Abstract
Bacillus Calmette–Guérin (BCG) is widely used in the clinic to effectively treat superficial urinary bladder cancer. However, a significant proportion of patients who fail to respond to BCG risk cystectomy or death. Though more than 3 million cancer treatments with BCG occur annually, surprisingly little is known about the initial signaling cascades activated by BCG. Here, we report that BCG induces a rapid intracellular Ca2+ (calcium ion) signal in bladder cancer cells that is essential for activating the transcription factor nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) and for synthesizing and secreting proinflammatory cytokines, including interleukin 8 (IL‐8). A similar Ca2+ response was observed when cells were exposed to the supernatant of BCG. Studying cellular molecular mechanisms involved in the BCG signaling event, we found pivotal roles for phospholipase C and the Toll‐like receptor 4. Further assessment revealed that this signaling pathway induces synthesis of IL‐8, whereas exocytosis appeared to be controlled by global Ca2+ signaling. These results shed new light on the molecular mechanisms underlying BCG treatment of bladder cancer, which can help in improving therapeutic efficacy and reducing adverse side effects.
Keywords: BCG, calcium signaling, TLR4, urinary bladder cancer
Abbreviations
- bBCGsn
boiled BCGsn
- BCG
Bacillus Calmette–Guérin
- BCGsn
Supernatant of BCG
- Ca2+
calcium ion
- ER
endoplasmic reticulum
- fBCGsn
frozen BCGsn
- GPCR
G protein‐coupled receptor
- IL
interleukin
- InsP3
inositol 1,4,5‐trisphosphate
- InsP3R
inositol 1,4,5‐trisphosphate receptor
- MSD
Meso Scale Discovery
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- PI3K
phosphatidylinositol 3‐kinase
- PLC
phospholipase C
- ppBCGsn
protein‐precipitated BCGsn
- qRT‐PCR
quantitative RT‐PCR
- TLR4
toll‐like receptor 4
1. Introduction
Bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, is arguably the most effective therapy for high‐risk non‐muscle‐invasive urinary bladder cancer, although it was originally developed with the intention to produce a vaccine against tuberculosis (Kassouf and Kamat, 2004; Liu et al., 2009; Morales et al., 1976). Bladder cancer is the fourth most commonly diagnosed cancer in men and tenth most common cancer in women in the United States (Siegel et al., 2017). Approximately 75% of bladder cancers do not invade the smooth muscle (Kaufman et al., 2009; Simons et al., 2008) and are considered non‐muscle‐invasive bladder cancers. The standard treatment for non‐muscle‐invasive bladder cancer is transurethral resection of the bladder tumor, followed by intravesical therapy with BCG (Chang et al., 2016). However, a significant proportion of patients fail to respond to BCG therapy; their tumors are refractory or relapse and may become invasive or metastatic (Kaufman et al., 2009).
Since the first report of intravesical use of BCG, there have been strong efforts to understand the pharmacology and toxicology of this treatment, which have hitherto been elusive. BCG is a mixture containing viable microorganisms, bacterial fragments, intracellular content, and soluble secreted compounds. It is currently accepted that the antitumor activity of BCG is derived by a local non‐specific immunological boost that recruits immunocompetent cells (Redelman‐Sidi et al., 2014). Several studies report that BCG instillation results in a significant cell‐mediated response in the bladder, characterized by secretion of cytokines and recruitment of immune cells (Redelman‐Sidi et al., 2014). However, the exact sequence of events from the BCG instillation to tumor eradication has been only partially addressed, which has limited the development of new BCG derivatives with enhanced clinical efficacy and fewer adverse side effects.
Calcium (Ca2+) signaling has been reported to regulate cytokine release and tumor growth (Berridge et al., 1998; Monteith et al., 2017; Roderick and Cook, 2008; Uhlen et al., 2000). Through the concert of actions between Ca2+ channels and pumps, intracellular signals can be shaped in infinite ways (Uhlen and Fritz, 2010). Release of Ca2+ from intracellular endoplasmic reticulum (ER) Ca2+ stores mainly occurs through inositol 1,4,5‐trisphosphate (InsP3) receptors (InsP3Rs), which are activated when phosphatidylinositol 4,5‐bisphosphate is cleaved by phospholipase C (PLC) into InsP3 and diacylglycerol. The subsequent elevation of the cytosolic Ca2+ concentration can activate downstream effectors that control numerous biological processes in cells (Mengel et al., 2010).
2. Methods
2.1. Cell cultures
Human bladder tumors were collected from patients undergoing transurethral resection in the Urology Unit of Karolinska University Hospital. Primary cell cultures were prepared as previously described (Rahman et al., 1987). Briefly, tissue was finely minced, enzymatically digested, pipetted to disperse clumps, washed in phosphate‐buffered saline, and cultured in a specialized medium. All experiments were ethically approved (Dnr 2011/421‐31/1), and written consent was obtained from all participants. The study methodologies conformed to the standards set by the Declaration of Helsinki.
The urinary bladder cancer cell lines T24 (human), RT4 (human), and MB49 (mouse) were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and were occasionally tested for mycoplasma (last tested in 2015). Cells were propagated according to their instructions, and all experiments were performed using cells between passage numbers 3 and 20.
2.2. BCG treatment
Bacillus Calmette–Guérin (2 × 108–3 × 109 cfu, Medac, Chicago, IL, USA) RIVM‐derived strain (1173‐P2) was resuspended in 5 mL of the cell line‐specific culture medium. The BCG mixture was then diluted 10 times to the final concentration of 4 × 106–6 × 107 cfu·mL−1 and treated to the cells for the indicated time period. To fractionate the BCG, it was centrifuged at 3000 g for 30 min to separate a BCG pellet and BCG supernatant (BCGsn). The BCGsn was frozen (fBCGsn) at ‐20 °C overnight, boiled (bBCGsn) at 100 °C for 1 h, or treated with acetone to precipitate protein (ppBCGsn). Proteins within ppBCGsn were separated into mass fractions: > 100, > 50, > 30, > 10, and > 3 kDa (Amicon tubes, Millipore, St Charles, MO, USA).
2.3. Reagents
The following reagents were used: cyclopiazonic acid (CPA, 50 μm, Tocris Bioscience, Bristol, UK); 2‐aminoethoxydiphenylborane (2APB, 100 μm, Tocris); U73122 (2–4 μm, Tocris, Abingdon, UK); U73343 (2–4 μm, Tocris); Edelfosine (ET‐18‐OCH3, 2–4 μm, Tocris); Wortmannin (1 μm, Tocris); Pertussis toxin (PTX, 1 μg·mL−1, Tocris); and 6‐(phenylsulfinyl)tetrazolo[1,5‐b]pyridazine (Ro 106‐9920, 0.01–100 μm, Tocris), Xestospongin D (Xesto, 5 μm, Tocris).
2.4. Calcium imaging
Cells were loaded with the Ca2+‐sensitive fluorescence indicator Fluo‐3/AM (5 μm, Invitrogen, Carlsbad, CA, USA) in Krebs‐Ringer solution (119 mm NaCl, 2.5 mm KCl, 2.5 mm CaCl2, 1.3 mm MgCl2, 1.0 mm NaH2PO4, 20.0 mm HEPES (pH 7.4), and 11.0 mm dextrose) at 37 °C with 5% CO2 for 30 min prior to experiments. The Ca2+ imaging was conducted at 37 °C in a heat‐controlled chamber (QE‐1, Warner Instruments, New Haven, CT, USA) with a confocal microscope Zeiss LSM510NLO META (Carl Zeiss, Jena, Germany) equipped with a 20x/0.8NA dipping lens (Carl Zeiss). Excitation was set at 488 nm and emission was detected at 510 nm. The sampling frequency was set to 0.1 Hz. carl zeiss software (Carl Zeiss) was used to analyze the acquired images. Experiments were performed in a Krebs‐Ringer buffer and all drugs were bath‐applied.
2.5. Cytokine measurements
The level of secreted cytokines in response to BCG was measured with a Meso Scale Discovery (MSD) MULTI‐SPOT Assay System (Meso Scale Discovery, Rockville, MD, USA). Cytokines were analyzed in the supernatants of human primary bladder cancer cultures by the Human ProInflammatory 9‐Plex Ultra‐Sensitive Kit and in mouse MB49 cells by the Mouse ProInflammatory 9‐Plex Ultra‐Sensitive Kit, following instructions from the manufacturer. Controls for standard curves were included with each plate. Data are presented as the means ± standard error of the mean of a minimum of four experiments.
2.6. Small interfering RNA
siRNA against PLCβ3 and PLCγ (Dharmacon, Lafayette, CO, USA) were used to knock down proteins. T24 cells were seeded in 25‐mm plates to 60% confluency, and siRNA (100 μm) was transfected into cells with Lipofectamine 2000 (Invitrogen) in Opti‐MEM (Invitrogen), according to the manufacturers’ instructions. The knockdown efficiency of siRNA was confirmed by western blotting (Fig. S1A,B).
2.7. Western blotting
Cells were lysed in a modified RIPA buffer for 20 min at 4 °C, and equal amounts of protein were separated on a 10% sodium dodecyl sulfate gel electrophoresis, followed by transfer to a PVDF membrane. The membranes were blocked in 5% milk or bovine serum albumin in TRIS‐buffered saline solution with 0.5% Tween‐20 for 1 h before incubation with primary antibodies (PLCβ3, 1 : 1000; PLCγ, 1 : 1000; β‐actin, 1 : 1000, Abcam, Cambridge, UK) overnight at 4 °C and further incubation with secondary antibodies (1 : 5000) for 1 h. Bands were detected with a chemiluminescence kit (Amersham Biosciences, GE Healthcare UK Limited, Bucks, UK) and an imaging system (Bio‐Rad, Hercules, CA, USA).
2.8. Luciferase reporter assays
The activity of nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) was determined with a NF‐κB‐firefly‐luciferase reporter and CMV‐Renilla‐luciferase reporter constructs (Promega, Madison, WI, USA). MB49 cells were seeded at 1–2 × 104 cells per well in 96‐well culture plates. On the following day, cells were co‐transfected with a firefly‐luciferase reporter construct with an interleukin 8 (IL‐8) promoter (or five copies of NF‐κB response element; 0.1 μg·well−1) and pCMV‐Renilla‐luciferase (0.005 μg·well−1) using Lipofectamine and PLUS reagents, according to the manufacturer's protocol (Invitrogen). Three hours after transfection, the medium was removed and replaced with complete Dulbecco's modified Eagle's medium for 24 h. The cells were preincubated with inhibitors for 24 h and then stimulated with BCG for 18 h. Then, cells were washed once with phosphate‐buffered saline and lysed in 25 μL Tropix lysis solution per well. Luciferase activity was determined with a Dual Luciferase Reporter Gene Assay (Promega) and a Wallac Victor2 1420 Multilabel Counter according to the manufacturer's instructions (Wallac, Gaithersburg, MD, USA). Renilla‐luciferase activity was analyzed to verify the reproducibility between quadruplicate transfections in all experiments.
2.9. Lentiviral vector production and in vitro transduction
Short hairpin RNA (shRNA) targeting the mouse Toll‐like receptor 4 (TLR4) mRNA, and a non‐related sequence (Control) were cloned into the lentiviral vector pLL3.7‐mRuby (Rubinson et al., 2003). The shRNA sequences were sh1TLR4: 5′‐ GCATAGAGGTAGTTCCTAATA ‐3′, sh2TLR4: 5′‐CTTCACTACAGAGACTTTA‐3′, and shControl: 5′‐TTCTCCGAACGTGTCACGT‐3′. Lentivirus was produced by co‐transfecting the packaging vector pΔ8.91 and the envelope vector pCMV‐VSVg with the pLL3.7.mRuby2 into HEK293FT cells (Invitrogen). The resulting supernatant was harvested after 60 h, concentrated by ultracentrifugation (95 min at 103.864 g in a Beckman JS‐24.38 rotor) and resuspended in 100 μL of RPMI 1640 medium. MB49 cells were transduced and selected by cell sorting (BD FACS Aria II cell sorter) targeting the mRuby reporter. Downregulation of TLR4 was confirmed by quantitative RT‐PCR (qRT‐PCR; Fig. S1C). Briefly, total RNA from transduced MB49 cells was isolated with the RNeasy Mini Kit (QIAGEN, Hilden, Germany) and reverse transcribed with the Superscript II Kit (Invitrogen). qRT‐PCR assays were performed with the SYBR® green PCR master mix (Applied Biosystems, Foster City, CA, USA) in an ABI PRISM 7900HT sequence detection system (Applied Biosystems). The expression of the target mRNA was normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). The primers used were mouse TLR4 forward: 5′‐CACTGTTCTTCTCCTGCCTGAC‐3′, mouse TLR4 reverse: 5′‐CCTGGGGAAAAACTCTGGATAG‐3′, mouse GAPDH forward: 5′‐TGACCTCAACTACATGGTCTACA‐3′, and mouse GAPDH reverse: 5′‐ CTTCCCATTCTCGGCCTTG‐3′. sh2TLR4 was selected for further experiments.
2.10. Statistical analysis
The Ca2+ recording data were normalized and cells were considered responsive to a treatment if the mean fluorescence was increased by at least 50% over the baseline. All the data are presented as the means ± SEM. Unless otherwise stated, at least three biological repeats were performed for all of the cell culture experiment. Groups were compared by Student's two‐tailed unpaired t‐test or one‐way ANOVA, with a P‐value < 0.05 as the limit for statistical significance.
3. Results
3.1. BCG elevates the cytosolic Ca2+ concentration
To test the influence of BCG on Ca2+ homeostasis in urinary bladder cancer cells, we collected tumor samples from patients treated with transurethral resection. We prepared primary cultures of human bladder cancer cells and loaded them with Fluo‐3/AM to monitor the cytosolic Ca2+ concentration with time‐lapse fluorescence microscopy. First, the basal Ca2+ level was recorded for ~ 5 min, and then, a clinically relevant preparation of BCG (4 × 106–6 × 107 cfu·mL−1) was applied to the cells. A rapid cytosolic Ca2+ increase was observed when primary bladder cancer cells were exposed to BCG (Fig. 1A). Next, we assayed the Ca2+ response to BCG in T24 cells, a human cell line derived from poorly differentiated (grade III) bladder carcinoma (Bubenik et al., 1973), RT4 cells, a human cell line derived from a grade I urothelial carcinoma (Franks and Rigby, 1975), and MB49 cells, a murine model of bladder cancer (Summerhayes and Franks, 1979). BCG triggered Ca2+ responses in all these cell types (Figs 1B, S2A,B, and Movie S1). Interestingly, the Ca2+ responses induced by BCG showed oscillatory behaviors. To determine whether the rise in cytosolic Ca2+ was due to influx from the extracellular milieu, we repeated the experiment in a Ca2+‐free medium. Eliminating extracellular Ca2+ had no apparent effect on the BCG‐induced Ca2+ response (Fig. 1C,D), suggesting that the major Ca2+ source was intracellular stores.
Figure 1.
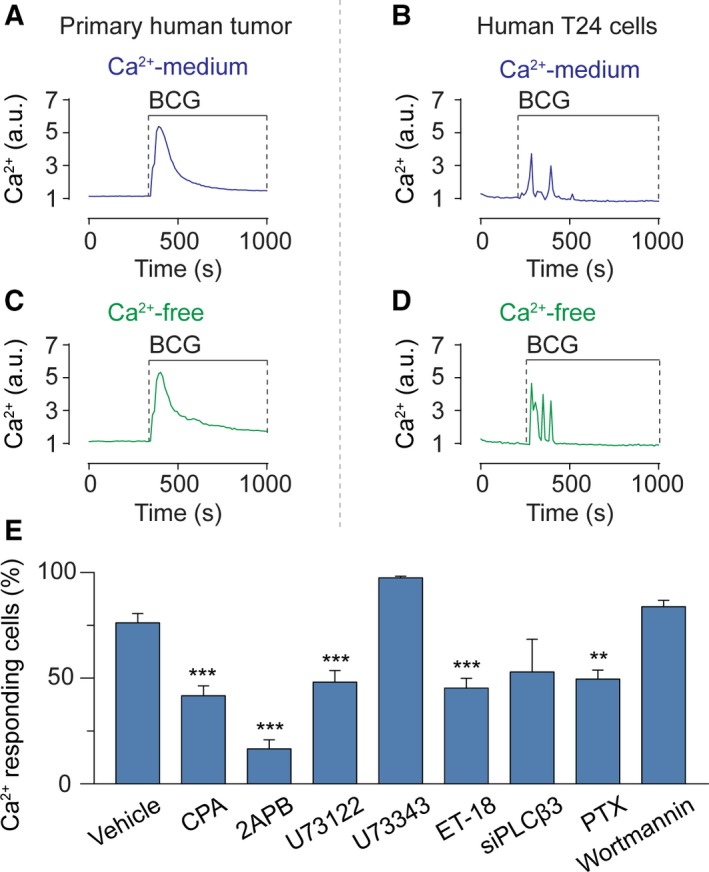
BCG evokes intracellular Ca2+ signaling in bladder cancer cells. Primary human bladder cancer cells (A) and human T24 cells (B) exposed to BCG (4 × 106–6 × 107 cfu·mL−1) in a Ca2+‐containing buffer exhibit Ca2+ signaling. Primary human bladder cancer cells (C) and human T24 cells (D) exposed to BCG (4 × 106–6 × 107 cfu·mL−1) in a Ca2+‐free buffer also exhibit Ca2+ signaling. (E) The number of human T24 cells responding to BCG with Ca2+ signaling was significantly reduced by the inhibitors CPA, 2APB, U73122, ET‐18‐OCH3 (ET‐18), and PTX, whereas wortmannin failed to significantly reduce the number of active cells. Results are means ± SEM of measurements from at least three separate cell cultures. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t‐test).
The intracellular Ca2+ signaling pathway triggered by BCG was further scrutinized in human T24 cells, of which 76.2 ± 4.4% (n = 2231, N = 21) showed a Ca2+ response when exposed to BCG (Fig. 1E). First, internal ER Ca2+ stores were depleted by blocking the SERCA pump with CPA, which abolished the BCG‐induced Ca2+ signal (Figs 1E and S3A). Then, we inhibited InsP3Rs with 2APB or Xestospongin D, which abolished the response (Figs 1E and S3B,C). Additionally, blocking PLC with U73122 or ET‐18‐OCH3 completely eliminated the BCG‐induced response (Figs 1E and S3D). U73343, the inactive analog of U73122, had no effect (Figs 1E and S3E).
We next applied RNA silencing (siRNA) to pinpoint which isoform of PLC was involved in the signaling event. siRNA‐based knockdown of PLCβ3 inhibited the BCG‐induced Ca2+ response (Fig. S3F), whereas siRNA knockdown of PLCγ did not (Fig. S3G). Since G protein‐coupled receptors (GPCRs) and receptor tyrosine kinases interact with PLC, we then tested whether they played a role in BCG‐induced Ca2+ signaling by treating cells with PTX and the phosphatidylinositol 3‐kinase (PI3K) inhibitor wortmannin. PTX abolished the Ca2+ response (Figs 1E and S3H), while wortmannin did not (Figs 1E and S3I). The wortmannin experiment demonstrated that PI3K signaling was not required for the Ca2+ mobilization. Together, these data suggest that BCG triggers an intracellular Ca2+ response that is mediated by the release of Ca2+ from internal ER stores through a mechanism dependent on PLCβ3 and GPCRs.
3.2. BCG‐induced cytokine release is Ca2+ dependent
We next sought out to test whether BCG‐induced cytokine release (Redelman‐Sidi et al., 2014) in bladder cancer cells was dependent on Ca2+ signaling. We decided to carry out these experiments on primary cultures of bladder cancer cells and MB49 cells, which is a widely used murine model of bladder cancer that is also sensitive to immunotherapy (Gunther et al., 1999; Kobayashi et al., 2015). Culturing primary human bladder cancer cells from a male tumor and a female tumor, we found that BCG induces release of a wide range of cytokines (Fig. 2A,B). In particular, IL‐6 and IL‐8 were released at significant levels in primary human bladder cancer cells as well as in mouse MB49 cells (Fig. 2C). BCG also induced a strong release of IFN‐γ, TNF‐α, IL‐1β, IL‐10, and IL‐12p70 in human bladder cancer cells. To determine whether the cytokine production was dependent on Ca2+ signaling, we measured IL‐8 release in MB49 cells pretreated with the inhibitors 2APB or U73122. Both drugs significantly hampered the BCG‐induced secretion of IL‐8 (Fig. 3A). Since NF‐κB is known to be regulated by Ca2+ signaling (Smedler and Uhlen, 2014) and to modulate IL‐8 (Karin et al., 2002), we then examined whether NF‐κB activation was induced by BCG. We determined that BCG induced a time‐dependent transcriptional activation of NF‐κB that leveled off after 6 h (Fig. 3B). This activation was indeed Ca2+ dependent, as 2APB and U73122 were able to hinder NF‐κB transcription induced by BCG (Fig. 3C).
Figure 2.
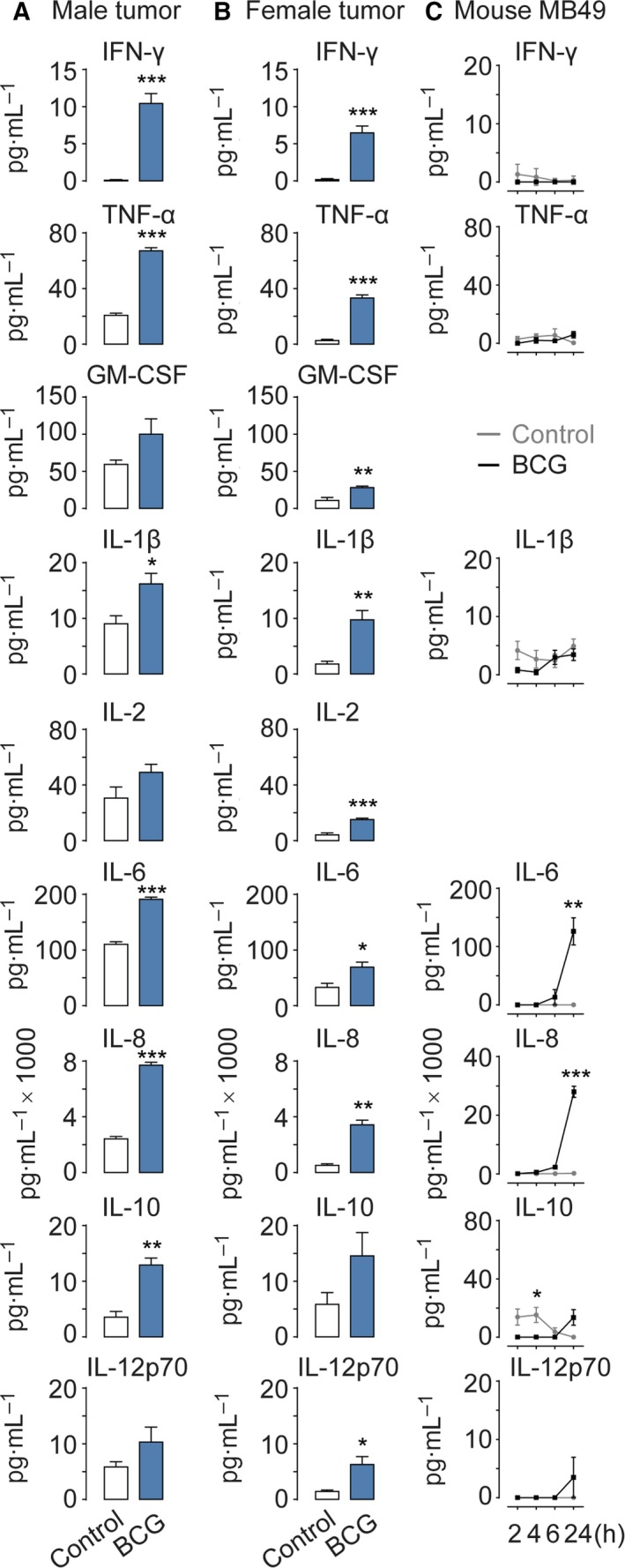
BCG triggers cytokine release in bladder cancer cells. Primary human bladder cancer cells derived from one male tumor (A) and one female tumor (B) or mouse MB49 cells (C) exposed to BCG (4 × 106–6 × 107 cfu·mL−1) secrete multiple cytokines, as compared to a control group. Results are means ± SEM of measurements from four separate cell cultures. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t‐test)
Figure 3.
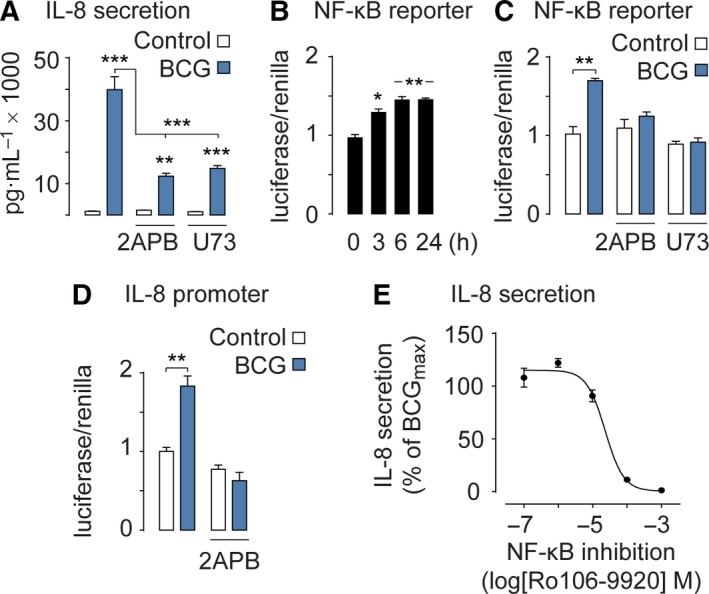
BCG activates NF‐κB and IL‐8. (A) BCG‐stimulated IL‐8 secretion is reduced when Ca2+ signaling is inhibited by 2APB or U73122 (U73). (B) NF‐κB reporter gene assay shows that NF‐κB is fully activated after 6 h of BCG treatment. (C) NF‐κB reporter gene assay shows that the NF‐κB activation is reduced when Ca2+ signaling is inhibited by 2APB or U73122 (U73). (D) BCG‐stimulated IL‐8 transcription is blocked when Ca2+ signaling is inhibited by 2APB. (E) BCG‐stimulated IL‐8 secretion levels are NF‐κB dependent as the inhibitor Ro106‐9920 hampers IL‐8 secretion in a dose‐dependent manner. Results are means ± SEM of measurements from at least three separate cell cultures. *P < 0.05, **P < 0.01, ***P < 0.001 (one‐way ANOVA)
Next, we analyzed the IL‐8 promoter activity. Inhibiting the intracellular Ca2+ release with 2APB abrogated IL‐8 transcription induced by BCG (Fig. 3D). To test whether the secretion of IL‐8 was dependent on NF‐κB activation, we gradually inhibited NF‐κB by varying the concentration of the NF‐κB inhibitor Ro106‐9920 together with a constant maximum dose of BCG. This experiment revealed a positive correlation between the NF‐κB activation and IL‐8 secretion (Fig. 3E).
These findings indicate that BCG‐evoked cytokine secretion is dependent on Ca2+ signaling and subsequent NF‐κB transcription.
3.3. The supernatant of BCG and TLR4 are activating IL‐8
The BCG mixture used in the clinic contains viable microorganisms, bacterial fragments, intracellular content, and soluble secreted compounds. We decided to screen for key bioactive compounds within the BCG mixture responsible for triggering the Ca2+ signaling response. First, we prepared a BCGsn. To our surprise, exposing cells to the BCGsn resulted in a similar Ca2+ response to that observed with the entire BCG mixture (Fig. 4A). In contrast, exposing cells to the BCG pellet failed to elicit Ca2+ signaling (Fig. 4B). To test whether the response required enzymatic activity we prepared fBCGsn and to test whether it was protein dependent, we prepared bBCGsn and protein‐precipitated BCGsn (ppBCGsn). All three preparations were found to evoke Ca2+ responses in bladder cancer cells (Fig. 4C,E). Next, we fractionated ppBCGsn according to the following molecular weights: > 100, > 50, > 30, > 10, and > 3 kDa. All fractions except > 100 kDa triggered Ca2+ responses (Fig. 4F), suggesting that the bioactive compound(s) triggering the Ca2+ response was lighter than 100 kDa. Indeed, when performing a polyacrylamide gel electrophoresis of the different BCG fractions, a majority of the bands detected were lighter than 100 kDa (Fig. S4).
Figure 4.
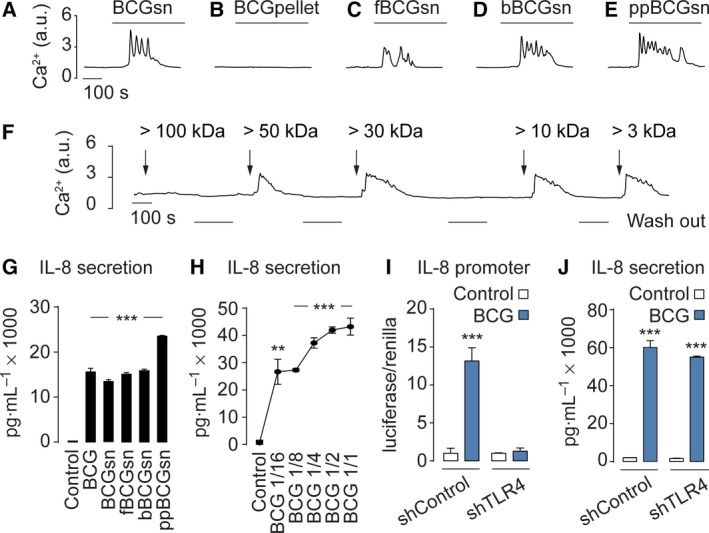
The BCGsn and TLR4 are key for BCG‐induced Ca2+ signaling. Assessment of Ca2+ signaling in mouse MB49 cells exposed to BCG preparations BCGsn (A), BCG pellet (B), fBCGsn (C), bBCGsn (D), ppBCGsn (E), or various weight fractions (F). (G) The BCG preparations that triggers Ca2+ signaling also stimulate IL‐8 secretion. (H) BCG at dilutions of 1/16, 1/8, 1/4, 1/2, and 1/1 activate IL‐8 in a dose‐dependent manner. TLR4 knockdown with shRNA (shTLR4) abolishes BCG‐induced IL‐8 transcription (I) but not IL‐8 secretion (J), compared to scramble shRNA controls (shControl). Results are means ± SEM of measurements from at least three separate cell cultures. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t‐test).
We then tested whether the various BCG fractions could also activate IL‐8. Treating cells with BCGsn, fBCGsn, bBCGsn, or ppBCGsn showed a similar IL‐8 response as treating with whole BCG (Fig. 4G). When exposing cells to a series of different BCG concentrations, we found that the IL‐8 secretion stimulated by BCG was dose dependent (Fig. 4H). Next, we investigated whether TLR4 was involved the release of IL‐8. We used lentivirus encoding shRNA against TLR4 (shTLR4), as well as scramble shRNA as a control (shControl). Infecting cells with shTLR4 and exposing them to BCG completely abolished the IL‐8 promoter activity (Fig. 4I). In contrast, measuring IL‐8 secretion under the same conditions demonstrated no effect of TLR4 knockdown (Fig. 4J). Together, these observations indicate that bioactive compounds within the BCGsn are evoking Ca2+ signaling and that TLR4 is controlling the synthesis, not the exocytosis, of IL‐8.
4. Discussion
Despite the frequent clinical usage of BCG to eradicate bladder cancer, little is known about its mechanism of action. Nevertheless, several studies report that BCG causes a local immune response reaction in the bladder (de Boer et al., 1997; Jackson et al., 1995; Redelman‐Sidi et al., 2014; Thalmann et al., 2000). BCG induces cancer cells in the bladder to secrete cytokines and chemokines, which attract cells of the immune system (Horinaga et al., 2005; Kamat et al., 2009; Liu et al., 2009). The cancer cells are subsequently killed by cytotoxic cells recruited to the site of the inflammatory response. IL‐6 and IL‐8 rapidly appear in the urine of BCG‐instilled patients and are believed to initiate the host immune response (Kamat et al., 2016). In this study, we shed new light on the signaling mechanisms involved in the eradication of bladder cancer following BCG therapy.
The BCG mixture injected into the bladder of cancer patients contains a myriad of compounds, including fragments of bacteria and even semi‐live bacteria. Early publications report that one immunostimulatory compound in BCG is non‐methylated CG‐rich DNA fragments that can induce a potent immune activation (Yamamoto et al., 1992a,b). However, most likely several compounds within the BCG mixture are involved in the overall response. Interestingly, our data show that the BCG supernatant sBCG can stimulate cytosolic Ca2+ signaling and cytokine release. We identified TLR4 as an essential receptor for BCG‐induced Ca2+ signaling. Previously, expression of both TLR4 and TLR9 has been reported in human bladder cancer cells (Olbert et al., 2015). Our data show that inhibiting Ca2+ signaling with 2APB or knocking down TLR4 with shRNA completely blocks IL‐8 promoter activity. Surprisingly, knocking down TLR4 had no effect on IL‐8 secretion, whereas inhibiting Ca2+ signaling partly reduced secretion. This indicates that global Ca2+ signaling regulates exocytosis. However, more information about the various compounds within BCG and their actions on cells is necessary to fully understand the overall cellular response. We speculate that bioactive compounds within the BCG mixture can be counterproductive. Identifying such compounds can help reduce adverse side effects of BCG therapy as well as decrease the risk of cancer relapse or progression to more invasive disease.
Our data show that pertussis toxin and PLC can inhibit BCG‐induced Ca2+ signaling. It has been demonstrated that mice with orthotopic bladder tumors that receive a recombinant BCG expressing pertussis toxin have less tumor growth than animals receiving ordinary BCG (Chade et al., 2008). Interestingly, a new TLR transduction mechanism that involves Ca2+ signaling was recently reported (Shintani et al., 2013, 2014). TLR9 stimulation reduced ER Ca2+‐ATPase activity, modulating Ca2+ handling between the ER and mitochondria, which resulted in decreased mitochondrial ATP levels and activation of protective cellular machineries. It is possible that when treating bladder cancer patients with BCG, parallel protective machineries are activated that could hinder the cancer eradication process and explain why some patients fail to respond to BCG. One such parallel process might be the secretion of IL‐8, which can act as a tumorigenic and pro‐angiogenic factor, driving tumor growth (Singh and Lokeshwar, 2011; Xie, 2001; Zhao et al., 2017).
A major challenge with the BCG therapy is its severe side effects, such as BCG sepsis, immunosuppression, hematuria, active urinary tract infection, and mild cystitis (Kresowik and Griffith, 2009). Another disadvantage with the therapy is that a significant proportion (~ 40%) are refractory cancers that fail to respond to the BCG therapy (O'Donnell and Boehle, 2006; Packiam et al., 2017). Why some patients fail to respond is largely unknown. Based on our results, we speculate that concerted actions of multiple signaling pathways define the efficiency of the BCG therapy. BCG triggers a long chain of events that critically regulates each other. Patients with a malfunction in this cascade of events may have refractory cancer. We here present a much more comprehensive picture of the initial signaling event triggered by BCG, containing multiple interlinked signaling pathways. At the molecular level, it appears that bioactive compounds present in the BCGsn interact with TLR4 to trigger a Ca2+ response, which subsequently activates NF‐κB.
5. Conclusion
Our work opens the door to more extensive studies of the mechanism of action of BCG therapy for bladder cancer. By better understanding the BCG‐mediated signaling cascades, we will be better able to design more efficient therapeutic strategies with less adverse side effects when treating patients with bladder cancer.
Ethics approval and consent to participate
All experiments were ethically approved by the Karolinska University Hospital Ethical Committee (Dnr 2011/421‐31/1) and all patients were informed.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
CI, MK, SC, and MVG designed research, performed research, and analyzed data. SZ, LL, NS, RK, and TKL performed research. ArH and AbH collected the tumor samples and clinical information. AbH, PW, SM, and MO advised the design of research. CI, MVG, SC, SM, AM, and PU prepared the manuscript. AM and PU designed the project. All authors read and approved the final manuscript.
Supporting information
Fig. S1. Validating knock‐down efficiency of siRNA and shRNA.
Fig. S2. BCG evokes intracellular Ca2 + signaling in bladder cancer cells.
Fig. S3. Scrutinizing the BCG‐induced Ca2 + signal cascade in bladder cancer cells.
Fig. S4. Screening substances in BCG. Polyacrylamide gel electrophoresis of the BCG mixture.
Movie S1. BCG‐induced Ca2 + signaling in bladder cancer cells.
Acknowledgements
The authors would like to thank Dr Benedict Chambers for helpful discussions and Nasrin Bavand, Alessandra Nanni, and Johnny Söderlund for technical and administrative assistance. This work was supported by the Swedish Research Council (grants 2009‐3364, 2013‐3189, and 2017‐00815 to PU), the Swedish Strategic Foundation (MultiBIO 2010 to PU), the Swedish Cancer Society (grant CAN 2013‐802 and CAN 2016‐801 to PU), Linnaeus Center in Developmental Biology for Regenerative Medicine (DBRM), the Knut and Alice Wallenberg Foundation (grants CLICK and Research Fellow to PU), the Karolinska Institutet's KID doctoral program (to MK), Åke Wiberg's Foundation (to PU), Magnus Bergvall's Foundation (to PU), Fredrik and Ingrid Thuring's Foundation (to PU), Sigrid Jusélius Foundation (to LL), Hillevi Fries’ Foundation (to AM), the Olle Engkvist Foundation (to PU), and the Swedish Society for Medical Research (to PU, SC and TKL).
Contributor Information
Ayako Miyakawa, Email: ayako.miyakawa@ki.se.
Per Uhlén, Email: per.uhlen@ki.se.
References
- Berridge MJ, Bootman MD and Lipp P (1998) Calcium–a life and death signal. Nature 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H and Donner J (1973) Established cell line of urinary bladder carcinoma (T24) containing tumour‐specific antigen. Int J Cancer 11, 765–773. [DOI] [PubMed] [Google Scholar]
- Chade DC, Borra RC, Nascimento IP, Villanova FE, Leite LC, Andrade E, Srougi M, Ramos KL and Andrade PM (2008) Immunomodulatory effects of recombinant BCG expressing pertussis toxin on TNF‐alpha and IL‐10 in a bladder cancer model. J Exp Clin Cancer Res 27, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD et al (2016) Diagnosis and treatment of non‐muscle invasive bladder cancer: AUA/SUO guideline. J Urol 196, 1021–1029. [DOI] [PubMed] [Google Scholar]
- de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH and Schamhart DH (1997) Role of interleukin‐8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res 25, 31–34. [DOI] [PubMed] [Google Scholar]
- Franks LM and Rigby C (1975) Letter: HeLa cells and RT4 cells. Science 188, 168. [DOI] [PubMed] [Google Scholar]
- Gunther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D and Bohle A (1999) Optimizing syngeneic orthotopic murine bladder cancer (MB49). Cancer Res 59, 2834–2837. [PubMed] [Google Scholar]
- Horinaga M, Harsch KM, Fukuyama R, Heston W and Larchian W (2005) Intravesical interleukin‐12 gene therapy in an orthotopic bladder cancer model. Urology 66, 461–466. [DOI] [PubMed] [Google Scholar]
- Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD and James K (1995) Changes in urinary cytokines and soluble intercellular adhesion molecule‐1 (ICAM‐1) in bladder cancer patients after Bacillus Calmette‐Guerin (BCG) immunotherapy. Clin Exp Immunol 99, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras Gonzalez GM, Anderson R, Grossman HB, Prat F and Dinney CP (2016) Cytokine Panel for Response to Intravesical Therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus calmette‐guerin. Eur Urol 69, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat AM, Tharakan ST, Sung B and Aggarwal BB (2009) Curcumin potentiates the antitumor effects of Bacillus Calmette‐Guerin against bladder cancer through the downregulation of NF‐kappaB and upregulation of TRAIL receptors. Cancer Res 69, 8958–8966. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR and Li ZW (2002) NF‐kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2, 301–310. [DOI] [PubMed] [Google Scholar]
- Kassouf W and Kamat AM (2004) Current state of immunotherapy for bladder cancer. Expert Rev Anticancer Ther 4, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Shipley WU and Feldman AS (2009) Bladder cancer. Lancet 374, 239–249. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Owczarek TB, McKiernan JM and Abate‐Shen C (2015) Modelling bladder cancer in mice: opportunities and challenges. Nat Rev Cancer 15, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresowik TP and Griffith TS (2009) Bacillus Calmette‐Guerin immunotherapy for urothelial carcinoma of the bladder. Immunotherapy 1, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, O'Donnell MA, Chen X, Han R and Luo Y (2009) Recombinant bacillus Calmette‐Guerin (BCG) expressing interferon‐alpha 2B enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro . Cancer Immunol Immunother 58, 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel B, Hunziker A, Pedersen L, Trusina A, Jensen MH and Krishna S (2010) Modeling oscillatory control in NF‐kappaB, p53 and Wnt signaling. Curr Opin Genet Dev 20, 656–664. [DOI] [PubMed] [Google Scholar]
- Monteith GR, Prevarskaya N and Roberts‐Thomson SJ (2017) The calcium‐cancer signalling nexus. Nat Rev Cancer 17, 367–380. [DOI] [PubMed] [Google Scholar]
- Morales A, Eidinger D and Bruce AW (1976) Intracavitary Bacillus Calmette‐Guerin in the treatment of superficial bladder tumors. J Urol 116, 180–183. [DOI] [PubMed] [Google Scholar]
- O'Donnell MA and Boehle A (2006) Treatment options for BCG failures. World J Urol 24, 481–487. [DOI] [PubMed] [Google Scholar]
- Olbert PJ, Kesch C, Henrici M, Subtil FS, Honacker A, Hegele A, Hofmann R and Hanze J (2015) TLR4‐ and TLR9‐dependent effects on cytokines, cell viability, and invasion in human bladder cancer cells. Urol Oncol 33(110), e119–e127. [DOI] [PubMed] [Google Scholar]
- Packiam VT, Johnson SC and Steinberg GD (2017) Non‐muscle‐invasive bladder cancer: intravesical treatments beyond Bacille Calmette‐Guerin. Cancer 123, 390–400. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Reedy EA and Heatfield BM (1987) Isolation and primary culture of urothelial cells from normal human bladder. Urol Res 15, 315–320. [DOI] [PubMed] [Google Scholar]
- Redelman‐Sidi G, Glickman MS and Bochner BH (2014) The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 11, 153–162. [DOI] [PubMed] [Google Scholar]
- Roderick HL and Cook SJ (2008) Ca2 + signalling checkpoints in cancer: remodelling Ca2 + for cancer cell proliferation and survival. Nat Rev Cancer 8, 361–375. [DOI] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT et al (2003) A lentivirus‐based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 33, 401–406. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Drexler HC, Kioka H, Terracciano CM, Coppen SR, Imamura H, Akao M, Nakai J, Wheeler AP, Higo S et al (2014) Toll‐like receptor 9 protects non‐immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep 15, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Kapoor A, Kaneko M, Smolenski RT, D'Acquisto F, Coppen SR, Harada‐Shoji N, Lee HJ, Thiemermann C, Takashima S et al (2013) TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci U S A 110, 5109–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD and Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67, 7–30. [DOI] [PubMed] [Google Scholar]
- Simons MP, O'Donnell MA and Griffith TS (2008) Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol 26, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK and Lokeshwar BL (2011) The IL‐8‐regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res 71, 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedler E and Uhlen P (2014) Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840, 964–969. [DOI] [PubMed] [Google Scholar]
- Summerhayes IC and Franks LM (1979) Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro . J Natl Cancer Inst 62, 1017–1023. [PubMed] [Google Scholar]
- Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG and Studer UE (2000) Urinary Interleukin‐8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette‐Guerin. J Urol 164, 2129–2133. [PubMed] [Google Scholar]
- Uhlen P and Fritz N (2010) Biochemistry of calcium oscillations. Biochem Biophys Res Commun 396, 28–32. [DOI] [PubMed] [Google Scholar]
- Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A and Richter‐Dahlfors A (2000) Alpha‐haemolysin of uropathogenic E. coli induces Ca2 + oscillations in renal epithelial cells. Nature 405, 694–697. [DOI] [PubMed] [Google Scholar]
- Xie K (2001) Interleukin‐8 and human cancer biology. Cytokine Growth Factor Rev 12, 375–391. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O and Tokunaga T (1992a) Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN [correction of INF] and augment IFN‐mediated [correction of INF] natural killer activity. J Immunol 148, 4072–4076. [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T and Tokunaga T (1992b) DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol 36, 983–997. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wang S, Lin Y, Miao Y, Zeng Y, Nie Y, Guo P, Jiang G and Wu J (2017) Epithelial‐mesenchymal transition in cancer: role of the IL‐8/IL‐8R axis. Oncol Lett 13, 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Validating knock‐down efficiency of siRNA and shRNA.
Fig. S2. BCG evokes intracellular Ca2 + signaling in bladder cancer cells.
Fig. S3. Scrutinizing the BCG‐induced Ca2 + signal cascade in bladder cancer cells.
Fig. S4. Screening substances in BCG. Polyacrylamide gel electrophoresis of the BCG mixture.
Movie S1. BCG‐induced Ca2 + signaling in bladder cancer cells.


