brain, 86 (41.7%) in bone, 35 (17.0%) in adrenal glands, 18 (8.7%) in liver, 40 (19.4%) in lung, 10 (4.9%) in chest wall, 9 (4.4%) in neck lymph nodes, and 1(0.5%) in intestine. Only 32 (15.5%) patients underwent surgical treatment, including 12 cases of brain, 10 cases of adrenal glands, 4 cases of bone, 3 cases of lung, 2 cases of liver, 1 case of intestine. 107 (51.9%) patients received first-line gefitinib therapy, 47 (22.8%) received erlotinib therapy and 52 (25.3%) received icotinib. 118 (57.3%) patients received second-line platinum-based combination chemotherapy, 46 (22.3%) patients received single-agent chemotherapy and 31 (15.1%) patients received Chinese traditional herbal medicine (CTHM) treatment. Only 11 (5.3%) patients received second or third-line treatment of third-generation EGFR-TKI osimertinib (Table 1).
PFS and OS
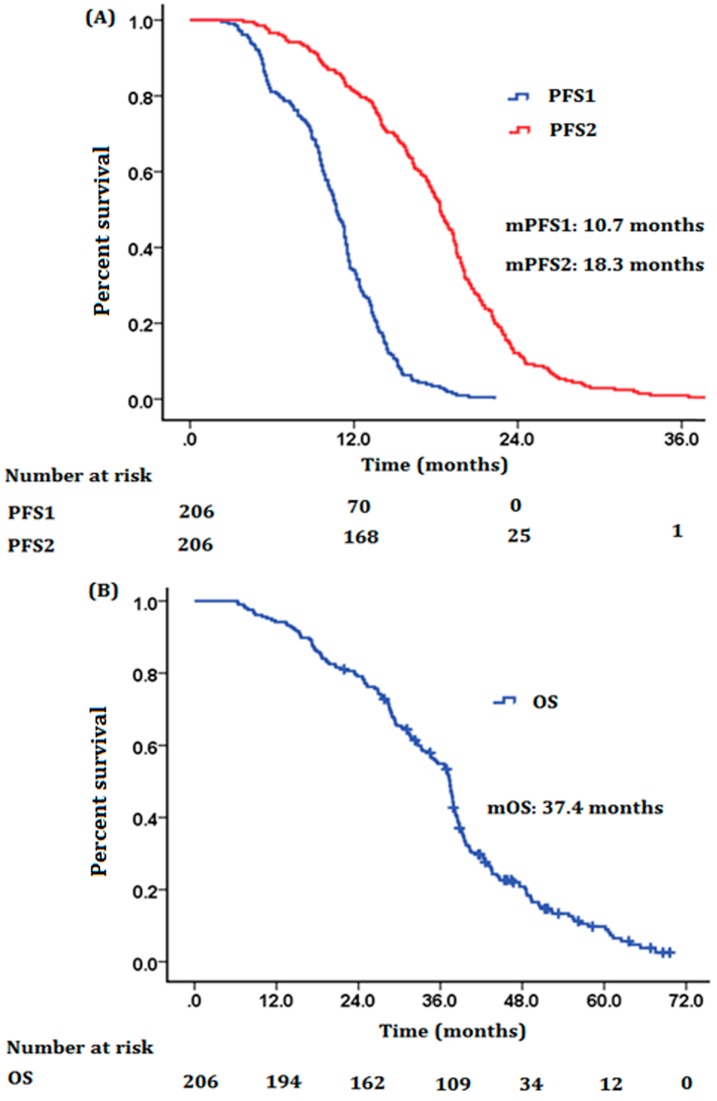
The median follow-up time was 42 months (20.0-69.6 months). The median PFS1 (mPFS1), median PFS2 (mPFS2) and median OS (mOS) for the related cohort were 10.7 months (95% CI, 10.1-13.3 months), 18.3 months (95% CI, 17.4-19.2 months) and 37.4 months (95% CI, 35.9-38.9 months), respectively (Figure 1). Survival rates of 1-year, 2-year and 3-year were 94.1%, 78.9%, and 54.7%, respectively. For the 124 patients who had brain oligometastases, the mPFS1, mPFS2 and mOS were 11.3 months (95% CI, 10.7-11.8 months), 19.0 months (95% CI, 17.9-20.0 months) and 37.7 months (95% CI, 36.8-38.6 months), respectively. For the 86 patients who had bone oligometastases, the mPFS1, mPFS2 and mOS were 10.5 months (95% CI, 9.4-11.6 months), 17.9 months (95% CI, 16.9-18.9 months) and 36.6 months (95% CI, 32.5-40.7 months) respectively. For the 35 patients who had adrenal glands oligometastases, the mPFS1, mPFS2 and mOS were 11.4 months (95% CI, 9.3-13.5 months), 19.5 months (95% CI, 16.9-22.1 months) and 38.5 months (95% CI, 34.3-42.7 months) respectively. For the 35 patients who had liver oligometastases, the mPFS1, mPFS2 and mOS were 7.6 months (95% CI, 4.1-11.1 months), 13.8 months (95% CI, 10.9-16.7 months) and 24.7 months (95% CI, 7.8-41.5 months) respectively. The mPFS1, mPFS2 and mOS for those 32 patients who had surgical treatment were 11.7 months (95% CI, 9.7-13.7 months), 18.9 months (95% CI, 16.3-22.5 months) and 39.2 months (95% CI, 34.5-43.9 months) respectively. Patients who received gefitinib, erlotinib and icotinib had comparable mPFS1 (10.7, 10.2 and 10.7 months, P = 0.97), mPFS2 (18.8, 17.9 and 18.4 months, P = 0.99) and mOS (37.1, 34.7 and 37.8 months, P = 0.96).
Figure 1.
Kaplan-Meier plot of (A) PFS1, PFS2 and (B) OS for all patients in this study cohort. Abbreviations: mPFS, media progression-free survival; mOS, media overal survival.
Subgroup analysis on PFS1, PFS2 and OS
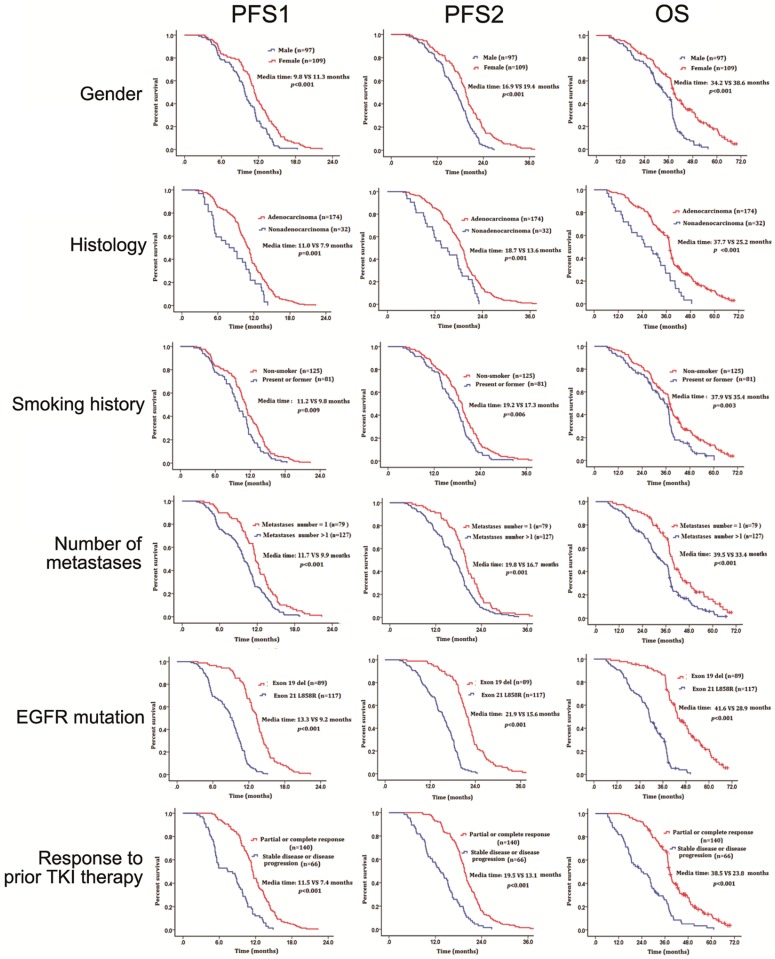
Univariate analysis revealed that male patients had significantly shorter mPFS1, mPFS2 and mOS than female patients (mPFS1: HR=0.59, 95% CI: 0.43-0.77, P < 0.001, mPFS2: HR=0.55, 95% CI: 0.41-0.73, P < 0.001, mOS: HR=0.47, 95% CI: 0.34-0.64, P < 0.001). Patients with adenocarcinoma had significantly longer mPFS1, mPFS2 and mOS than those with other histological types (mPFS1: HR=1.94, 95% CI: 1.32-2.84, P = 0.001, mPFS2: HR=1.87, 95% CI: 1.27-2.74, P = 0.001, mOS: HR=1.98, 95% CI: 1.36-2.89, P < 0.001). Never-smokers had significantly longer mPFS1, mPFS2 and mOS than former/current-smoker patients (mPFS1: HR=0.69, 95% CI: 0.52-0.92, P = 0.009, mPFS2: HR=0.68, 95% CI: 0.50-0.91, P = 0.006, mOS: HR=0.63, 95% CI: 0.47-0.86, P =0.003). Patients with 1 metastatic lesion had significantly better mPFS1, mPFS2 and mOS than patients with more than 1 metastatic lesion (mPFS1: HR=0.59, 95% CI: 0.44-0.79, P < 0.001, mPFS2: HR=0.62, 95% CI: 0.47-0.83, P = 0.001, mOS: HR=0.56, 95% CI: 0.41-0.76, P < 0.001). Patients with EGFR exon 19 deletion had significantly better mPFS1, mPFS2 and mOS than those with EGFR exon 21 L858R (mPFS1: HR=4.93, 95% CI: 3.53-6.89, P < 0.001, mPFS2: HR=5.10, 95% CI: 3.64-7.11, P < 0.001, mOS: HR=5.4, 95% CI: 3.77-7.74, P < 0.001). Patients who had partial or complete response to first-line EGFR TKIs had significantly better mPFS1, mPFS2 and mOS than patients who had stable disease or disease progression (mPFS1: HR=0.34, 95% CI: 0.25-0.46, P < 0.001, mPFS2: HR=0.34, 95% CI: 0.25-0.46, P < 0.001, mOS: HR=0.35, 95% CI: 0.25-0.48, P < 0.001) (Figure 2 and Table 2). In multivariate analysis, the variables independently associated with prolonged mPFS1, mPFS2 and mOS were female, EGFR exon 19 mutation, 1 metastatic lesion, partial or complete responses to EGFR TKIs therapy in 6 months (Table 3).
Figure 2.
The effect of different clinical factors on survival. Abbreviations: PFS, progression-free survival; OS, overal survival; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Table 2.
Univariable analysis of clinical factors potentially associated with PFS and OS.
| Characteristic | mPFS1 (m) | HR (95%CI) | p Value | mPFS2 (m) | HR (95%CI) | p Value | mOS (m) | HR (95%CI) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Age (year) | |||||||||
| <65 | 10.8 | 1.25 (0.92-1.68) |
0.148 | 18.6 | 1.26 (1.01-1.63) |
0.092 | 37.6 | 1.29 (0.96-1.74) |
0.131 |
| ≥65 | 10.5 | 17.4 | 37.1 | ||||||
| Gender | |||||||||
| Male | 9.8 | 0.59 (0.43-0.77) |
<0.001 | 16.9 | 0.55 (0.41-0.73) |
<0.001 | 34.2 | 0.47 (0.34-0.64) |
<0.001 |
| Female | 11.3 | 19.4 | 38.6 | ||||||
| ECOG performance status | |||||||||
| 0~1 | 10.7 | 1.08 (0.79-1.49) |
0.614 | 18.4 | 1.06 (0.77-1.45) |
0.739 | 37.5 | 0.99 (0.71-1.38) |
0.745 |
| 2 | 10.7 | 18.3 | 37.3 | ||||||
| Histology | |||||||||
| Adenocarcinoma | 11.0 | 1.94 (1.32-2.84) |
0.001 | 18.7 | 1.87 (1.27-2.74) |
0.001 | 37.7 | 1.98 (1.36-2.89) |
<0.001 |
| Nonadenocarcinoma | 7.9 | 13.6 | 25.2 | ||||||
| Disease stage | |||||||||
| ⅢB | 11.5 | 1.38 (0.97-1.96) |
0.068 | 19.4 | 1.35 (0.95-1.91) |
0.096 | 38.4 | 1.43 (0.97-2.10) |
0.069 |
| Ⅳ | 10.5 | 18.2 | 37.2 | ||||||
| Smoking status | |||||||||
| Non-smoker | 11.2 | 0.69 (0.52-0.92) |
0.009 | 19.2 | 0.68 (0.51-0.90) |
0.006 | 37.9 | 0.63 (0.47-0.86) |
0.003 |
| Present or former smoker | 9.8 | 17.3 | 35.4 | ||||||
| Metastases number | |||||||||
| 1 | 11.7 | 0.59 (0.44-0.79) |
<0.001 | 19.8 | 0.62 (0.47-0.83) |
0.001 | 39.5 | 0.56 (0.41-76) |
<0.001 |
| >1 | 9.9 | 16.7 | 33.4 | ||||||
| EGFR mutation | |||||||||
| Exon 19 deletion | 13.3 | 4.93 (3.53-6.89) |
<0.001 | 21.9 | 5.1 (3.64-7.11) |
<0.001 | 41.6 | 5.4 (3.77-7.74) |
<0.001 |
| Exon 21 L858R | 9.2 | 15.6 | 28.9 | ||||||
| Response to first-line EGFR TKIs | |||||||||
| Partial or complete response | 11.5 | 0.34 (0.25-0.46) |
<0.001 | 19.5 | 0.34 (0.25-0.46) |
<0.001 | 38.5 | 0.35 (0.25-0.48) |
<0.001 |
| Stable disease or disease progression | 7.4 | 13.1 | 23.8 | ||||||
| Oligoprogressive symptom | |||||||||
| Symptomatic | 10.7 | 0.98 (0.70-1.36) |
0.550 | 16.7 | 0.85 (0.58-1.12) |
0.080 | 34.2 | 0.91 (0.62-1.30) |
0.215 |
| Asymptomatic | 10.8 | 18.4 | 37.6 |
Abbreviations: m, months; HR, hazard ratio; mPFS, media progression-free survival; mOS, media overal survival; ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Table 3.
Multivariable analysis of covariables associated with PFS and OS.
| Variable | PFS1 | PFS2 | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender (male VS. female) | 0.69 (0.50-0.95) |
0.025 | 0.61 (0.43-0.82) |
0.016 | 0.612 (0.43-0.87) |
0.006 |
| Histology (adenocarcinoma vs. nonadenocarcinoma) | 1.27 (0.83-1.94) |
0.281 | 0.85 (0.55-1.30) |
0.445 | 1.70 (1.10-2.62) |
0.016 |
| Smoking status (non-smoker vs. present or former) | 1.08 (0.78-1.51) |
0.648 | 0.95 (0.69-1.31) |
0.753 | 1.02 (0.72-1.44) |
0.916 |
| EGFR mutation (exon19 vs. exon 21) | 4.06 (2.86-5.76) |
<0.001 | 3.90 (2.75-5.98) |
<0.001 | 4.68 (3.19-6.87) |
<0.001 |
| Metastases number (1 vs.>1) | 1.64 (1.19-2.16) |
0.002 | 0.642 (0.48-0.86) |
0.003 | 1.76 (1.28-2.40) |
<0.001 |
| Response to first-line EGFR TKIs (Partial or complete response vs. Stable disease or disease progression) | 2.92 (1.37-2.69) |
<0.001 | 0.49 (0.36-0.69) |
<0.001 | 1.93 (1.38-2.70) |
<0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; OS, overal survival; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Toxicity
The most common toxicities of EGFR TKIs therapy included skin rash, diarrhea, neutropenia, fatigue, nausea, vomiting, and pneumonitis. The majority of toxicities were grade 1 to 2 (G1-2). G3 skin rash occurred in 11 (5.3%) patients. G3 diarrhea occurred in 8 (3.9%) patients. G3 pneumonitis was observed in 3 (1.5%) patients. No G4 or G5 toxicity was recorded. The majority of adverse events attributing to LATs occurred to lung. G3 radiation pneumonia was reported in 3 patients within 3 months after SBRT. There was no other documented G3/4 adverse event due to the addition of radiotherapy.
Discussion
Almost all patients receiving EGFR-TKI therapy will eventually experience disease progression. Premature discontinuation of EGFR TKI therapy may result in rapid progression of symptoms and tumor regrowth, while reintroduction of TKI therapy could slow tumor growth again.20 Preclinical data indicated that coexistence of EGFR-dependent and EGFR-independent clones arising in vitro from the same environmental stress.21 On removal of selective pressure by discontinuation of EGFR-TKI therapy, the TKI-sensitive clones may regrow and result in rapid PD with the potential risk of flare-up of symptoms. Therefore, continue EGFR-TKI treatment beyond RECIST progression may prolong survival benefits in clinically selected patients. ASPIRATION has demonstrated a potential improvement in median PFS with continuation of erlotinib beyond progression.22 However, the characteristics of those patients who continued erlotinib therapy and those who did not were not balanced. Significantly more patients who had recurrent disease, ECOG PS 0 or 1 at PFS1, longer median PFS1, improved depth of response, and a longer median time from best overall response to PFS1 continued erlotinib therapy than those who did not. IMPRESS reported that continuation of gefitinib after radiological disease progression on first-line gefitinib did not prolong PFS in patients who received platinum-based doublet chemotherapy as subsequent line of treatment.23 Therefore, the patient population who could benefit from continuing EGFR-TKI therapy remained controversial.
Patients who developed local or slow/minimal progression (oligoprogression) during EGFR-TKI treatment present unique clinical characteristics which could benifit from continual EGFR-TKI plus LAT. Lo 13 et al showed that continuation of EGFR-TKI therapy plus locoregional treatment (surgery and radiotherapy) after PD, delayed the need for second-line chemotherapy by 3 months. In a retrospective analysis by Weickhardt 24 et al, 25 paitents (15 ALK positive cases, 10 EGFR mutant cases) continuing TKI therapy with LAT beyond PD significantly extended PFS. However, the sample size of these studies was too small. To our knowledge, this is one of the largest single institutional analyses in the literature of NSCLC patients with EGFR mutant who developed oligoprogresson and received LAT during first-line EGFR-TKI therapy. The median PFS1 from the study was consistent with previous studies of first-line EGFR-TKI therapy in EGFR mutation-positive NSCLC. 6-12 The median PFS2 was 18.3 months and the median OS was 37.4 months in this whole cohort, which were similar to those observed in the retrospective studies. 24-26 The results of mPFS2 and mOS in the current study were longer than that in the ASPIRATION study (14.1 months in median PFS2, 31.0 months in median OS),22 suggesting that first-line EGFR TKIs therapy plus LATs might provide better outcome in EGFR-mutant NSCLC patients who had oligoprogressive disease. Therefore, continual EGFR TKI as systemic treatment plus additional LAT for progressive sites may be appropriate in EGFR-mutant patients with oligoprogresson in clinical practice.
With the development of TKI that target T790M mutation, the optimal strategy and timing for switching treatment will be an important challenge26 Although LAT seemed to be effective for EGFR-mutant patients with oligoprogression, the criteria of patient selection remained uncertain. To identify the subgroup of patients who can benefit the most along with less toxicities become a controversial topic. In our study, only 11 (5.3%) patients who had T790M mutation received second or third-line osimertinib treatment after re-biopsy, and had achieved a mOS of 41.2 months. The following osimertinib treatment in patient with T790M mutation could be delayed by extending the duration of treatment with EGFR-TKI plus LAT, which contributed to a longer mPFS and mOS. Whether or not asymptomatic oligoprogression requires ongoing local treatment remains controversial. In many retrospective studies, continuing EGFR-TKI therapy in combination with local therapy for isolated CNS disease or non-CNS tumors in selected patients could be beneficial.25, 28-29 In our study, patients with symptomatic oligoprogression had marginally worse PFS2 than those with asymptomatic oligoprogression. Our study suggested that patients should immediately recieved LAT with asymptomatic or symptomatic oligoprogression.
There are several limitations that should be acknowledged. Firstly, this is a single arm retrospective study in a single institutional, which inevitably results in a selection bias. Secondly, this study did not use predefined treatment strategies according to the different progression pattern types. The actual treatment strategy utilized with regard to continuing EGFR TKI treatment plus LAT or switching to a cytotoxic chemotherapy or third-generation EGFR TKI after the RECIST-PD assessment was at the discretion of the physician. Thirdly, re-biopsy was not performed in most patients to investigate possible mechanisms of acquired resistance such as the presence of EGFR T790M mutation, MET amplification, or transformation to small cell lung cancer. Perhaps most importantly, we do not have a comparator to judge the true benefit from EGFR-TKIs continuation treatment or LAT.
This study provided rationale for considering the approach of continuation of first-line EGFR-TKI therapy plus LAT in EGFR-mutant NSCLC patients after oligoprogression. Combination of EGFR TKI plus LAT could provide additional benefits for these patients. Further multicenter, prospective studies are needed to confirm the benefit of continuation of first-line EGFR TKI and LAT after oligoprogression.
Acknowledgments
This study was supported by Shanghai Science and Technology Commission Guiding Project (16411964400) and Shanghai Municipal Health and Family Planning Commission Foundation (201440385).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–1132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Moran T, Queralt C. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 4.Shigematsu H, Lin L, Takahashi T. et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 5.Wu YL, Zhong WZ, Li LY. et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Han JY, Park K, Kim SW. et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Morita S, Yatabe Y. et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M, Inoue A, Kobayashi K. et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Wu YL, Chen G. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positivenon-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label,randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Zhang L, Liu X. et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Yang JC, Yamamoto N. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 13.Lo PC, Dahlberg SE, Nishino M. et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer. 2015;121:2570–2577. doi: 10.1002/cncr.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishie K, Kawaguchi T. et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7:1722–1727. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Yu CJ, Kim SW. et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016;2(3):305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 16.Campo M, Al-Halabi H, Khandekar M. et al. Integration of Stereotactic Body Radiation Therapy With Tyrosine Kinase Inhibitors in Stage IV Oncogene-Driven Lung Cancer. Oncologist. 2016;21(8):964–973. doi: 10.1634/theoncologist.2015-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basler L, Kroeze SG, Guckenberger M. SBRT for oligoprogressive oncogene addicted NSCLC. Lung Cancer. 2017;106:50–57. doi: 10.1016/j.lungcan.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Hoang CD, Kesarwala AH. et al. Role of Local Ablative Therapy in Patients with Oligometastatic and Oligoprogressive Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(2):179–193. doi: 10.1016/j.jtho.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Ren R, Ren S. et al. Peripheral blood for epidermal growth factor receptor mutation detection in non-small cell lung cancer patients. Transl Oncol. 2014;7(3):341–348. doi: 10.1016/j.tranon.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riely GJ, Kris MG, Zhao B. et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13(17):5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Ko J, Cui Z. et al. The EGFR T790M mutation in acquired resistance to an irreversible second-generation EGFR inhibitor. Mol Cancer Ther. 2012;11(3):784–791. doi: 10.1158/1535-7163.MCT-11-0750. [DOI] [PubMed] [Google Scholar]
- 22.Park K, Yu CJ, Kim SW. et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol. 2016;2(3):305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 23.Soria JC, Wu YL, Nakagawa K. et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 24.Weickhardt AJ, Scheier B, Burke JM. et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu HA, Sima CS, Huang J. et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu B, Liang Y, Li Q, Local Therapy for Oligoprogressive Disease in Patients With Advanced Stage Non-small-cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutation. Clin Lung Cancer; 2017. S1525-7304(17)30107-9. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu Y-L, Ahn M-J. et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukuya T, Takahashi T, Naito T. et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer. 2011;74(3):457–461. doi: 10.1016/j.lungcan.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Conforti F, Catania C, Toffalorio F. et al. EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer. 2013;81(3):440–444. doi: 10.1016/j.lungcan.2013.05.019. [DOI] [PubMed] [Google Scholar]