Abstract
Objectives
To detect systolic dysfunction in heart failure with preserved ejection fraction (HFpEF) patients by using global longitudinal strain (GLS).
Methods
This study included 46 heart failure patients: 24 with heart failure with reduced ejection fraction (HFrEF) and 22 with heart failure with preserved ejection fraction (HFpEF), and 20 patients with similar risk factor but no symptoms or signs of heart failure, matched for age and sex, as controls. All patients were screened by echocardiography. The ejection fraction of left ventricle was measured using Simpson’s method and the GLS of the left ventricle was measured by using two-dimensional speckle tracking.
Results
Left ventricular ejection fraction (LVEF) was 61.90 ± 2.94% in the controls, 60.45 ± 7.4% in the HFpEF group (p = 0.421), and 32.75 ± 8.45% in the HFrEF group (p = 0.001). The value of left ventricle (LV) GLS (controls = −19.74 ± 1.12%, HFpEF = −15.03 ± 2.03%, HFrEF = −10.72 ± 1.99%, p = 0.0001) was significantly impaired in the HFpEF group despite normal LVEF.
Conclusion
There is significant left ventricular systolic impairment detected by GLS despite preserved LVEF.
Keywords: Global longitudinal strain, Heart failure, Preserved ejection fraction
Abbreviations
- AFI
Automated function image
- AUC
Area under the curve
- BNP
B type natriuretic peptide
- GLS
Global longitudinal strain
- HF
Heart failure
- HFmrEF
Heart failure with midrange ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- LA
Left atrium
- LAVI
Left atrial volume index
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- LVMI
Left ventricular mass index
- LVOT
Left ventricular outflow tract
- MI
Myocardial infarction
- NT-proBNP
N-terminal-proB type-natriuretic peptide
- ROC
Receiver operating characteristics
- TDI
Tissue Doppler image
1. Introduction
Heart failure (HF) is a clinical syndrome caused by a functional and/or structural cardiac abnormality that results in a reduction of cardiac output and/or elevation in cardiac filling pressure. This syndrome is characterized by typical symptoms (e.g., fatigue, shortness of breath, and ankle swelling) and may be accompanied by signs (e.g., elevated jugular venous pressure, pulmonary crackles, and leg edema) [1].
The prevalence of HF in the United States is derived from the National Health and Nutrition Examination Survey (NHANES). It is estimated that 5.1 million Americans from age 20 years and older have heart failure.
The European Society of Cardiology in their guidelines published in 2016 recommended the following conditions should be fulfilled for the diagnosis of heart failure with preserved ejection fraction (HFpEF) [2]: (1) the presence of symptoms and/or signs of HF; (2) a ‘preserved’ ejection fraction [(EF) defined as LVEF ≥ 50% or 40–49% for heart failure with midrange ejection fraction (HFmrEF); (3) elevated levels of natriuretic peptides [(NPs),B type natriuretic peptide (BNP). 35 pg/mL and/or N-terminal-proB type-natriuretic peptide (NT-proBNP) 125 pg/mL); and (4) objective evidence of other cardiac functional and structural alterations underlying HF.
Because of the characteristics of noninvasiveness and availability, echocardiography plays a major role in the diagnosis of left ventricle (LV) diastolic dysfunction in patients with HFpEF. There are several echocardiographic parameters, such as Doppler study of mitral inflow pattern, Doppler study of pulmonary veins inflow, and tissue Doppler, that can be used to help in the diagnosis of diastolic dysfunction; however, none of these are diagnostic. It is recommended that the presence of multiple abnormalities is strongly suggestive of diastolic dysfunction. Tissue Doppler Image (TDI) has proven to be more accurate than other parameters for detecting impaired diastolic function in HFpEF patients. Key functional alterations are an Early diastolic flow velocity/Diastolic flow velocity measured by tissue Doppler (E/e′) ≥13 and a mean e′ septal and lateral wall, 9 cm/s [3], [4], [5], [6].
Speckle tracking echocardiography measures the myocardial deformation (strain) and is superior to myocardial velocities in assessment of LV systolic function because it is angle independent and easy to calculate compared with tissue Doppler counterpart. Global longitudinal strain (GLS) is calculated from the mean of 18 cardiac segments obtained from apical four-chamber, three-chamber and two-chamber views [7], normal value of GLS from –18% to –21.5% [8].
2. Materials and methods
2.1. Study population
In this study 66 patients were enrolled for evaluation of ejection fraction and GLS between January 2016 and December 2016. All participants attended Al-Najaf Cardiac Center, Najaf, Iraq for evaluation of chest pain, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and/or leg edema. Framingham criteria were used for clinical diagnosis of heart failure. Validated congestive heart failure is considered if two major or one major and two minor criteria are present concurrently. Major criteria include: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, rales, third heart sound (s3), cardiomegaly, or pulmonary edema. Minor criteria include: peripheral edema, night cough, dyspnea on exertion, hepatomegaly, pleural effusion, heart rate > 120/min, weight loss ≥ 4.5 kg in 5 days with diuretic. Study population were classified into three groups according to clinical and echocardiographic findings: (1) Group 1: 24 patients with overt heart failure (fulfilled Framingham criteria) with LVEF < 40% (HFrEF); (2) Group 2: 22 patients with symptoms and or signs of heart failure (fulfilled Framingham criteria) with EF > 50% and left atrial volume index (LAVI) 34 mL/m2 or a left ventricular mass index (LVMI) ≥ 115 g/m2 for males and ≥ 95 g/m2 for females and/or E/e′ ≥13 [according to European Society of Cardiology (ESC) criteria], and a mean e′ septal and lateral wall 9 cm/s (HFpEF). Diagnostic criteria for this group did not include measurement of BNP, because assessment of natriuretic peptides is not routinely done in clinical practice [2]; and (3) Group 3: 20 patients with risk factor for heart failure who presented with atypical chest pain or mild dyspnea who did not have specific symptoms and signs of heart failure and echocardiography showed preserved LVEF > 50% with E/e < 8 with no structural abnormality (considered as control group).
2.2. Echocardiographic measures
All patients were examined at the Al-Najaf Cardiac Center by a Vivid E9 GE healthcare echocardiographic machine using a 3.5 MHz probe; quantitative measure of LVEF, LV dimension, and LA dimension were performed according to the recommendation of American Echocardiographic Society [8]. All patients were examined in the left lateral position. LVEF was determined using Simpson’s method by measuring LV volume in systole and diastole obtained from apical four- and two-chamber views. LV diastolic function was measured by using Pulse-waved Doppler inflow examination for the assessment of peak early flow velocity (E) and deceleration time, the sample volume was placed perpendicular on the tip of mitral valve. Tissue Doppler Imaging (TDI) of mitral annulus was measured from apical four chamber view, sample volume was placed 1 cm at the septal and lateral annulus of mitral valve, early diastolic peak E′ was measured at septal and lateral annulus of mitral valve. The E/e′ ratio was measured from the average of the septal and lateral E′. Two-dimensional tissue speckle tracking images were acquired in the apical four-chamber, apical three-chamber, and apical two-chamber long axis views at a frame rate of 40–90 frames/s.
2.3. Echocardiographic analysis
The analysis of data was done by the same cardiologist using Automated Functional Images (AFI) software from a vivid E9 GE Healthcare echocardiographic machine (GE Healthcare, USA). The endocardium was tracked algorithmically throughout the cardiac cycle in a single frame. The area of interest was manually adjusted to ensure that all layers of myocardium are involved. The subendocardium and trabeculae of the left ventricle are not included. By using AFI software the value of longitudinal strain derived from the apical three-chamber view, apical two-chamber view, and apical four-chamber view were automatically calculated and the average of the three was regarded as left ventricular GLS. If two segments were not visualized, AFI was considered not valid.
2.4. Ethical considerations
Formal approval was obtained from the committee of the Iraqi Board of Medical Specializations, and legal agreements were obtained from related offices. Informed consent was obtained from participants after clear explanation of the purpose of the study and the type of data required, and respondents were assured of data confidentiality and anonymity.
2.5. Statistical analysis
SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA) was used for performing statistical analysis. Continuous data are presented as mean ± standard deviation and qualitative data are presented as n (%). Comparison of study groups were carried out using Student t test. Pearson product–moment correlation coefficient was also used to compute the relationship between continuous variables. A p value < 0.05 was considered statistically significant. Power calculation for estimation of sample size was used, it was calculated that analysis of variance (ANOVA) with three different groups, given the power of 0.9 and effect size of 0.5 with a significance level of 0.05 the sample size would be 17.91, therefore, 20 participants were studied in each of the three study groups (total of 60 participants).
3. Results
This research included 66 patients: 22 with HFpEF, 24 with HFrEF, and 20 controls. All patients had undergone echocardiography, but six patients were excluded (2 with HFpEF, 4 with HFrEF) due to inadequate images for reliable AFI analysis. Characteristics of the patients are represented in Table 1.
Table 1.
Demographic description and echo findings of the study subjects. The p value was obtained using comparison between HFpEF group and control group.
| Variable | HFrEF (n = 20) | HFpEF (n = 20) | Control (n = 20) | p |
|---|---|---|---|---|
| Age (y) | 61 ± 5 | 60 ± 7 | 56 ± 4 | 0.068 |
| Sex, female | 5 (25) | 8 (60) | 9 (45) | 0.749 |
| Body mass index (kg/m2) | 26.10 ± 3.78 | 27.41 ± 4.14 | 25.90 ± 3.21 | 0.204 |
| Diabetes mellitus | 14 (70) | 6 (30) | 12 (60) | 0.057 |
| Hypertension | 18 (90) | 12 (60) | 17 (85) | 0.077 |
| Systolic blood pressure (mmHg) | 154.00 ± 12.10 | 149.40 ± 15.25 | 145.50 ± 10.12 | 0.347 |
| Diastolic blood pressure (mmHg) | 97.25 ± 10.70 | 94.35 ± 10.21 | 88.25 ± 6.54 | 0.030 |
| LVEF (%) | 32.75 ± 8.45 | 60.45 ± 7.40 | 61.90 ± 2.94 | 0.421 |
| LV mass index (g/m2) | 102.25 ± 20.21 | 115.85 ± 11.08 | 92.40 ± 4.44 | <0.001 |
| LA volume index (mL/m2) | 34.85 ± 3.94 | 35.80 ± 3.19 | 29.00 ± 2.25 | <0.001 |
| LA parasternal (mm) | 39.24 ± 12.73 | 42.75 ± 4.10 | 20.75 ± 17.72 | <0.001 |
| E/E‘ | 13.42 ± 1.92 | 13.87 ± 1.13 | 6.74 ± 1.08 | <0.001 |
| GLS (%) | −10.72 ± 1.99 | −15.03 ± 2.03 | −19.74 ± 1.12 | <0.001 |
Data are presented as mean ± standard deviation or n (%).
GLS = global longitudinal strain; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LA = left atrium; LV = left ventricle; LVEF = left ventricular ejection fraction.
Comparison between Group 1 (HFrEF) and Group 2 (HFpEF) regarding the echocardiographic finding have shown that there was significant difference in LVEF and LV mass index but there was no significant difference in LA volume index and LA dimension and E/e′ ratio (Table 2).
Table 2.
Comparison of echo findings and GLS value between HFrEF and HFpEF group.
| Variable | HFrEF (n = 20) | HFpEF (n = 20) | p |
|---|---|---|---|
| LVEF (%) | 32.75 ± 8.45 | 60.45 ± 7.40 | <0.001 |
| LV mass index (g/m2) | 102.25 ± 20.21 | 115.85 ± 11.08 | 0.012 |
| LA volume index (mL/m2) | 34.85 ± 3.94 | 35.80 ± 3.19 | 0.407 |
| LA parasternal (mm) | 39.24 ± 12.73 | 42.75 ± 4.10 | 0.248 |
| E/E′ | 13.42 ± 1.92 | 13.87 ± 1.13 | 0.378 |
| GLS (%) | −10.72 ± 1.99 | −15.03 ± 2.03 | <0.001 |
Data are presented as mean ± standard deviation.
GLS = global longitudinal strain; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LA = left atrium; LV = left ventricle; LVEF = left ventricular ejection fraction.
The comparison of echocardiographic change between Group 2 (HFpEF) and control Group 3 have shown no significant difference in LVEF but there was significant difference in LA volume index, LA dimension, and E/e′ ratio (Table 1).
Regarding GLS value there was significant difference between Group 1 (HFrEF) and Group 2 (HFpEF) despite normal LVEF, however the reduction was severe in Group 1 (Table 2), whereas study of GLS value in Group 2 (HFpEF) and Group 3 (control) revealed significant impairment of GLS in Group 2 in comparison with control Group 3 despite normal LVEF (Table 1).
A Pearson product–moment correlation coefficient was computed to assess the relationship between the LVEF and GLS value in both HFpEF group and control group. There was a negative correlation between the two variables in HFpEF (r = −0.69, n = 20, p = 0.001). A scatter diagram summarizes the results (Fig. 1).
Figure 1.
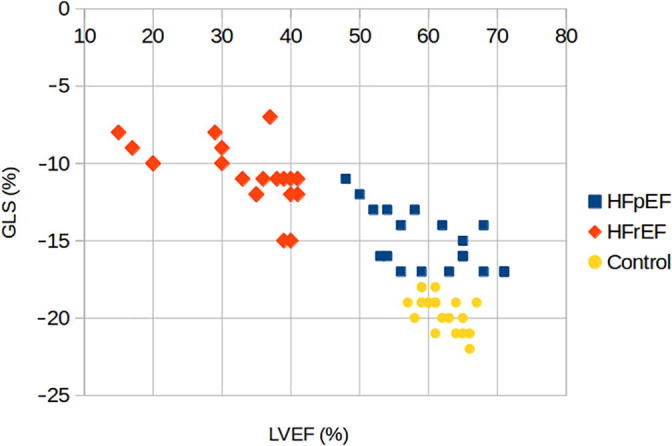
Scatter diagram showing the relationship between LVEF and GLS value in HFpEF group, HFrEF group, and Control group. GLS = global longitudinal strain; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction.
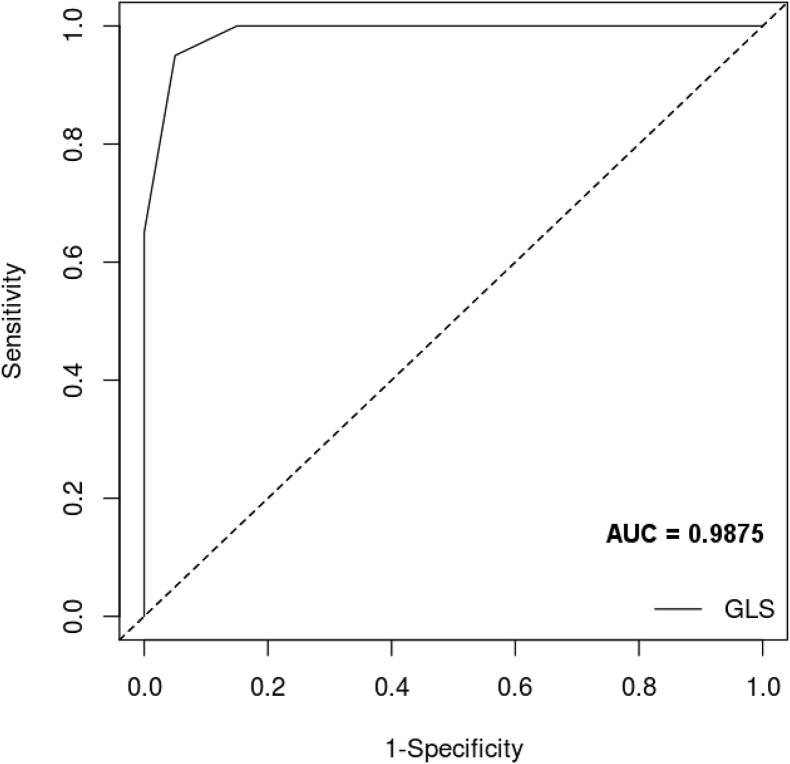
There was also a negative correlation between the two variables in the control group (r = −0.55, n = 20, p = 0.011) summarized in the scatter diagram in Fig. 1. Receiver operating characteristics (ROC) curve analysis was performed, with area under the curve (AUC) of 0.9875 (Fig. 2).
Figure 2.
Receiver operating characteristics curve analysis for the accuracy of GLS in differentiating HFpEF group from control group. AUC = area under curve; GLS = global longitudinal strain; HFpEF = heart failure with preserved ejection fraction.
4. Discussion
In this study it was observed that the patients with HFpEF have LV systolic dysfunction detected by GLS. GLS decrement in patients with HFpEF probably caused by regional decline in longitudinal strain of left ventricle. By using GLS of the left ventricle HFpEF can be distinguished from the control. AFI is good and accurate for measuring of GLS of the left ventricle with a higher diagnostic value than other echocardiographic parameters such as LVEF and E/E′ ratio in assessment of patients with HFpEF and in differentiation between patients with HFpEF from controls [7], [9].
It is controversial whether impairment in the systolic function can be detected in patients with HFpEF. Several studies have found a reduction in global or regional systolic peak velocities in patients with HFpEF [10], [11].
Yip et al. [12] demonstrate that there was LV systolic dysfunction measured by TDI of mitral annular peak velocity and amplitude in patients with HFpEF and LV hypertrophy.
Wang et al. [11] found a reduction in LV longitudinal and radial strain in patients with HFpEF. Although there is a significant difference in the GLS between patients with HFpEF and normal control groups, the control group were younger than HFpEF and did not have any risk factors such as ischemic heart disease (IHD), diabetes mellitus, and hypertension which could result in reduction in the value of GLS between the group [11]. It could not be ascertained that the reduction in the GLS was due to heart failure, aging, or from other concomitant risk factor. In this study the GLS was measured by speckle tracking echocardiography and calculated using AFI in patients with HFpEF and compared with age- and sex-matched control group.
There was no difference in LVEF between the HFpEF group and control group but there was significant difference in the GLS value between these groups i.e., higher in the control group.
Based on (single syndrome) hypothesis of heart failure [13], [14] HFpEF and HFrEF are regarded to be different phenotypes of the same disease, thus patients with HFpEF presented with regional dysfunction in the function of longitudinal axis and diastolic dysfunction [15].
Patients with HFpEF are usually old, obese, and have multiple comorbidity i.e., hypertension, diabetes mellitus, ischemic heart disease. These diseases can result in micro- and macrovascular dysfunction as well as muscle fibrosis.
Because the endocardium of the left ventricle is more sensitive to the harmful effect of ischemia or hypertrophy, the decreased LV longitudinal function can be detected early by using GLS [11], [16].
All patients with HFpEF have a reduction in the GLS value of the left ventricle. This indicates that impairment of LV systolic function can be detected by AFI in those with HFpEF whether ischemia is present or not, the longitudinal fiber of the subendocardial layer in position making them more liable for ischemia, ventricular hypertrophy, and any abnormality in contraction and relaxation [17], [18].
In this study all layers of myometrium were assessed by AFI for measuring LV GLS, so that despite subendocardium ischemia and hypoperfusion that play a role in systolic dysfunction of the left ventricle, the other mechanisms are not yet recognized [15], [19]. The exact mechanism requires further investigation.
The study has some limitations. Measurement of BNP or pro-BNP were not performed for the study participants and diagnosis was based on clinical history, physical examination, electrocardiogram, and echocardiography. ESC guidelines suggest measuring circulating NP levels for patients, however, if NP level was not available; it is indicated to perform echocardiography for the patient.
5. Conclusion
GLS can be considered a significant measure for diagnosis of left ventricular systolic dysfunction in patients with symptoms of heart failure with normal LVEF.
Acknowledgments
We would like to express our thanks to Dr. Mustafa Wahhudi for his assistance in performing statistical analysis of this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Naqvi T.Z. Echocardiography in heart failure. In: St. John Sutton M., Wiegers S., editors. Distinguishing systolic versus diastolic heart failure: a practical approach by echocardiography. Elsevier; Amsterdam: 2012. pp. 1–34. [Google Scholar]
- 2.European Society of Cardiology ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Ghio S. Role of echo Doppler techniques in the evaluation and treatment of heart failure patients. Eur Heart J Suppl. 2006;8(Suppl E):E28–E31. [Google Scholar]
- 4.Oh J.K. Echocardiography in heart failure: Beyond diagnosis. Eur J Echocardiogr. 2007;8:4. doi: 10.1016/j.euje.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Paulus W.J., Tschope C., Sanderson J.E., Rusconi C., Flachskampf F.A., Rademalers F.E. How to diagnose diastolic heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 6.Tschope C., Kasner M., Westermann D., Gaub R., Poller W.C., Schultheiss H.P. The role of NT-proBNP in the diagnosis of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J. 2005;26:2277. doi: 10.1093/eurheartj/ehi406. [DOI] [PubMed] [Google Scholar]
- 7.Hill J.C., Palma R.A. Doppler tissue imaging for the assessment of left ventricular diastolic function: a systemic approach for the sonographer. J Am Soc Echocardiogr. 2005;18:80. doi: 10.1016/j.echo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Cardiology/European Association of Cardiovascular Imaging Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Kasner M., Westermann D., Steendijk P., Gaub R., Wilkenshoff U., Weitmann K. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 10.Yu C.M., Lin H., Yang H., Kong S.L., Zhang Q., Lee S.W. Progression of systolic abnormalities in patients with ‘‘isolated’’ diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Khoury D.S., Yue Y., Torre-Amione G., Nagueh S.F. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 12.Yip G., Wang M., Zhang Y., Fang J.W., Ho P.Y., Sanderson J.E. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Keulenaer G.W., Brutsaert D.L. Diastolic heart failure: a separate disease or selection bias? Prog Cardiovasc Dis. 2007;49:275. doi: 10.1016/j.pcad.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.De Keulenaer G.W., Brutsaert D.L. Systolic and diastolic heart failure: different phenotypes of the same disease? Eur J Heart Fail. 2007;9:136. doi: 10.1016/j.ejheart.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Belghiti H., Brette S., Lafitte S., Reant P., Ricard F., Serri K. Automated function imaging: a new operator-independent strain method for assessing left ventricular function. Arch Cardiovasc Dis. 2008;101:163. doi: 10.1016/s1875-2136(08)71798-4. [DOI] [PubMed] [Google Scholar]
- 16.Martinez D.A., Guhl D.J., Stanley W.C., Vailas A.C. Extracellular matrix maturation in the left ventricle of normal and diabetic swine. Diabetes Res Clin Pract. 2003;59:1. doi: 10.1016/s0168-8227(02)00178-x. [DOI] [PubMed] [Google Scholar]
- 17.Streeter D.D., Jr, Vaishnav R.N., Patel D.J., Spotnitz H.M., Ross J., Jr, Sonnenblick E.H. Stress distribution in the canine left ventricle during diastole and systole. Biophys J. 1970;10:345. doi: 10.1016/S0006-3495(70)86306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marwick T.H., Leano R.L., Brown J., Sun J.P., Hoffmann R., Lysyansky P. Myocardial strain measurement with two-dimensional speckle tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Henein M.Y., Gibson D.G. Long axis function in disease. Heart. 1999;81:229. doi: 10.1136/hrt.81.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]