Abstract
Background: The radioresistance of nasopharyngeal carcinoma (NPC) was the main cause of radiotherapy failure and it was still a challenge in the treatment of advanced NPC patients. Previous clinical studies demonstrated that sodium glycididazole(CMNA) can enhance the radiosensitivity of NPC, but the corresponding cellular mechanisms or processes remains largely unclear.
Methods: To clarify the radiosensitizing effects of CMNA on NPC cells and reveal its cellular mechanisms, its effect on cell survival of NPC cells was assessed by MTT and clonogenic assay, with or without radiation. The potential cellular mechanisms such as cell cycle distribution, apoptosis and DNA damage were assessed. A retrospective analysis of the outcome of patients with III-IV stage NPC who undergo same radiochemotherapy with or without concurrent CMNA treatment was performed to elucidate the role of CMNA in the improvement of the curative effects.
Results: The treatment with CMNA at the concentration lower or close to the clinical dosage had little effect on cell survival, cell cycle distribution and a weak effect on DNA damage and cell apoptosis of NPC cells. The combination of CMNA and radiation significantly increased the DNA damage and enhanced the apoptosis of NPC cells, but did not significantly alter the cell cycle distribution as compared with the irradiation (IR) alone. A total of 99 patients who underwent radiochemotherapy were categorized into those with (treatment group, n=52) and without (control group, n=47) the treatment with CMNA. The complete response rates of patients in treatment group were significantly higher than in control group.
Conclusions: Our results suggested that CMNA enhance the sensitivity of the NPC cells to radiation via enhancing DNA damage and promoting cell apoptosis. It provides clues for further investigation of the molecular mechanism of the radiosensitization of CMNA on NPC cells.
Keywords: nasopharyngeal carcinoma, sodium glycididazole, radiotherapy, sensitization
Introduction
Nasopharyngeal carcinoma is a most common malignant cancer in Southeast Asia, which accounts for more than 70% of the cases worldwide in 2012. The majority of nasopharyngeal carcinoma patients are initially diagnosed with locally advanced cancer 1, 2. Radiotherapy is a primary and essential treatment of non-disseminated nasopharyngeal carcinoma. However, due to radioresistance, certain NPC patients exhibit local recurrences and distant metastases within 2 years after treatment 3, 4. Moreover, the radiotherapeutic effect for NPC patients in an advanced stage is not efficient as that in primary stage mostly because of radiation resistance 5-8. How to improve efficacy of treatment for patients with locally advanced nasopharyngeal carcinoma as well as reduce side effects is still one of the research hot spots. Enhancing radiation sensitivity of the tumor cells is one of attractive strategies for achieving the goal, therefore an effective radiotherapy sensitization agent will benefit a large number of patients.
Sodium glycididazole (CMNA) is a new nitroimidazole compound which has a radiation-enhancing effect in vivo and in vitro 9, 10. CMNA exhibited the effect of enhancing radiation in the treatment of patients with various tumors, such as non-small-cell lung cancer, esophageal carcinoma, differentiated thyroid carcinoma, in clinical trial 11-13. CMNA combined chemotherapy or radiotherapy in treating patients with locally advanced nasopharyngeal carcinoma could improve curative effects without increasing adverse reactions, and significantly increase survival rates of the patients 14. But the cellular mechanisms or processes that sodium glycididazole take effect in enhancing radiosensitivity of NPC remain largely unclear.
The most deleterious damage of irradiation is DNA double-strand breaks (DSBs), which induce harmful lesions and causes cell-cycle arrest or cell death 15-18, therefore, most radiation sensitizers target the cellular mechanisms involved in the cell cycle, DNA repair, or apoptosis pathways 19. In this study, a retrospective analysis was performed to demonstrate that CMNA could improve therapeutic effects of patients with locally advanced NPC. Then, we used in vitro cell models of human NPC to validate the effect of sodium glycididazole on radiation of NPC and uncover the cellular mechanisms through which CMNA take effect as a radiation sensitizer.
Methods
Cell culture and reagents
The nasopharyngeal carcinoma cell line 6-10B and HNE2 were obtained from the cell bank of Xiangya School of Medicine(Changsha, China) and were cultured in RPMI 1640 medium (Gibco) with 10% fetal bovine serum (FBS; Gibco), 100U/ml penicillin, and 100mg/ml streptomycin (Gibco), under conditions of 5% CO2 in an incubator at 37°C. Sodium glycididazole (CMNA) was produced by Shandong Luye Pharma Group Ltd (Yantai, China). Sodium glycididazole was dissolved and diluted in RPMI 1640 medium with 10% fetal bovine serum when used.
MTT
Cells were plated at the concentration of 1×103 cells/well in 96-well plates and incubated for 12h. The medium was removed and replaced with or without sodium glycididazole (0-5mmol/L), and the cells were incubated for 96 hs. Next, 20ul of MTT (5mg/ml; Sigma-Aldrich) was added to each well, and cells were incubated for 4 hs at 37°C. The medium containing MTT solution was removed, and adding 150ul DMSO, and the plates were shocked for 10 min at table concentrator. The absorbance was measured at a wavelength of 490nm. All experiments here were repeated three times.
Colony formation assay
Cells were seeded onto six-well dishes and incubated overnight. Cells were treated with sodium glycididazole (1, 3, 5mmol/L) or control for 1 h. The cells were then irradiated at a dose of 0, 2, 4, 6, 8Gy with 6-MV X-rays, 4.0Gy/min. The cell culture medium was washed away and replaced with new medium (10% FBS) after irradiation (IR) for 24 hs. The cells were then cultured in 5% CO2 incubator at 37°C for 7 to 8 days. The colonies were fixed by methanol and stained with crystal violet. The number of colonies containing at least 50 cells was determined.
Cell cycle
Cells were seeded in 6-well plates and treated with sodium glycididazole (3mmol/L) for 1 h before irradiation (4Gy) and were harvested at 24h after irradiation. The cells were fixed in 70% ice-cold ethanol and stored at -4°C overnight. Then, the cells were pelleted, washed, and stained with PI/ ribonuclease staining buffer (BD, 550825) for 15 minutes at room temperature. All experiments were performed at least three times.
Cell apoptosis
6-10B and HNE2 cells were irradiated at a single dose of 4 Gy after treatment with sodium glycididazole (3mmol/L) or cell culture medium for 1h. Cell proteins were extracted after radiation for 48h. Protein content was quantified by the BCA Protein Assay Reagent Kit. The primary antibodies were rabbit anti-c-PARP (1:1000, 5625T, CST) and rabbit anti-caspase 3 (1:800, 19677-1-AP, Proteintech).
γ-H2AX assay
Cells were plated in chamber slides, incubated overnight, and pretreated with sodium glycididazole (3mmol/L) 1 h before irradiation (4Gy). At 5h post-irradiation, the cells were fixed with 2% paraformaldehyde for 15min at room temperature. The fixed cells were permeabilized with 1% Triton-X-100 and subsequently blocked with goat serum blocking solution at room temperature for 30 mins. The cells were then incubated overnight at 4°C with primary monoclonal antibodies against γ-H2AX (1:300, ab22551, Abcam). After primary antibody incubation, the cells were washed with PBST and incubated with secondary antibody conjugated to FITC (1:200) for 1h at room temperature. Nuclei were counterstained with mounting medium DAPI (1 μg/ml). 6-10B and HNE2 cells were irradiated at a single dose of 4 Gy after treatment with sodium glycididazole (3mmol/L) or control for 1h. Cell proteins were extracted after radiation for 5 hs. Protein content was quantified by the BCA Protein Assay Reagent Kit. The primary antibody was mouse anti-γ-H2AX (1:1000, ab22551, Abcam). For quantitative analysis, ≥100 cells were chosen at random and nuclei counted manually to determine the percentage positive for γ-H2AX (having ≥5 discrete foci/nucleus). Results were averaged from 3 biological replicates.
Retrospective Analysis
In order to evaluate the impact of CMNA on the curative effects of radiotherapy, we retrospectively enrolled the patients with locally advanced NPC who underwent radiochemotherapy at Xiangya hospital of Central South University between February 2017 to May 2018. The inclusion criteria of the study were as follows: (1) patients with NPC aged less than 70 years. (2) pathologically diagnosed with undifferentiated carcinoma, differentiated squamous cell carcinoma. (3) patients with locally advanced nasopharyngeal carcinoma who were in clinical stage III or IV. (4) patients received the same radiochemotherapy regimen. In the present study, all patients received 70.4 Gy of radiation dose and were treated with cis-platinum from d1 to d3. Chemotherapy was started from the first week of radiotherapy and repeated every three weeks. Exclusion criteria: diagnosed with other malignant or metastatic NPC. A total of 99 patients who met abovementioned criteria were categorized into the control or the treatment group. For the treatment group, the patients were given CMNA 800mg/m2 before radiotherapy. Response was classified according to according to the Response Evaluation Criteria in Solid Tumors (RECIST) 20. The study was approved by the Institutional Review Board of Xiangya hospital of Central South University.
Statistical analysis
The measurement data are presented as the mean ± standard deviation and the significance of difference between mean values was determined by Student's t-test. The counting data were compared with Wilcoxon rank sum test. A p value < 0.05 was considered statistically significant.
Results
Effect of CMNA alone or combined with radiation on cell growth
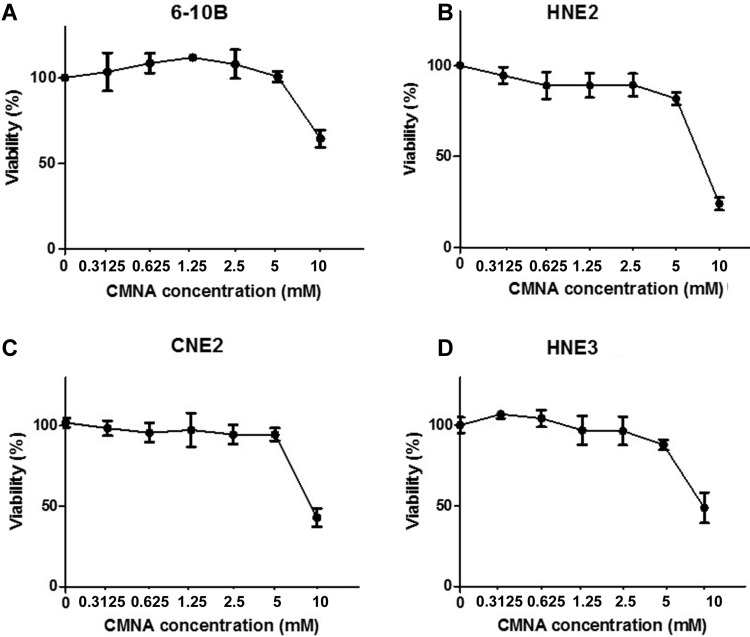
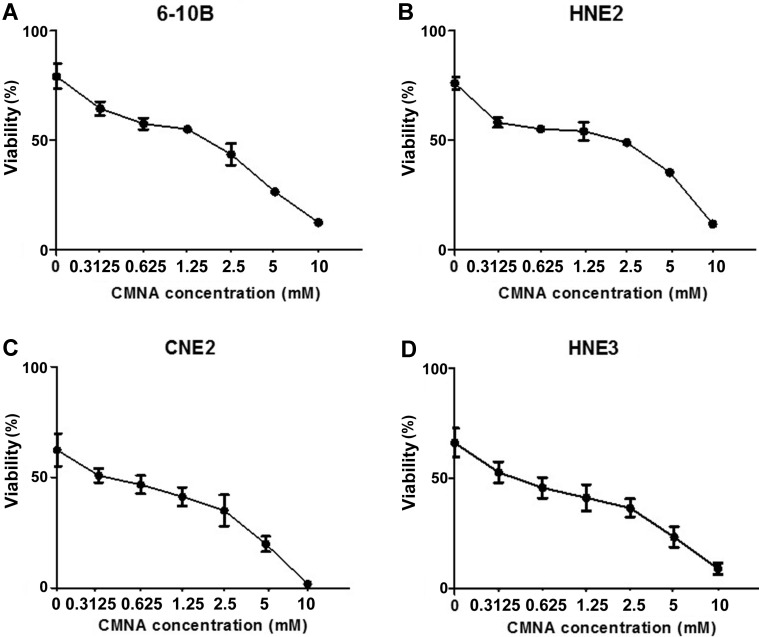
To evaluate the impact of CMNA on growth of NPC cells, MTT assay was performed to determine the toxicity of CMNA on 6-10B, HNE2, CNE2 and HNE3 cells. As shown in Figure 1, CMNA showed low-toxicity effect on 6-10B, HNE2, CNE2 and HNE3 cells at the concentration of less than 5mM. To determine the effect of CMNA on the radiosensitivity of NPC cells, we pre-treated the four NPC cell lines with vehicle (control) or various concentration of CMNA for one hour, and then exposed cells to 4 Gy dose of irradiation. The cell viability was evaluated by MTT assay. As shown in Figure 2, the viability of the cells decreased as the concentration of CMNA increased, which demonstrated that CMNA could increase the sensitivity of cells to radiation. It was found that the cell viability of CNE2 and HNE3 was reduced by nearly half, while the cell viability of 6-10b and HNE2 was reduced by only 10-20% after exposed to radiation when no CNMA was used. It demonstrated that CNE2 and HNE3 are more sensitive to radiation than 6-10B and HNE2. Therefore, the cell lines 6-10B and HNE2 were selected for follow-up experiments as they were more suitable for discovery and proof-of-concept studies because of being more resistant to radiation.
Figure 1.
Effect of CMNA on the viability of 6-10B, HNE2, CNE2 and HNE3 cells. The viability of the cells at 96h post treatment with the indicated concentrations of CMNA were evaluated by MTT assay (n=3).
Figure 2.
CMNA enhances the radiosensitivity of the NPC cells. After pre-treated with vehicle or various concentrations of CMNA (0.3125, 0.625, 1.25, 2.5, 5,10 mmol/L) and then irradiated at the dose of 4Gy, the viability was tested by MTT assay.
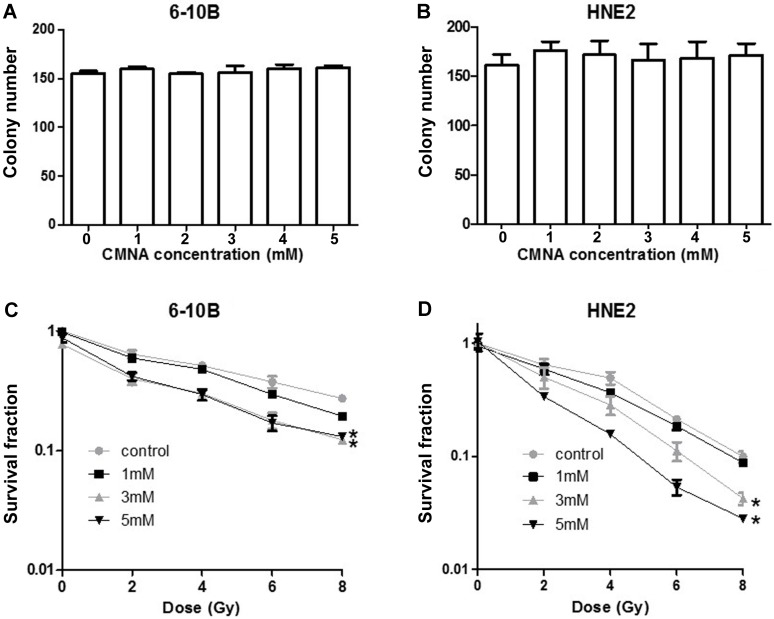
To further evaluate the long-term toxicity of CMNA, the effect of various concentrations of CMNA on clonogenic ability of 6-10B and HNE2 cells was detected. As seen in Figure 3a and 3b the colony number was comparable in various concentrations, which demonstrated that CNMA have no apparent inhibitory effect on colony-forming ability of 6-10B and HNE2.
Figure 3.
Effect of CMNA alone or combined radiation on cell clonogenic ability. The clonogenic ability of 6-10B(a) and HNE2(b) cells at 24h post treatment with vehicle or various concentrations (1, 2, 3, 4, 5mmol/L) of CMNA (n=3). After pre-treated with vehicle or various concentrations of CMNA (1, 3, 5mmol/L), and then irradiated with a series of doses, the survival fraction of 6-10B and HNE2 cells at 7 days post initial treatment were determined by clonogenic assay. (n=3, * P<0.05 for CMNA at concentration (3 or 5mM) as compared to control)
CMNA enhances the radiosensitivity of 6-10B and HNE2 cell
To further determine the effect of CMNA on the radiosensitivity of NPC cells, we pre-treated 6-10B and HNE2 cells with various concentration of CMNA for 1h, and then exposed cells to different doses of irradiation. Clonogenic survival assay was performed to assess the radiosensitizing effect of CMNA. As shown in Figure 3c and 3d, the addition of CMNA further reduced the survival fraction at various dose of radiation and increase the inhibitory effects of radiation. In addition, along with the increasing of drug concentrations the inhibitory effects were more significant, which suggested that CMNA can enhance the radiosensitivity of NPC cells and the effect was dose dependent.
According to the above result, the optimized CMNA concentration of 3 mM and the propriate dose of irradiation of 4Gy were determined to investigate the cellular mechanisms behind the effect of CMNA on radiosensitivity of NPC cells in the following experiments.
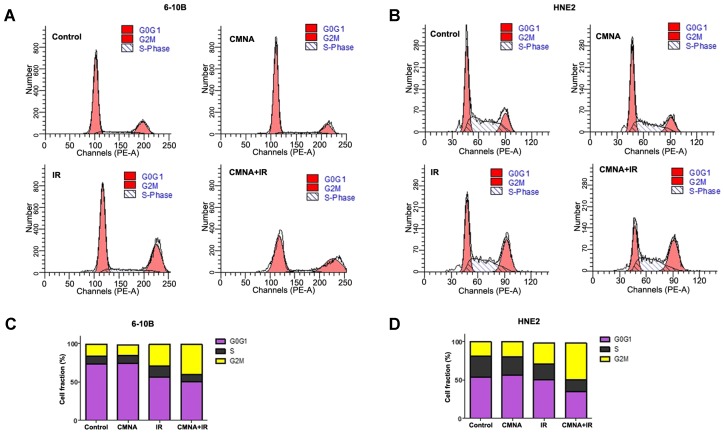
CMNA regulates the cell cycle in 6-10B and HNE2 cells
To clarify whether CMNA-mediated radiosensitization was due to its impact on the cell cycle redistribution, the cell cycle of 6-10B and HNE2 after irradiation with or without the CMNA pre-treatment were analyzed by flow cytometry. As shown in Figure 3, after treated with CMNA alone for 24 hs, the cell cycle distribution in both examined cell lines did not markedly change as compared to the controls. Cells were treated with CMNA for 1 h and then expose to radiation, the cell cycle analysis after 24 hs demonstrated the percentage of cells at G2/M phase significantly increased after radiation, which was accompanied by a significantly decreased G0/G1 phase compared to the controls (p<0.05). In both cell lines, however, the percentage of G2/M-phase cells had no significant alteration between the irradiation with or without CMNA treatment (p>0.05).
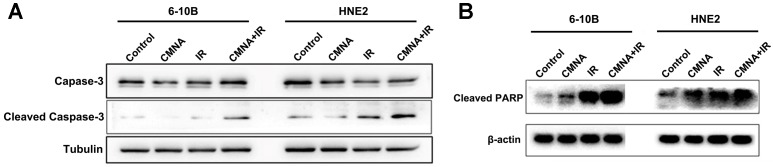
Radiotherapy combined CMNA promotes apoptosis
We next investigated whether CMNA enhanced radiosensitivity of NPC cells was associated with the increase of apoptosis induced by irradiation. Cells were exposed to 3mM CMNA for 1h, and then irradiated with a dose of 4 Gy. Expression of apoptosis markers, active caspase-3 and cleaved forms of PARP, were assessed in 6-10B and HNE2 cells at 48 hs after treatment. As shown in Figure 4, irradiation increased the expression of the apoptosis marker activated caspase-3 as well as cleaved PARP, whereas CMNA pre-treatment resulted in greater expression of activated caspase-3 and cleaved forms of PARP induced by irradiation in both 6-10B and HNE2 cells (Figure 4). The result suggested that CMNA could enhance the apoptosis induced by irradiation.
Figure 4.
Cell cycle distribution after irradiation is not altered by CMNA. (a, b) Propidium iodide staining and flow-cytometry analysis 24 hs after the treatment in 6-10B and HNE2 cells. (c, d) The percentage of cells in G0/G1, S, and G2/M phase of the cell cycle before and after radiation (4 Gy) with and without pretreatment with CMNA for 1 h.
CMNA regulates DNA repair processes in 6-10B and HNE2 cell
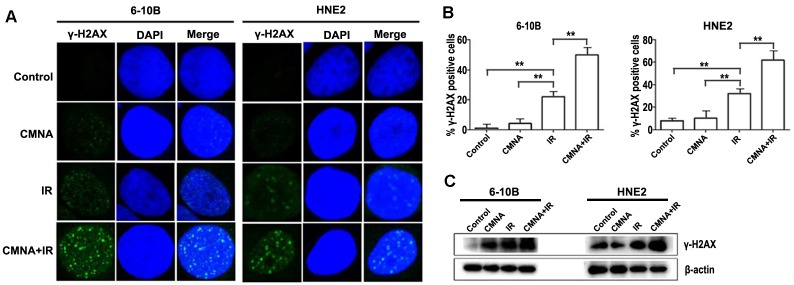
DNA damage caused by radiation play a key role in radiotherapy. Theoretically, what can amplify DNA damage induced by radiation could increase the radiosensitivity. Whether could CMNA enlarge the extent of DNA damage induced by radiation? Previous study reported that the nuclear foci of γ-H2AX is one of the markers for evaluating the level of DNA damage 21. To address the question, the formation of γ-H2AX foci in cell nuclei was evaluated. Cells were treated with or without CMNA for 1 h prior to irradiation (4 Gy) and fed with CMNA-free medium, and the average number of foci per cell was measured at 5h. As shown in Figure 5, the results demonstrated that exposure of NPC cells to CMNA result in no marked alteration in the number of γ-H2AX foci as compared to the control, while exposure to either radiation or the combination of radiation and CMNA lead to a significant increase of γ-H2AX foci as compared to the control. More importantly, the combination of CMNA and radiation resulted in a greater number of g-H2AX foci than either CMNA or irradiation alone.
Figure 5.
Cell apoptosis after irradiation with or without pretreatment with CMNA. Western blots show that sodium glycididazole pretreatment increases the expression of the apoptosis markers (cleaved forms of PARP and caspase3) induced by irradiation.
We further assessed the level of phosphorylated histone H2AX (γ-H2AX), which forms foci at DNA double strand breaks (DSB) and recruits DSB repair proteins. As shown in Figure 5c, radiation induced γ-H2AX formation in both NPC cell lines, and the addition of CMNA further increase the level of γ-H2AX. These results demonstrated that CMNA could enlarge the extent of DNA damage induced by radiation.
Patient characteristics
According to pathological diagnosis, there were 99 patients (66 male and 33 female) with locally advanced (T1-4 N0-3, III-IV) NPC. 47 patients were diagnosed with clinical stage III, 52 patients with stage IV. All patients were categorized into treatment group (radiochemotherapy plus CMNA) and control group (radiochemotherapy). Clinical characteristics of two groups were shown in Table 1, there was no statistically significant difference between two groups (p>0.05).
Table 1.
Characteristics of patients
| Treatment group (n=52) | Control group (n=47) | p value | |
|---|---|---|---|
| Gender | 0.811 | ||
| M | 34 | 32 | |
| F | 18 | 15 | |
| Age | 0.781 | ||
| <50 | 32(61.5%) | 27(57.4%) | |
| >50 | 20(38.5%) | 20(42.6%) | |
| Pathological type | 0.084 | ||
| Undifferentiated carcinoma | 37(71.1%) | 45(95.7%) | |
| Keratinizing carcinoma | 10(19.2%) | 0(0%) | |
| Non-keratinizing carcinoma | 5(9.7%) | 2(5.3%) | |
| T stage | 0.946 | ||
| T1 | 2(3.8%) | 3(6.4%) | |
| T2 | 6(11.5%) | 7(14.9%) | |
| T3 | 25(48.1%) | 20(42.6%) | |
| T4 | 19(36.5%) | 17(36.2%) | |
| N stage | 0.431 | ||
| N0 | 2(3.8%) | 5(10.6%) | |
| N1 | 15(28.8%) | 15(31.9%) | |
| N2 | 26(50.0%) | 15(31.9%) | |
| N3 | 9(17.4%) | 12(25.5%) | |
| Clinical stage | 0.422 | ||
| III | 27(51.9%) | 20(42.6%) | |
| IV | 25(48.1%) | 27(57.4%) |
Response rate
Evaluated by CT on NPC, (CR+PR) in two groups were 96.2% and 82.9% when the radiation dose reached 70.4 Gy. Complete response rate (CR) of treatment group was higher than control group, and the difference was statistically significant (p<0.05, Table 2).
Table 2.
A comparison on response rate between treatment and control group
| Prognosis | Treatment group(n=52) | Control group(n=47) | p value |
|---|---|---|---|
| CR rate | 17(32.7%) | 2(4.2%) | 0.020 |
| PR rate | 33(63.5%) | 37(78.7%) | |
| SD rate | 2(3.8%) | 8(17.1%) |
*CR=complete response, PR=partial response, SD=stable disease
Discussion
Although NPC is highly radiosensitive and radiotherapy is the primary and only curative treatment for nasopharyngeal carcinoma, radioresistance inevitably occurred in many cases 22, 23. Researchers are trying to find new substances that will make tumors more sensitive to radiation without affecting normal tissues 24. Previous study showed that sodium glycididazole has enhancing effect on radiosensitivity of nasopharyngeal carcinoma cells 14. A clinical trial also demonstrated that CMNA enhanced the sensitivity to radiation therapy and improved survival rates with minimal side effects in nasopharyngeal carcinoma 18. CMNA enhancing effect on radiosensitivity has also been observed in other tumors including non-small-cell lung cancer, laryngeal cancer and esophageal carcinoma 11, 12, 25. Retrospective analysis also demonstrated that CMNA could increase the CR ratio and improved the curative effect of radiotherapy on NPC. It is unambiguous that CMNA has radiation-enhancing effect. However, the underlying mechanisms that CMNA enhance the radiosensitivity of tumor cells remained largely unknown, although it has been reported that CMNA enhances the radiosensitivity of laryngeal cancer cells through downregulation of ATM signaling pathway, which may regulate the DNA repair of radiation response 25.
The present study demonstrated that the treatment of NPC cells with CMNA alone had no significant effect on tumor cell proliferation, while it can enhance the radiosensitivity of the NPC cell lines. The 6-10B and HNE2 cells treated with the combination of CMNA and irradiation were shown significant growth inhibition. In order to clarify the underlying mechanism that CMNA enhance the radiosensitivity of NPC cells, we firstly determined whether CMNA enhances the radiosensitivity of NPC through redistributing cell cycle growth phases. We observed that CMNA alone had no significant effect on the cell cycle in NPC cells. Intriguingly, CMNA combined with ionizing radiation did not induced a significantly higher G2/M arrest in 6-10B and HNE2 cells compared with radiation alone. The result indicated that CMNA alone did not redistribute the cell cycle of NPC cells. Moreover, the cell cycle distribution between irradiated NPC cells with and without CMNA treatment had no significant difference. This phenomenon was also found in the study about another potential radiation sensitizer 26. Accordingly, we speculated that it was not through redistributing the cell cycle that CMNA enhances the radiosensitivity of NPC.
Radiation therapy achieves its therapeutic effects by inducing apoptosis or non-apoptotic cell death 27. Therefore, we want to know whether CMNA enhances the radiosensitivity of NPC through promoting apoptosis of NPC cells. To answer this question, western blotting assay were performed on irradiated NPC cells that were exposed to CMNA to evaluate the expression of apoptotic markers. We observed that CMNA alone was not able to induce apoptosis of cancer cells. However, the combination of CMNA and radiation led to more apoptosis of NPC cells than radiation alone. Consistent with the present result, earlier study also demonstrated that CMNA increased apoptosis induced by irradiation in laryngeal cancer cells 25.
Agents that increase the extent of DNA damage or that inhibit DNA double strand break repair often sensitize tumor cells to irradiation 28. To further investigate the effects of CMNA treatment on DNA damage and repair, an immunofluorescent staining assay were performed to detect gamma-H2AX levels and measure DNA double strand breaks at 5 hs. As indicated in Fig. 6, it suggested that CMNA alone caused no change in gamma-H2AX levels, but radiation or the combination of CMNA and radiation did. It was worth noting that the cells treated with the combination CMNA and irradiation had higher γ-H2AX levels than those treated with radiation alone. Indeed, previous study demonstrated that the CMNA can regulate the ATM pathway, which is key pathway that mediates the radiation-induced DNA damage and repair 25.
Figure 6.
CMNA regulates DNA repair processes in 6-10B and HNE2 cell. (a). Irradiation with 4GY of ionizing radiation and pretreatment with CMNA (3mmol/L) induces γ-H2AX foci formation in both NPC cells. γ-H2AX foci per cell increases 5h after radiation and is further enhanced by pretreatment with CMNA in 6-10B and HNE2. (b). Mean percent of cells with ≥ 5 γ-H2AX foci, ±SEM; data were combined from three experiments. **p < 0.01. (c). Western blots of γ-H2AX show that irradiation increases the DNA damage (the level of γ-H2AX), which is further enhanced by irradiation combined sodium glycididazole.
The data presented here showed that the pretreatment of irradiated NPC cells with CMNA resulted in a dose-dependent induction of clonogenic cell death. Our results demonstrate that CMNA can enhance the radiosensitivity of NPC via promote apoptosis and impairing DNA damage and repair. These findings provided clues for further revealing the molecular mechanisms behind radiosensitization of CMNA and optimization of irradiation treatment strategy of NPC.
Acknowledgments
This work was supported by Natural Science Foundation of China [81372904], and Natural Science Foundation of Hunan Province of China [2015JJ2163].
References
- 1.Ouyang P Y, Su Z, Ma X H. et al. Comparison of TNM staging systems for nasopharyngeal carcinoma, and proposal of a new staging system. Br J Cancer. 2013;109(12):2987–97. doi: 10.1038/bjc.2013.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L, Tang M, Hu Z. et al. miR-504 mediated down-regulation of nuclear respiratory factor 1 leads to radio-resistance in nasopharyngeal carcinoma. Oncotarget. 2015;6(18):15995–6018. doi: 10.18632/oncotarget.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sarraf M, Reddy M S. Nasopharyngeal carcinoma. Curr Treat Options Oncol. 2002;3(1):21–32. doi: 10.1007/s11864-002-0038-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Tang M, Li H. et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett. 2016;380(1):191–200. doi: 10.1016/j.canlet.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Chan A T, Gregoire V, Lefebvre J L. et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii83–5. doi: 10.1093/annonc/mds266. [DOI] [PubMed] [Google Scholar]
- 6.Chan A T, Felip E. Nasopharyngeal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):123–5. doi: 10.1093/annonc/mdp150. [DOI] [PubMed] [Google Scholar]
- 7.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85(2):74. [PubMed] [Google Scholar]
- 8.Zhao Y, Shen L, Huang X. et al. High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2017;494(1-2):390–396. doi: 10.1016/j.bbrc.2017.09.118. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y C, Wu R, Xu Z G. et al. Safety and radiation-enhancing effect of sodium glycididazole in locoregionally advanced laryngeal cancers previously treated with platinum-containing chemotherapy regimens: A preliminary report. Cancer Radiother. 2010;14(1):59–64. doi: 10.1016/j.canrad.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Gao J, Zhang H. et al. Radiosensitizing efficiency of sodium glycididazole on v(79) cells in vitro. J Radiat Res Radiat Proc. 1995;13(4):213–218. [Google Scholar]
- 11.Yang J, Liu M Z, Cai L. et al. [Phase II clinical trial of sodium glyci-didazole (CM-Na) combined with concurrent radiochemotherapy for advanced esophageal carcinoma] Ai Zheng. 2008;27(6):622–6. [PubMed] [Google Scholar]
- 12.Zhang Q, Wang D Q, Wu Y F. Sodium glycididazole enhances the efficacy of combined iodine-125 seed implantation and chemotherapy in patients with non small-cell lung cancer. Oncol Lett. 2015;9(5):2335–2340. doi: 10.3892/ol.2015.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen Q, Ma Q, Bai L. et al. Glycididazole sodium combined with radioiodine therapy for patients with differentiated thyroid carcinoma (DTC) Int J Clin Exp Med. 2015;8(8):14095–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Li M Y, Liu J Q, Chen D P. et al. Glycididazole sodium combined with radiochemotherapy for locally advanced nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15(6):2641–6. doi: 10.7314/apjcp.2014.15.6.2641. [DOI] [PubMed] [Google Scholar]
- 15.Huang S M, Harari P M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6(6):2166–74. [PubMed] [Google Scholar]
- 16.Rastogi R P, Richa, Kumar A. et al. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson S P, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun S, Jung Y S, Suh H N. et al. LIG4 mediates Wnt signalling-induced radioresistance. Nat Commun. 2016;7:10994. doi: 10.1038/ncomms10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citrin D E, Mitchell J B. Altering the response to radiation: sensitizers and protectors. Semin Oncol. 2014;41(6):848–59. doi: 10.1053/j.seminoncol.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer E A, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson D O, Sekiguchi J M, Frank K M. et al. The interplay between nonhomologous end-joining and cell cycle checkpoint factors in development, genomic stability, and tumorigenesis. Cold Spring Harb Symp Quant Biol. 2000;65:395–403. doi: 10.1101/sqb.2000.65.395. [DOI] [PubMed] [Google Scholar]
- 22.Chua M, Wee J, Hui E P. et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Yang L, Li J J. et al. Potential use of nucleic acid-based agents in the sensitization of nasopharyngeal carcinoma to radiotherapy. Cancer Lett. 2012;323(1):1–10. doi: 10.1016/j.canlet.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Begg A C, Stewart F A, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Y C, Xing R, Zeng J. et al. Sodium glycididazole enhances the radiosensitivity of laryngeal cancer cells through downregulation of ATM signaling pathway. Tumour Biol. 2016;37(5):5869–78. doi: 10.1007/s13277-015-4278-1. [DOI] [PubMed] [Google Scholar]
- 26.Gordon I K, Graves C, Kil W J. et al. Radiosensitization by the novel DNA intercalating agent vosaroxin. Radiat Oncol. 2012;7:26. doi: 10.1186/1748-717X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balcer-Kubiczek E K. Apoptosis in radiation therapy: a double-edged sword. Exp Oncol. 2012;34(3):277–85. [PubMed] [Google Scholar]
- 28.Thoms J, Bristow R G. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol. 2010;20(4):217–22. doi: 10.1016/j.semradonc.2010.06.003. [DOI] [PubMed] [Google Scholar]