Abstract
Treatment of young adults with colorectal cancer (CRC) represents an unmet clinical need, especially as diagnosis in this population might lead to the greatest loss of years of life. Since 1994, CRC incidence in individuals younger than 50 years has been increasing by 2% per year. The surge in CRC incidence in young adults is particularly alarming as the overall CRC frequency has been decreasing. Early‐onset CRC are characterized by a more advanced stage at diagnosis, poorer cell differentiation, higher prevalence of signet ring cell histology, and left colon‐sided location of the primary tumor. Among EO‐CRC, approximately 30% of patients are affected by tumors harboring mutations causing hereditary cancer predisposing syndromes, and 20% have familial CRC. Most notably, the remaining 50% of EO‐CRC patients have neither hereditary syndromes nor familial CRC, thus representing a formidable challenge for research. In this review article we summarize epidemiology, clinical and molecular features, heredity and outcome of treatments of EO‐CRC, and provide considerations for future perspectives.
Keywords: colorectal cancer, familial colorectal cancer, hereditary colorectal cancer, sporadic early‐onset colorectal cancer, young adults
Abbreviations
- AYA
adolescent and young adult
- CIMP
CpG island methylator phenotype
- CIN
chromosomal instability
- CRC
colorectal cancer
- EO‐CRC
early‐onset colorectal cancer
- FAP
familial adenomatous polyposis
- FOBT
fecal occult blood test
- LS
Lynch syndrome
- MACS
microsatellite and chromosome stable
- MAP
MutYH‐associated polyposis
- mCRC
metatastic colorectal cancer
- MMR
mismatch repair
- MSI
microsatellite instability
- NAP
NTHL1‐associated polyposis
- OS
overall survival
- PAPP
polymerase proofreading‐associated polyposis
- PFS
progression‐free survival
- RR
Response rate
- SEER
surveillance, epidemiology and end results program
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and cause of cancer death worldwide in both genders (Siegel et al., 2018). At diagnosis, the median age of patients with colon cancer is 68 and 72 years in men and women, respectively; the median age of patients with rectal cancer is 63 years in both genders (American Cancer Society. Colorectal Cancer Facts & Figures, 2017; Siegel et al., 2018). In recent years, the overall CRC incidence and mortality in the USA and Europe has dropped (Malvezzi et al., 2018; Siegel et al., 2018). Since the mid‐2000s, CRC incidence in the USA has decreased by 2–3% per year in men and women (American Cancer Society. Colorectal Cancer Facts & Figures, 2017; Siegel et al., 2018). This reduction has been mainly correlated with the spread of screening tests [fecal occult blood test (FOBT) and colonoscopy] allowing detection and excision of premalignant lesions, and to a higher awareness of CRC risk factors among the population (Holme et al., 2013; Siegel et al., 2018; Welch and Robertson, 2016). Since 2012 in Europe, the CRC mortality rate decreased by 6.7% in men and 7.5% in women, whereas between 2008 and 2016, CRC incidence increased by 6% annually (Malvezzi et al., 2018; Vuik et al., 2018). Overall survival (OS) at 5 years from diagnosis is around 60% considering all stages of disease (Siegel et al., 2018; Van Cutsem et al., 2014). Metastatic CRC, despite therapeutic advances, exhibits poor prognosis; with the present state of the art, only 14% of patients are alive after 5 years from diagnosis (Siegel et al., 2018; Van Cutsem et al., 2014).
In 2010, CRC among patients < 50 years old accounted for 4.8% and 9.5% of colon and rectal cancers, respectively (Bailey et al., 2015; Silla et al., 2014). Interestingly, recent studies on several continents have reported an increase in CRC incidence in this age subset and especially in individuals < 40 years (Bailey et al., 2015; Bleyer et al., 2017; Exarchakou et al., 2018; Hessami Arani and Kerachian, 2017; Troeung et al., 2017), including also the metropolitan area of Milan, Italy (A.‐G. Russo, A. Andreano, A. Sartore‐Bianchi, G. Mauri, A. Decarli & S. Siena, personal communication). To date, the actual magnitude and the underlying etiologies of this increase are unclear. Diagnostic and therapeutic protocols dedicated to early‐onset CRC (EO‐CRC) in young individuals are a currently unmet clinical need. Also, there is no consensus on whether EO‐CRCs are undistinguishable or are a distinct molecular/immunologic entity from CRC in older patients (Bleyer et al., 2017; Luzzatto and Pandolfi, 2015; Tomasetti and Vogelstein, 2015; Tricoli et al., 2016).
In this article, we critically review clinical and molecular characteristics of EO‐CRC based on retrospective analyses, clinical trials, books and reviews currently available in the scientific literature. A systematic review approach has been applied for prognosis and standard metastatic colorectal cancer (mCRC) molecular biomarkers (RAS and BRAF) prevalence among EO‐CRC.
2. Materials and methods
We first performed a narrative review of clinical and pathological characteristics of EO‐CRC, retrieving articles through PubMed database, American Society of Clinical Oncology, European Society for Medical Oncology, and United European Gastroenterology meeting libraries, websites www.cancer.org and https://seer.cancer.gov/statfacts, and the book Cancer in Adolescents and Young Adults – Chapter 13. Secondly, according to PRISMA Criteria Statement 2009 (Fig. 1; Moher et al., 2009) we systematically retrieved articles describing EO‐CRC prognosis compared with older counterparts, and ther prevalence of RAS and BRAF mutations through PubMed database using [young(Title) OR younger(Title) OR early‐onset(Title) OR adolescent(Title) OR association of age(Title)] AND [colon(Title) OR rectal(Title) OR colorectal(Title)] AND [cancer(Title)] as search terms. Articles published in languages other than English, published earlier than year 2000, articles with a follow up shorter than 2 years, articles including patients older than 50 years of age in the younger group, and articles not comparing young with old patients in the same cohort were not included in the systematic review. This search was implemented by further manual revision of the bibliography of the included articles.
Figure 1.
PRISMA 2009 flow diagram depicting the systematic review process performed to retrieve articles on prognosis and RAS and BRAF prevalence among EO‐CRC.
2.1. Definition of age groups
An unequivocal definition of ‘EO‐CRC’, or ‘young adult CRC’, is currently needed, as no clear and widely accepted consensus is available in literature or guidelines. According to a non‐pediatric oncology definition, the definition generally comprises all CRCs diagnosed before the screening age, i.e. < 50 years of age. Most screening programs start from this age chosen based on cost‐effective analyses of healthcare system sustainability. In contrast, in Adolescent and Young Adult (AYA) Oncology, it comprises CRC patients diagnosed at 15–29 years of age (Bleyer et al., 2017). Nevertheless, in the context of some AYA clinical trials, the age range has been extended to 50 years by the Children's Oncology Group (Bleyer et al., 2017). The US National Cancer Institute (NCI) Progress Review Group of AYA Oncology in 2006 proposed the age range of 15–39 years (Bleyer et al., 2017; Coccia et al., 2018). The definition of ‘very EO‐ CRC’ has also no clear consensus in the literature, with extreme variability among different publications. Therefore, definition of age groups among CRC patients is currently based on non‐specific epidemiologic screening or clinical trial accrual criteria. Current age‐group subdivision is indeed a limitation for interpreting results obtained from published molecular and clinical studies among EO‐CRCs. In this review, we included all publications dealing with EO‐CRC diagnosed before 50 years of age.
A reasonable solution to improve future retrospective analyses of previously published studies in such a population of EO‐CRCs might be to consider age a continuous variable rather than using arbitrary predefined age cut‐offs (Lieu et al., 2014). The open question remains as to how to define age cut‐off for clinical trials.
2.2. Epidemiology
According to the Surveillance, Epidemiology and End Results Program (SEER) database in the USA, around 5% of all CRC are diagnosed in patients < 45 years old (Colorectal Cancer ‐ Cancer Stat Facts, 2018). Rectal cancer is diagnosed in up to 18% of cases < 50 years old in men and women alike (Ahnen et al., 2014). EO‐CRC is more commonly diagnosed among minorities and uninsured populations (You et al., 2012).
At the beginning of the 21st century, several studies showed evidence that CRC incidence was changing across different age groups. Indeed, in the USA an increase in the number of CRC diagnoses in the population younger than 50 years has been described and, in particular, this increase was observed in patients aged 20–35 years (Bailey et al., 2015; Bhandari et al., 2017; Edwards et al., 2014; Meyer et al., 2010; O'Connell et al., 2003). Epidemiological evidence indicates that since 1994, CRC incidence in those < 55 years is increasing by 2% per year (Figs 2 and 3; Fast Stats, 2018; Siegel et al., 2018). Bailey et al., based on CRC incidence trends between 1975 and 2010 in the USA, predicted a nearly doubled incidence rate of CRC by 2030 in the population aged < 35 years, and the highest increase is expected for recto‐sigmoid cancers between the ages of 18 and 35 years (Bailey et al., 2015). By 2030 in the USA, 10% of all colon and 22% of all rectal cancers are expected to be diagnosed in patients < 50 years old, a provocative prediction if compared with 4% and 9% for colon and rectal cancer, respectively, 10 years ago (Fig. 3; Bailey et al., 2015). These data indicate that EO‐CRC is a current public health issue in the USA (Ahnen et al., 2014; Bailey et al., 2015) and elsewhere (Fig. 4; Abou‐Zeid et al., 2017; Brenner et al., 2017; Exarchakou et al., 2018; Gandhi et al., 2017; Hessami Arani and Kerachian, 2017; Malekzadeh et al., 2009; A.‐G. Russo, A. Andreano, A. Sartore‐Bianchi, G. Mauri, A. Decarli & S. Siena, personal communication; Troeung et al., 2017; Vuik et al., 2018). Following this trend, in the USA it has been proposed that CRC screening should start at 45 years rather than 50 years, but further data are needed to explore this option in real life (Peterse et al., 2018).
Figure 2.
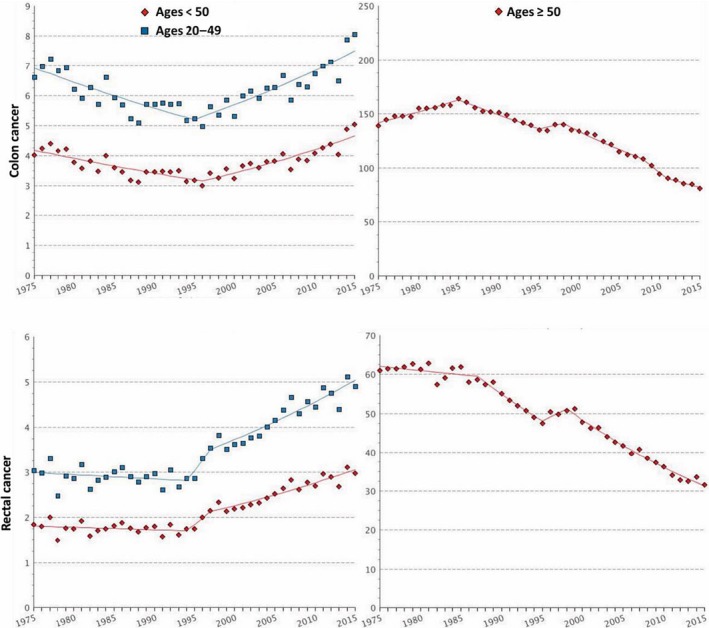
Graphs report age‐adjusted SEER (Surveillance, Epidemiology and End Results) incidence rates of colon (upper panels) and rectal (lower panels) cancer from 1975 to 2015 among individuals younger (left panels) and older (right panels) than 50 years. On the Y‐axis is reported incidence rate per 100 000 and on the X‐axis is reported the year of diagnosis. Data were plotted by accessing SEER website at the weblink https://seer.cancer.gov/faststats/selections.php?series=cancer
Figure 3.
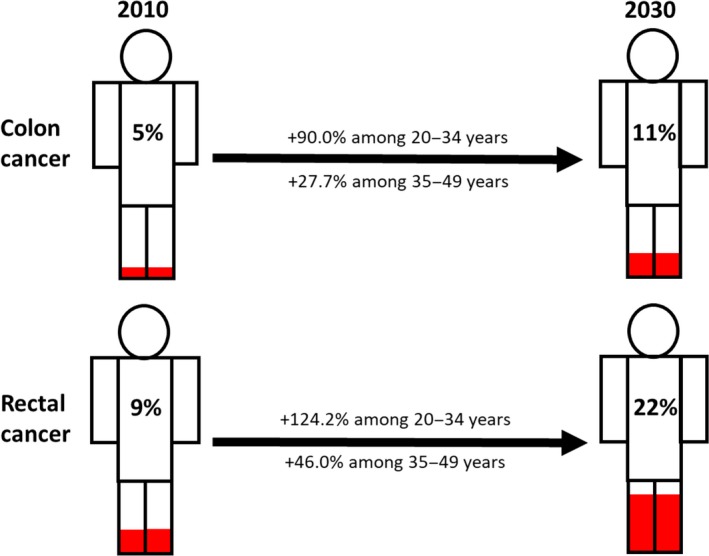
Incidence rates increase in colon and rectal cancer in patients younger than 50 years from 2010 to 2030 according to Bailey et al. (2015). Data and projections are confirmed for men and women in the USA). Figures colored in red represent percentages of CRC diagnosed under the age of 50.
Figure 4.
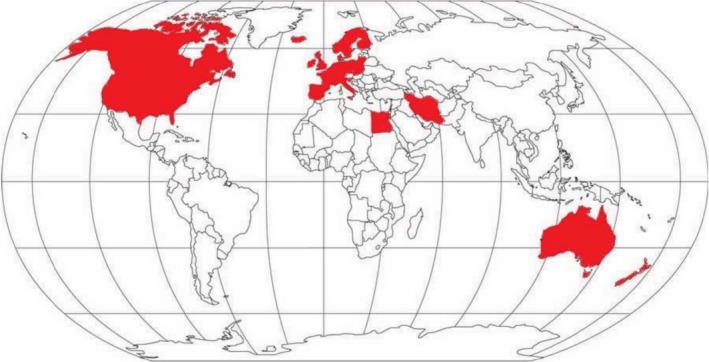
Map of the world and countries (shown in red) in which increased incidence of CRC among patients younger than 50 years old has been documented as described in the text (Abou‐Zeid et al., 2017; Ahnen et al., 2014; Bailey et al., 2015; Brenner et al., 2017; Exarchakou et al., 2018; Gandhi et al., 2017; Hessami Arani and Kerachian, 2017; Malekzadeh et al., 2009; A.‐G. Russo, A. Andreano, A. Sartore‐Bianchi, G. Mauri, A. Decarli & S. Siena, personal communication; Troeung et al., 2017; Vuik et al., 2018).
Similarly, recent data from Europe indicate an annual 1.5% increase in rectal cancer incidence between 1990 and 2008, and an annual 7.4% increase in colon cancer incidence between 2008 and 2016 (Vuik et al., 2018). In the Western world, it is reasonable to assume that access to screening procedures, i.e. FOBT and recto‐sigmoidoscopy or colonoscopy in the population > 50 years, can explain only partially the decreasing trend in CRC incidence (Edwards et al., 2014; Welch and Robertson, 2016). Indeed, the trend towards CRC incidence increase among young individuals has been reported not only in the USA and Europe but also in Iran and Egypt (Abou‐Zeid et al., 2017; Hessami Arani and Kerachian, 2017; Malekzadeh et al., 2009).
Finally, based on current data and projections for the next years it is reasonable to predict an increase of CRC cases diagnosed worldwide in individuals < 50 years for reasons that need to be investigated appropriately.
2.3. Clinical presentation
Excluding patients adhering to specific screening programs, CRC diagnosis in young individuals is usually carried out when symptoms appear (Hill et al., 2007; Karnak et al., 1999; Riaz et al., 2017). Painless bleeding could anticipate other CRC symptoms by 2–3 years (Riaz et al., 2017). However, CRC diagnosis in young adults is carried out on average 6 months later than symptom onset due to low level of suspiciousness by probands and clinicians, sense of invincibility in young adults, and lack of medical insurance (Bleyer et al., 2008; Hill et al., 2007; O'Connell et al., 2004b).
In 61% of patients < 50 years (Kneuertz et al., 2015) and up to 76% of patients < 30 years (Indini et al., 2017; Kam et al., 2004; Khan et al., 2016), CRC is diagnosed as stage III or IV, strikingly different from older CRC patients (46–50% diagnosed as stage III or IV; Ferrari et al., 2008; Khan et al., 2016; Kneuertz et al., 2015). EO‐CRCs are more frequently poorly differentiated G3 tumors, left‐sided and rectal (Chang et al., 2012; Indini et al., 2017; Kam et al., 2004; Khan et al., 2016; Kneuertz et al., 2015; Sultan et al., 2010; Wang et al., 2015a). Signet ring cell CRC, accounting for < 1% of all CRC, among younger patients account for 3–13% of cases, especially in those younger than 30 years (Chang et al., 2012; Khan et al., 2016; Kneuertz et al., 2015; Wang et al., 2015a; Yantiss et al., 2009). Young patients usually have a Charlson comorbidity index of 0 and no other medical issues (Kneuertz et al., 2015).
2.4. Risk factors
Non‐Mediterranean Western diet, obesity, little physical activity, high consumption of red and processed meat, and low fiber intake are the most relevant risk factors for developing CRC (Castelló et al., 2018; Edwards et al., 2014; Hessami Arani and Kerachian, 2017; Slattery et al., 1998, 2003; Thune and Lund, 1996). There is a recently increased incidence of obesity in the USA, especially among young patients, and, being a well‐known risk factor for CRC, this may have played a role in reducing the age of CRC onset (Aleksandrova et al., 2013; Bailey et al., 2015; Bassett et al., 2010; Hedley et al., 2004). The net contribution of these factors cannot be assessed (Bailey et al., 2015; Kim et al., 2018).
2.5. There are CRC risk factors well‐known to play a more important role among young individuals:
Inflammatory bowel diseases increase of by two‐ to threefold the risk of CRC compared with general population, especially when diagnosed in early age (Triantafillidis et al., 2009).
Known hereditary cancer‐predisposing syndromes or familial CRC is higher among EO‐CRCs (see next paragraph; Hampel et al., 2005; Liang et al., 2003; Losi et al., 2005; Sinicrope, 2018).
Low adherence to specific screening programs in individuals with known cancer syndromes or familial CRC is also a major point in countries with a private health system or among populations with a low socioeconomic level (Hampel et al., 2005; Shin et al., 2017; U.S. Preventive Services Task Force, 2002; Vale Rodrigues et al., 2018).
Prior abdominal irradiation (i.e. radiotherapy for curable pediatric malignancies): colonoscopy is recommended starting from 35 years of age or a decade following > 30 Gy radiation treatment to the pelvis (Hill et al., 2007).
2.6. Molecular etiology, hereditary and familial syndromes
In the general population of CRC there are three main pathways of carcinogenesis involved in the onset and development of CRC: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP).
Among EO‐CRCs, as well as in the general population, CIN is the most common cause of CRC (Goel et al., 2010; Markowitz and Bertagnolli, 2009). In a variable percentage of cases depending on disease stage, CRC is caused by MSI (Lanza et al., 2006; Tran et al., 2011; Zaanan et al., 2018). There are two possible mechanisms leading to MSI‐CRC (Sinicrope, 2018):
hereditary germline mutations occurring in mismatch repair (MMR) genes;
tumor somatic hypermethylation of MLH1.
In all MSI‐CRC patients, referral for genetic counseling is recommended to screen Lynch syndrome (LS; Palomaki et al., 2009). LS predisposes to EO‐CRC and the percentage of MSI tumors has been observed to rise to up to 27%, especially in patients < 30 years (Khan et al., 2016). In contrast to the overall CRC population, among patients younger than 30 years the location of the primary tumor and histology of MSI‐CRCs do not differ from MSS‐CRC (Khan et al., 2016).
These mechanisms of carcinogenesis are not mutually exclusive and may coexist (Armaghany et al., 2012; Snover, 2011). A small proportion of MSI‐CRCs can also present CIN, whereas around 50% of MSS‐CRC are CIN‐negative (Banerjea et al., 2009; Sinicrope et al., 2006; Tang et al., 2004). The latter subgroup has been defined as Microsatellite and Chromosome Stable (MACS). These tumors are more frequently rectal or left‐sided, characterized by a poor prognosis and poor recognition by the immune system (Banerjea et al., 2009; Sinicrope et al., 2006; Tang et al., 2004). MACS‐CRCs have been described more frequently among young patients with a family history of CRC, but data in the literature are conflicting (Chan et al., 2001; Rex et al., 2012). MACS‐CRC has been associated with LINE‐1‐hypomethylation and CIMP‐low. Among MACS‐CRC, BRAF mutation or absent MLH1 expression are rare (Antelo et al., 2012; Cai et al., 2008; Silver et al., 2012). In addition, recent results from an extended somatic molecular investigation in a cohort of left‐sided and rectal EO‐CRC showed higher mutation rates of NF1, POLE, SMAD4 and BRCA2 (Puccini et al., 2018). Furthermore, mutations in left‐sided EO‐CRC genes involved in histone modification, such as KDM5C, KMT2A, KMT2C, KMT2D and SET2D, were reported more frequently (Puccini et al., 2018).
A prominent cause of EO‐CRC is the presence of a germline oncogene mutation, giving rise to a hereditary cancer syndrome. Prevalence of hereditary CRC syndromes among EO‐CRCs is influenced by the different age groups analyzed in different studies (Mork et al., 2015; Pearlman et al., 2017; Stoffel et al., 2018), with a higher prevalence among patients < 35 years old (Mork et al., 2015).
Four potential genetic scenarios should be considered among EO‐CRCs: known hereditary cancer syndromes, de novo germline hereditary cancer mutations, familial colorectal cancer and non‐hereditary and non‐familial CRC.
2.6.1. Hereditary cancer syndromes
Around 22% (10–33% according to different studies) of patients diagnosed with EO‐CRC are affected by hereditary cancer syndromes (Fig. 5). This proportion is significantly higher when compared with the 2–5% of hereditary cancer syndromes among the general CRC population (Chang et al., 2012; Jasperson et al., 2010; Mork et al., 2015). The most frequent hereditary cancer syndrome is hereditary non‐polyposis colorectal cancer, also known as LS (Mork et al., 2015; Pearlman et al., 2017; Sinicrope, 2018; Stoffel et al., 2018). LS patients have a lifetime risk of developing CRC of 70%, and in 40% of cases the onset of CRC is before age 40 (Lynch et al., 2008). LS is the most frequent CRC hereditary syndrome among patients younger than 50 years old and accounts for around one‐third of EO‐CRC in patients younger than 35 years old (Mork et al., 2015; Pearlman et al., 2017; Stoffel et al., 2018). Germline mutations in MLH1 and MSH2 genes are the most frequent, but MSH6 and PMS2 gene can present pathogenic mutations as well (Evans et al., 2007; Lynch et al., 2015). In addition, mutations occurring in EPCAM gene can also silence the MSH2 promoter region, leading to LS (Kempers et al., 2011; Ligtenberg et al., 2009, p. 2). In some cases of LS, homozygous or compound heterozygous (bi‐allelic) constitutive MMR gene mutations have been identified (Durno et al., 2005, 2015; Wimmer and Etzler, 2008). These lead to a very low age of CRC onset, usually earlier than 16 years old (Durno et al., 2005, Wimmer and Etzler, 2008). In contrast, a relevant proportion of young patients with MSI‐H tumors, usually MLH1 hypermethylated, do not harbor germline alterations in MMR genes (Pearlman et al., 2017; Zbuk et al., 2009).
Figure 5.
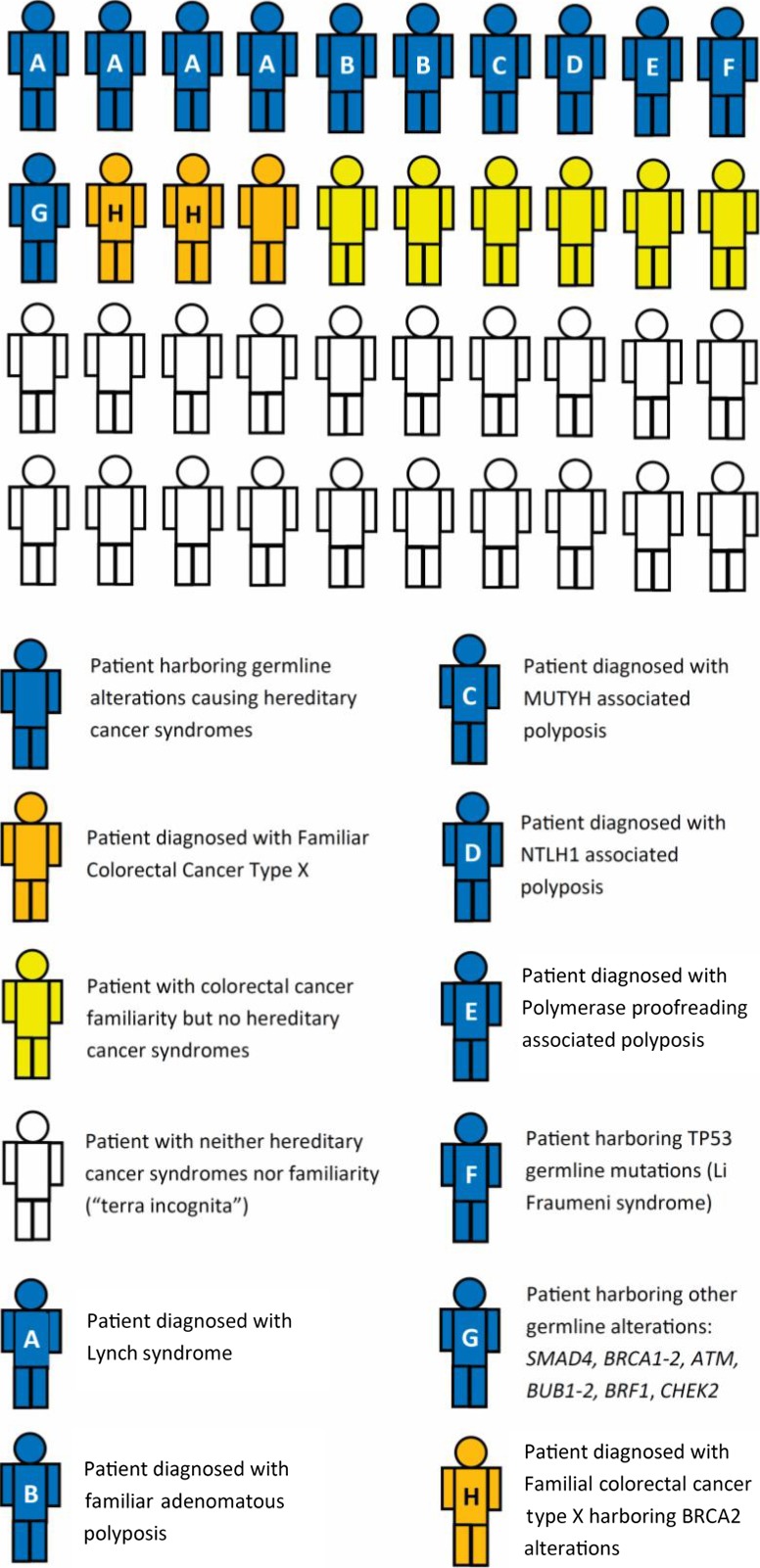
Prevalence of hereditary and familial syndromes, and neither hereditary nor familial syndromes (‘terra incognita’) among EO‐CRC in young individuals. Figures are derived from studies in the text (Bellido et al., 2018; Chang et al., 2012; Fong et al., 2009; Garre et al., 2015; Hahn et al., 2016; Jasperson et al., 2010; Ledermann et al., 2012; Lubbe et al., 2009; Mateo et al., 2015; Mork et al., 2015; Mur et al., 2018; Nejadtaghi et al., 2017; Palles et al., 2013; Pearlman et al., 2017; Robson et al., 2017; Sinicrope, 2018; Stoffel et al., 2018; Umar et al., 2004; Vasen et al., 1991, 1999; Weren et al., 2015, 2018; Yoshida et al., 2017; Yurgelun et al., 2015).
CRC may develop earlier in patients affected by adenomatous polyposis syndromes: familial adenomatous polyposis (FAP), polymerase proofreading‐associated polyposis (PAPP), MutYH‐associated polyposis (MAP) and NTHL1‐associated polyposis (NAP). FAP is the second most frequent hereditary cancer syndrome (Mork et al., 2015). Lifetime risk of developing CRC in FAP patients is 100% with a median age of CRC onset of 39 years old (Zbuk et al., 2009). Different APC mutations have been associated with different phenotypes, with mutations occurring in codon 1309 being associated with severe polyposis in the first decade of life (Caspari et al., 1994). FAP screening is recommended from the age of 10 years old and the treatment of choice is total proctocolectomy (Warrier and Kalady, 2012; Winawer et al., 2003). PAPP is an autosomal dominant genetic disease leading to increased risk of cancer and by germline mutations in POLE and POLD1 genes (Palles et al., 2013). PAPP confers a highly penetrant predisposition to develop polyposis (usually < 100) and EO‐CRCs (Palles et al., 2013; Valle et al., 2014). In addition, mutations occurring in POLE and POLD1 can occur de novo and in patients without polyposis (Valle et al., 2014). Thus, alterations in these genes could be found in all familial CRCs and are not restricted to polyposis cases (Valle et al., 2014). Differently, MAP and NAP are two autosomal recessive genetic diseases caused by germline mutation in MutYH and NTHL1 DNA glycosylase gene of the base excision repair (BER) pathway (Weren et al., 2015, 2018). These syndromes are characterized by the onset of 10–50 polyps around the age of 40 that cause an increased risk of CRC (Weren et al., 2015, 2018). MAP confers a 28‐fold higher lifetime risk of developing CRC at usually < 60 years old with a reported penetrance of 40–100% (Lubbe et al., 2009; Weren et al., 2018).
Other cancer syndromes, such as Li‐Fraumeni syndrome, characterized by germline TP53 mutations, have been related to EO‐CRC; they account for less than 1% of cases (Yoshida et al., 2017; Yurgelun et al., 2015; Fig. 5).
In patients with known hereditary syndromes, adherence to screening programs should be strongly encouraged, as in LS it has been demonstrated to reduce the risk of death by 65% (Järvinen et al., 2000). However, screening program adherence is low in youths, including in Western countries, and has to be enhanced to reduce late diagnosis and cancer deaths (Kim et al., 2017; Shaikh et al., 2018). Furthermore, a preventive aspirin regimen has been reported to significantly reduce CRC incidence among LS patients (Burn et al., 2011).
2.6.2. Other germline alterations associated with EO‐CRC
Many recent studies have tried to broaden the spectrum of germline alterations predisposing to EO‐CRC to expand the population admitted to screening programs. Several genes have been investigated, e.g. SMAD4, BRCA1‐2, ATM, BUB1‐2, BRF1 and CHEK2 (Bellido et al., 2018, p. 1; Hahn et al., 2016; Mur et al., 2018; Pearlman et al., 2017; Stoffel et al., 2018; Yurgelun et al., 2015); however, at around 2–10% they account for only a small proportion of EO‐CRC overall (Pearlman et al., 2017; Stoffel et al., 2018). Although rare, these genetic alterations should not be neglected, especially for those that can potentially be treated with drugs, such as BRCA2 alterations (Fong et al., 2009; Ledermann et al., 2012; Mateo et al., 2015; Robson et al., 2017). As pointed out by different studies, a large percentage of EO‐CRC patients with no CRC familiarity may be affected by hereditary cancer syndromes or at least harbor gene alterations potentially predisposing to CRC (Mork et al., 2015; Stoffel et al., 2018). Only half (43/85) of EO‐CRC patients presenting germline cancer‐predisposing alterations reported CRC in a first‐degree relative, therefore being the first to be diagnosed with a cancer‐predisposing syndrome (Stoffel et al., 2018). This evidence has led some authors to recommend genetic counseling for all patients diagnosed with extreme EO‐CRC (< 35 years old; Mork et al., 2015).
2.6.3. Familial EO‐CRC
The opposite situation is represented by patients characterized by a relevant familial CRC not affected by hereditary CRC syndromes. In particular, this is the case for patients fulfilling Amsterdam Criteria I or II but not affected by LS (Garre et al., 2015; Vasen et al., 1991, 1999). Today, the Amsterdam criteria have largely been replaced by the Bethesda criteria, which are more sensitive but less specific in identifying MSH2 and MLH1 germline mutations carriers, thus broadening the spectrum of familial CRC patients (Piñol et al., 2005; Umar et al., 2004). Amsterdam or Bethesda criteria‐positive patients without a diagnosis of LS are diagnosed with Familiar Colorectal Cancer Syndrome Type X (Nejadtaghi et al., 2017; Umar et al., 2004; Vasen et al., 1991, 1999). This peculiar population may be an ideal group in whom to extend molecular screening in order to find out potentially actionable gene alterations hopefully to confer survival benefit, if receiving appropriately targeted agents (Garre et al., 2015; Nejadtaghi et al., 2017). In this regard, Garre and coworkers documented that, in a population of EO‐CRC patients fulfilling Amsterdam criteria II, the prevalence of BRCA2 germline mutations, including point and frame mutations, was 60% (29/48; Garre et al., 2015), leading to the speculation that these patients might derive benefit from platinum‐ or PARP‐inhibitor‐based regimens in the metastatic setting (Garre et al., 2015; Mylavarapu et al., 2018).
In addition, CRC incidence in this subgroup may increase in the next years due to the activity of known carcinogenic agents in patients harboring a hypothetical genetic predisposition to develop CRC. At this regard, in a Japanese cross‐sectional study it has been recently reported that alcohol consumption might be associated with earlier CRC onset in patients affected by LS (Miguchi et al., 2018).
2.6.4. Non‐hereditary and non‐familial EO‐CRC
It is very surprising to note that although hereditary and familial cancer syndromes are more frequent among young individuals, half of EO‐CRC patients have neither (Mork et al., 2015; Stoffel et al., 2018). This implies that, since these patients are not included in screening programs, they are often diagnosed in later stages of the disease. Among this subset of patients, without known hereditary cancer syndromes, different alterations in TNFR1, EIF4E, LTBP4, CYR61, UCHL1, FOS and FOS B genes have been demonstrated between early‐ and late‐onset CRC. However, definite conclusions are still far from being reached, since study populations were limited to cohorts of 10–39 patients and the gene panels tested were heterogeneous (Berg et al., 2010; Hong et al., 2007; Kirzin et al., 2014).
In addition to somatic tumor mutations, tumor immune infiltration is currently under investigation in EO‐CRC. Recently, in EO‐CRC a different expression of immune activation genes (i.e. CLC and IFNAR1) has been reported when compared with the older counterparts (Ågesen et al., 2011). Overall, it is conceivable that among EO‐CRCs, more patients could potentially benefit from immunotherapy in the metastatic setting because of a higher prevalence of MMR, POLE and POLD1 aberrations, leading to a higher tumor mutational burden (Pai et al., 2017). However, data on peculiar features of interactions between the immune system and tumors in EO‐CRC are still to be addressed.
2.7. What could be responsible for sporadic EO‐CRC?
Environmental reasons for EO‐CRC are still unknown. In the last decades, dramatic environmental and behavioral changes may have contributed to lowering the age of CRC onset. Changes in microbiome induced by cesarean delivery or appendicectomy might play a role. Microbiome may also be altered by modern dietary regimens including colorants and preservatives. These substances may also play a role of direct carcinogenesis on intestine cells. The extensive use of antibiotics in agriculture and medicine, although immediately beneficial, could reasonably alter the gut microbiome. Reduced breast‐feeding may alter the development of the immune system and its capacity for cancer surveillance. However, we still do not know how these agents may alter cells pathways causing CRC. Large epidemiological and preclinical studies are warranted.
2.8. Prognosis, treatments, and outcome
We systematically retrieved 37 articles describing the prognosis of EO‐CRC compared with older patients (Abdelsattar et al., 2016; Blanke et al., 2011; Boyce et al., 2016; Chandrasinghe et al., 2017; Chou et al., 2011, 2017; Damodaran and Seshadri, 2016; Fu et al., 2014; Fu et al., 2013; Haleshappa et al., 2017; Hawk et al., 2015; Hubbard et al., 2012; Josifovski et al., 2004; Khan et al., 2016; Kim et al., 2016; Kneuertz et al., 2015; Kolarich et al., 2018; Li et al., 2014; Lieu et al., 2014; Manjelievskaia et al., 2017; McMillan and McArdle, 2009; Murata et al., 2016; O'Connell et al., 2004a; Orsini et al., 2015; Pokharkar et al., 2017; Quah et al., 2007; Rho et al., 2017; Rodriguez et al., 2018; Shen et al., 2018; Shida et al., 2018; Sultan et al., 2010; Vatandoust et al., 2016; Wang et al., 2015a,2015b; Yang et al., 2012; You et al., 2012; Zhao et al., 2017). EO‐CRC survival data are conflicting. Some studies indicate a poorer prognosis (Bleyer et al., 2008; Khan et al., 2016; Lieu et al., 2014; McMillan and McArdle, 2009; O'Connell et al., 2004b; Shida et al., 2018; Sultan et al., 2010), whereas others support a comparable or even better prognosis in comparison with older patients (Blanke et al., 2011; Hubbard et al., 2012; Kneuertz et al., 2015; Kolarich et al., 2018; McMillan and McArdle, 2009; O'Connell et al., 2004a; Quah et al., 2007; Rodriguez et al., 2018; Vatandoust et al., 2016; Wang et al., 2015a; You et al., 2011; Table 1). From 1973 to 2005, adult CRC survival outcome improved, whereas child and adolescent CRC survival have not (Sultan et al., 2010). A worse survival was observed in studies comparing survival between patients younger than 30 years old with those > 50 years (Khan et al., 2016; Lieu et al., 2014; Sultan et al., 2010). In particular, it was reported that the younger the patient, the worse the prognosis (Lieu et al., 2014). By contrast, publications comparing patients younger than 50 years of age with those > 50 years showed a better prognosis among the younger patients (Kneuertz et al., 2015; Kolarich et al., 2018; Vatandoust et al., 2016; Wang et al., 2015a). This suggests that in patients with very early‐onset CRC (< 35 years old) a different biological background likely underlies an earlier and faster CRC progression (Ferrari et al., 2008; Indini et al., 2017; Khan et al., 2016; Zhao et al., 2017).
Table 1.
Prognosis of early‐onset colorectal cancer patients according to age groups from systematically reviewed studies written in English, published later than 2000 with a follow up longer than 2 years and considering 50 years of age as upper cut‐off of early‐onset, according to the PRISMA Criteria of 2009 (Moher et al., 2009). Color codes: Green, significantly better prognosis; Yellow, similar prognosis; Red, significantly worse prognosis. ARR, adjusted relative risk; CSS, cancer‐specific survival; DSS, disease‐specific Survival; HR, hazard ratio; LC, Linköping cancer; MHS, Military Health System; mOS, median overall survival; NSS, not statistically significant; NSW, New South Wales; NCDB, National Cancer Data Base; OS, overall survival; PD, progressive disease; RER, relative excess risk; SAMCRC, South Australian Metastatic Colorectal Cancer; SEER, Surveillance, Epidemiology and End Results program
| Authors (references) | Data source | Stage of disease | Age groups (range in years of age) | Prognosis |
|---|---|---|---|---|
| Kneuertz et al. (2015) | NCDB | Stage II | 18–49 | 5‐year ARR 0.72 (0.58–0.88) |
| 65–75 | ||||
| Stage II | 18–49 | 5‐year ARR 0.90 (0.69–1.17) | ||
| 65–75 | ||||
| Stage III | 18–49 | 5‐year ARR 0.89 (0.81–0.97) | ||
| 65–75 | ||||
| Stage IV | 18–49 | 5‐year ARR 0.84 (0.79–0.90) | ||
| 65–75 | ||||
| McMillan and McArdle (2009) | Hospital records | All stages | < 45 | 10‐year CSS P = 0.275 |
| > 45 | ||||
| Khan et al. (2016) | Hospital records | All stages | < 30 | 5‐year DSS P < 0.0001 |
| > 50 | ||||
| Shen et al. (2018) | Hospital records | Stage I‐III | < 35 | 5‐year OS P = 0.0013a |
| > 35 | ||||
| O'Connell et al. (2004a) | SEER (1991–1999) | Stage I | < 40 | CSS P = NS |
| 60–80 | ||||
| Stage II | < 40 | CSS P = 0.01 | ||
| 60–80 | ||||
| Stage III | < 40 | CSS P = NS | ||
| 60–80 | ||||
| Stage IV | < 40 | CSS P < 0.0001 | ||
| 60–80 | ||||
| Sultan et al. (2010) | SEER (1973–2005) | All stages | < 20 | OS P < 0.001 |
| > 20 | ||||
| You et al. (2012) | Hospital records | Stage I | < 50 | 5‐year CSS P = NS |
| > 65 | ||||
| Stage II | < 50 | 5‐year CSS P = 0.012 | ||
| > 65 | ||||
| Stage III | < 50 | 5‐year CSS P = NS | ||
| > 65 | ||||
| Stage IV | < 50 | 5‐year CSS P = NS | ||
| > 65 | ||||
| Wang et al. (2015a) | SEER (1973–2011)/LC (1972–2009) | Stage I | < 50 | CSS P < 0.001 |
| > 50 | ||||
| Stage II | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Stage III | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Stage IV | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Wang et al. (2015b) | SEER (1988–2011) | Stage I | < 50 | CSS P < 0.001 |
| > 50 | ||||
| Stage II | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Stage III | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Stage IV | < 50 | CSS P < 0.001 | ||
| > 50 | ||||
| Quah et al. (2007) | Hospital records | Stage I‐III | < 40 | 5‐year DSS p.43 |
| > 40 | ||||
| Murata et al. (2016) | Hospital records | Stage I‐III | < 40 | 5‐year OS P = 0.93 |
| ≥ 40 | ||||
| Vatandoust et al. (2016) | SAMCRC database | Stage IV | < 40 | mOS HR 0.81 (0.56–1.16) |
| > 40 | ||||
| Kolarich et al. (2018) | NCDB | Stage I | < 50 | 5‐year OS P < 0.001 |
| > 50 | ||||
| Stage II | < 50 | 5‐year OS P < 0.001 | ||
| > 50 | ||||
| Stage III | < 50 | 5‐year OS P < 0.001 | ||
| > 50 | ||||
| Rodriguez et al. (2018) | Ontario registry | Stage I‐III | < 40 | 5‐year OS P < 0.001 |
| > 60 | ||||
| Orsini et al. (2015) | Netherlands cancer registry | Stage I‐III | ≤ 40 | RER of death 0.82 (0.71–0.94) |
| > 40 | ||||
| ≤ 40 | RER of death 1.04 (0.91–1.18) | |||
| > 40 | ||||
| Damodaran and Seshadri (2016) | Hospital records | Stage II‐III | ≤ 40 | 5‐year CSS P = NSS |
| > 40 | ||||
| Blanke et al. (2011) | Clinical trials | Stage IV | < 40 | mOS P = 0.61 |
| < 40 | ||||
| < 50 | mOS P = 0.48 | |||
| < 50 | ||||
| Hubbard et al. (2012) | Clinical trials | Stage II | < 40 | OS P < 0.01 |
| > 40 | ||||
| Stage III | < 40 | OS P < 0.01 | ||
| > 40 | ||||
| Shida et al. (2018) | Hospital records | Stage IV | < 40 | 5‐year OS P = 0.042 |
| > 40 | ||||
| Haleshappa et al. (2017) | Hospital records | All stages | < 40 | mOS P = 0.0029 |
| > 40 | ||||
| Pokharkar et al. (2017) | Hospital records | All stages | < 45 | 3‐year OS P = 0.302 |
| > 45 | ||||
| Chou et al. (2017) | Taiwan cancer registry | All stages | < 40 | 10‐year CRC related mortality P < 0.001 |
| 41–70 | ||||
| Chandrasinghe et al. (2017) | Hospital records | All stages | < 50 | 5‐year OS P = 0.03 |
| > 70 | ||||
| Rho et al. (2017) | Hospital records | All stages | 18–44 | Mortality risk HR 1.53 (0.91‐ 2.58) |
| > 44 | ||||
| Zhao et al. (2017) | Hospital records | Stage I‐III | ≤ 35 | 5‐year OS P = 0.010 |
| > 35 | ||||
| Manjelievskaia et al. (2017) | US MHS database | Stage I | 18–49 | 5‐year survival HR 0.29 (0.13–0.62)b |
| 50–64 | ||||
| Stage II | 18–49 | 5‐year survival HR 0.59 (0.31–1.14)b | ||
| 50–64 | ||||
| Stage III | 18–49 | 5‐year survival HR 0.01 (0.01–0.89)b | ||
| 50–64 | ||||
| Stage IV | 18–49 | 5‐year survival HR 0.47 (0.22–0.98)b | ||
| 50–64 | ||||
| Stage I‐IV | 18–49 | 5‐year survival HR: NSS c | ||
| 50–64 | ||||
| Boyce et al. (2016) | NSW central cancer registry | All stages | < 50 | 5‐year CSS P < 0.001 |
| > 50 | ||||
| Kim et al. (2016) | Hospital records | Stage I | 22–45 | 5‐year CSS P = 0.188 |
| 56–75 | ||||
| 22–45 | 5‐year CSS P = 0.771 | |||
| 56–75 | ||||
| 22–45 | 5‐year CSS P = 0.087 | |||
| 56–75 | ||||
| 22–45 | 5‐year CSS P = 0.142 | |||
| 56–75 | ||||
| Abdelsattar et al. (2016) | SEER (1998–2011) | Stage I‐II | < 50 | 5‐year CSS P < 0.001 |
| > 50 | ||||
| Stage III | < 50 | 5‐year CSS P < 0.001 | ||
| > 50 | ||||
| Stage IV | < 50 | 5‐year CSS P < 0.001 | ||
| > 50 | ||||
| Fu et al. (2014) | Hospital records | Stage I‐III | ≤ 35 | 10‐year OS P = NSS |
| > 35 | ||||
| Stage IV | ≤ 35 | 10‐year OS P = 0.046 | ||
| > 35 | ||||
| Li et al. (2014) | SEER (1988–2003) | Stage I‐III | ≤ 40 | 5‐year CSS P < 0.001 |
| > 40 | ||||
| Hawk et al. (2015) | SEER (1973–2008) | Stage IV | < 50 | OS HR 0.725 (0.703–0.749) |
| > 50 | ||||
| Fu et al. (2013) | Hospital records | Stage I‐II | ≤ 30 | 10‐year OS P = 0.899 |
| > 30 | ||||
| Stage III‐IV | ≤ 30 | 10‐year OS P = 0.024 | ||
| > 30 | ||||
| Yang et al. (2012) | Hospital records | All stages | ≤ 44 | 10‐year OS P = NSS |
| > 44 | ||||
| Chou et al. (2011) | Hospital records | All stages | ≤ 40 | 5‐year CSS P < 0.001 |
| ≥ 80 | ||||
| Josifovski et al. (2004) | Hospital records | All stages | < 40 | 5‐year OS P = 0.053 |
| > 65 | ||||
| Lieu et al. (2014) | Clinical trials | Stage IV | ≈ 18d |
+19% risk of death +22% risk of PD |
| ≈ 57–61d |
Worse 5‐year OS was observed only in female and young colorectal cancer patients.
Patients treated with surgery alone.
Patients treated with surgery and postoperative chemotherapy.
No age cut‐offs available: age used a continuous variable rather than using specified cut‐off points.
Clinical guidelines do not consider early age of onset of CRC as a criterion to drive treatment, either in the adjuvant or in the metastatic setting. To date, therapeutic options for early‐ and late‐onset CRC patients are the same according to the major oncology societies worldwide (Benson et al., 2017; Van Cutsem et al., 2014; Yoshino et al., 2018). However, many publications describe a more aggressive attitude of both clinicians and surgeons treating early‐onset stage III and IV CRC (Khan et al., 2016; Kneuertz et al., 2015). This aggressiveness has been demonstrated to not confer a significant survival benefit (Kneuertz et al., 2015). In particular, an adjuvant, more intensive schedule of chemotherapy or targeted agents failed to confer a survival benefit and, overall, a more aggressive surgical and adjuvant approach in young CRC patients should not be recommended, since it may lead to overtreatment (Alberts et al., 2012; Allegra et al., 2011; De Gramont et al., 2012; Kneuertz et al., 2015; Taieb et al., 2017). Combining neo‐adjuvant chemo‐radiotherapy with surgery failed to confer a survival advantage in rectal patients younger than 50 years of age when compared with surgery alone (Kolarich et al., 2018). Conversely, oxaliplatin added to standard chemoradiotherapy in patients affected by locally advanced rectal cancer seems to improve disease‐free survival and OS in those < 60 years of age (Hofheinz et al., 2018).
In the metastatic setting, two recent retrospective analyses have analyzed the outcome of EO‐CRC. A retrospective study including nine phase III trials stated that patients < 40 years old at diagnosis had a poorer progression‐free survival (PFS) but not OS or response rate (RR) in comparison with patients > 50 years (Blanke et al., 2011). More recently, Lieu and coworkers considered age as a continuous variable rather than a prespecified cut‐off and pooled results of 24 first line metastatic CRC trials including young patients treated with anti‐EGFR treatment according to CAIRO2, COIN, FIRE II and PRIME studies (Lieu et al., 2014). They reported that EO‐CRC patients diagnosed in the 1920s experienced a worse PFS and OS when compared with patients diagnosed in their middle ages. They stated that prognostic effect of age did not differ according to class of targeted therapy for either OS or PFS.
Finally, age‐grouped comparisons assessing the efficacy of triplet vs doublet drug combinations in young patients are still lacking. Even if Lieu et al. were the first to include patients treated with anti‐EGFR drugs, more molecularly focused analyses are warranted to clarify this complex picture. Whether age of onset in addition to sidedness is a factor involved in determining anti‐EGFR drug resistance is still unknown (Boeckx et al., 2017; Holch et al., 2017).
2.9. RAS and BRAF mutations among EO‐CRC
Similar to all‐age populations, anti‐EGFR drugs are a valid treatment option for RAS wild‐type EO‐CRC patients (Benson et al., 2017; Douillard et al., 2013; Van Cutsem et al., 2009, 2016; Yoshino et al., 2018). RAS mutations are identified in 40% of all CRC (Cancer Genome Atlas Network, 2012).
We systematically retrieved 11 articles describing RAS and BRAF prevalence among EO‐CRC; data are conflicting (Alsop et al., 2006; Chang et al., 2012; Goel et al., 2010; Khan et al., 2016; Kirzin et al., 2014; Magnani et al., 2015; Perea et al., 2017; Rho et al., 2017; Tsai et al., 2016; Watson et al., 2016; Yantiss et al., 2009; Table 2). Most studies revealed a lower prevalence of KRAS mutations among EO‐CRC (4–31%), but others reported a similar (35–39%) or higher (54%) prevalence to that in older patients (Alsop et al., 2006; Chang et al., 2012; Goel et al., 2010; Khan et al., 2016; Kirzin et al., 2014; Magnani et al., 2015; Perea et al., 2017; Rho et al., 2017; Watson et al., 2016; Yantiss et al., 2009; Table 2).
Table 2.
Assessment of primary tumor side, KRAS, NRAS, and BRAF mutations among early‐onset colorectal cancer (EO‐CRC)
| Author (references) | Age cut‐off | Primary tumor in left colon or rectum (%) | KRAS mutation prevalence (%) | KRAS codons analyzed | NRAS mutation prevalence (%) | NRAS codons analyzed | BRAF V600E mutation prevalence (%) |
|---|---|---|---|---|---|---|---|
| Chang et al. (2012) | 40 | 44/55 (80) | 2/45 (4) | 12, 13, 61 | N/A | N/A | 0/45 (0) |
| Yantiss et al. (2009) | 40 | 22/24 (91) | 6/24 (25) | 12, 13 | N/A | N/A | 2/24 (8) |
| Goel et al. (2010) | 50 | 54/75 (72) | 18/66 (27) | 12, 13 | N/A | N/A | 0/66 (0) |
| Alsop et al. (2006) | 45 | 4/6 (67) * | 6/101 (6) | 12, 13, 61 | N/A | N/A | N/A |
| Watson et al. (2016) | 40 | 42/68 (62) | 37/68 (54) | 12, 13, 61 | 1/14 (1) | 12, 13, 61 | 0/17 (0) |
| Khan et al. (2016) | 30 | 62/94 (66) | 26 (28) | 12, 13 | N/A | N/A | 8 (9) |
| Tsai et al. (2016) | 30 | N/A | N/A | N/A | N/A | N/A | 11/66 (19) |
| Kirzin et al. (2014) | 45 | 36/48 (75) | 17/48 (35) | 12, 13 | N/A | N/A | 0/48 |
| Rho et al. (2017) | 44 | 152/224 (68) | 24/77 (31) | N/A | N/A | N/A | N/A |
| Magnani et al. (2015) | 30 | 26/33 (79) | 10/33 (30) | 12,13, 61 | N/A | N/A | 0/33 (0) |
| Perea et al. (2017) | 45 | N/A ** | 27/69 (39) | 12, 13, 61 | 3/69 (0.5) | N/A | N/A |
*Sidedness was reported only for KRAS mutant early‐onset colorectal cancer.
**In the article a general left sided predominance is reported without figures of prevalence percentage.
N/A = not available.
[Correction added after online publication on 21 January 2019: Table 2 corrected]
NRAS prevalence was reported at around 1% in a small population (n = 69) of EO‐CRC (Perea et al., 2017). The prevalence of BRAF mutations is reported to be similar among EO‐CRC and the older onset patients, ranging from 0 to 19% (Chang et al., 2012; Goel et al., 2010; Khan et al., 2016; Kirzin et al., 2014; Magnani et al., 2015; Tsai et al., 2016; Watson et al., 2016; Yantiss et al., 2009).
In conclusion, as summarized in Table 2, there are no unequivocal data regarding the prevalence of RAS/RAF mutations in EO‐CRC.
2.10. Considerations for future perspectives
Most of the studies on EO‐CRC are retrospective and include a small number of patients of different ranges of ages. Despite the wide heterogeneity, the reviewed studies can be considered to generate hypotheses, even though no definitive conclusions or recommendations can be derived. Several studies demonstrate an increase incidence of EO‐CRC in different areas of the world, but the reasons behind this epidemiologic phenomenon are still to be unveiled. To counteract the greatest loss of life‐years in this younger than average CRC population, we need to achieve the best possible clinical results with the current treatments and to develop new therapies. Non‐hereditary CRC among the youngest (< 35 years old) represents the toughest challenge and this subset of EO‐CRC is expected to become even more prevalent in the next years. Available data suggest that survival is worst in patients younger than 30 years old, whereas it is comparable or even better among patients between 40 and 50 when compared with those older than 50 years. Given the clinical and molecular peculiarities of EO‐CRC, especially in patients younger than 30 years of age, a different molecular carcinogenesis might be speculated in those sporadic cases.
A mandatory step to answering questions of recognizing EO‐CRC as a common subdivision of age groups is necessary for comparison of EO‐CRC studies. In interpreting retrospective studies, considering age as a continuous variable may be an interesting solution. In addition, clinical criteria could be useful to identify EO‐CRC patients more likely to benefit from targeted therapy, such as MSS BRCA2 mutated patients fulfilling Amsterdam criteria, or immunological treatments, such as patients harboring POLE or POLD hypermutated tumors potentially benefitting from checkpoint inhibitors. The role of environmental risk factors and the microbiome remains to be clarified. Finally, but more compelling, there is still a scarcity of clinical trials focusing on EO‐CRC and these are warranted to improve care of these patients.
Conflict of interest
AS‐B has acted as a consultant/advisory member for Amgen, Bayer, Lilly and Merck‐Serono. AB has acted as a consultant/advisory member for Horizon Discovery, Biocartis and Trovagene. SS is an advisory board member for Amgen, Bayer, BMS, CheckmAb, Celgene, Incyte, Merck, Novartis, Roche and Seattle Genetics.
Acknowledgements
The authors are supported by grants from Associazione Italiana Ricerca Cancro grant AIRC 5× mille (Project ID 51000) Special Program Molecular Clinical Oncology, AIRC Investigator Grant (Project ID 20685), and AIRC Special Program 5 per mille Metastases Project ID 21091; CORDIS Community Research and Development Information Service, Horizon 2020 (Project ID 635342) grant, Molecularly Guided Trials with Specific Treatment Strategies in Patients with Advanced Newly Molecular Defined Subtypes of Colorectal Cancer (MoTriColor); Fondazione Oncologia Niguarda Onlus, grant Terapia Molecolare dei Tumori; and Studies to Develop Therapies Against Colorectal Cancer in Young Adults grant.
Gianluca Mauri and Andrea Sartore‐Bianchi contributed equally as first authors.
Alberto Bardelli and Salvatore Siena contributed equally as senior authors.
References
- Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM and Hendren S (2016) Colorectal cancer outcomes and treatment patterns in patients too young for average‐risk screening. Cancer 122, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou‐Zeid AA, Jumuah WA, Ebied EF, Abd El Samee Atia KS, El Ghamrini Y, Somaie DA (2017) Hereditary factors are unlikely behind unusual pattern of early‐onset colorectal cancer in Egyptians: a study of family history and pathology features in Egyptians with large bowel cancer (cross‐sectional study). Int J Surg 44, 71–75. [DOI] [PubMed] [Google Scholar]
- Ågesen TH, Berg M, Clancy T, Thiis‐Evensen E, Cekaite L, Lind GE, Nesland JM, Bakka A, Mala T, Hauss HJ et al (2011) CLC and IFNAR1 are differentially expressed and a global immunity score is distinct between early‐ and late‐onset colorectal cancer. Genes Immun 12, 653–662. [DOI] [PubMed] [Google Scholar]
- Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A and You YN (2014) The increasing incidence of young‐onset colorectal cancer: a call to action. Mayo Clin Proc 89, 216–224. [DOI] [PubMed] [Google Scholar]
- Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S et al (2012) Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 307, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova K, Pischon T, Buijsse B, May AM, Peeters PH, Bueno‐de‐Mesquita HB, Jenab M, Fedirko V, Dahm CC, Siersema PD et al (2013) Adult weight change and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer 49, 3526–3536. [DOI] [PubMed] [Google Scholar]
- Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM et al (2011) Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C‐08. J Clin Oncol 29, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop K, Mead L, Smith LD, Royce SG, Tesoriero AA, Young JP, Haydon A, Grubb G, Giles GG, Jenkins MA et al (2006) Low somatic K‐ras mutation frequency in colorectal cancer diagnosed under the age of 45 years. Eur J Cancer 42, 1357–1361. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Colorectal Cancer Facts & Figures . 2017‐2019. (2017). Acessed 5 April 2018. From https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html
- Antelo M, Balaguer F, Shia J, Shen Y, Hur K, Moreira L, Cuatrecasas M, Bujanda L, Giraldez MD, Takahashi M et al (2012) A high degree of LINE‐1 hypomethylation is a unique feature of early‐onset colorectal cancer. PLoS ONE 7, e45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaghany T, Wilson JD, Chu Q and Mills G (2012) Genetic alterations in colorectal cancer. Gastrointest Cancer Res GCR 5, 19–27. [PMC free article] [PubMed] [Google Scholar]
- Bailey CE, Hu C‐Y, You YN, Bednarski BK, Rodriguez‐Bigas MA, Skibber JM, Cantor SB and Chang GJ (2015) Increasing disparities in the age‐related incidences of colon and rectal cancers in the United States, 1975‐2010. JAMA Surg 150, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjea A, Hands RE, Powar MP, Bustin SA and Dorudi S (2009) Microsatellite and chromosomal stable colorectal cancers demonstrate poor immunogenicity and early disease recurrence. Colorectal Dis 11, 601–608. [DOI] [PubMed] [Google Scholar]
- Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL and Giles GG (2010) Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 19, 2978–2986. [DOI] [PubMed] [Google Scholar]
- Bellido F, Sowada N, Mur P, Lázaro C, Pons T, Valdés‐Mas R, Pineda M, Aiza G, Iglesias S, Soto JL et al (2018) Association between germline mutations in BRF1, a subunit of the RNA polymerase III transcription complex, and hereditary colorectal cancer. Gastroenterology 154, 181–194.e20. [DOI] [PubMed] [Google Scholar]
- Benson AB, Venook AP, Cederquist L, Chan E, Chen Y‐J, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A et al (2017) Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15, 370–398. [DOI] [PubMed] [Google Scholar]
- Berg M, Agesen TH, Thiis‐Evensen E, INFAC‐study group , Merok MA, Teixeira MR, Vatn MH, Nesbakken A, Skotheim RI and Lothe RA. (2010) Distinct high resolution genome profiles of early onset and late onset colorectal cancer integrated with gene expression data identify candidate susceptibility loci. Mol Cancer 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari A, Woodhouse M and Gupta S (2017) Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER‐based analysis with comparison to other young‐onset cancers. J Investig Med 65, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke CD, Bot BM, Thomas DM, Bleyer A, Kohne C‐H, Seymour MT, de Gramont A, Goldberg RM and Sargent DJ (2011) Impact of young age on treatment efficacy and safety in advanced colorectal cancer: a pooled analysis of patients from nine‐first‐line phase III chemotherapy trials. Clin Oncol 29, 2781–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A, Barr R, Hayes‐Lattin B, Thomas D, Ellis C and Anderson B, and Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology (2008) The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 8, 288–298. [DOI] [PubMed] [Google Scholar]
- Bleyer A, Barr R, Ries L, Whelan J. and Ferrari A, eds (2017) Cancer in Adolescents and Young Adults. Cham: Springer International Publishing. [Google Scholar]
- Boeckx N, Koukakis R, Opde Beeck K, Rolfo C, Van Camp G, Siena S, Tabernero J, Douillard JY, André T and Peeters M (2017) Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first‐line panitumumab studies. Ann Oncol 28, 1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S, Nassar N, Lee CYY, Suen MK, Al Zahrani S and Gladman MA (2016) Young‐onset colorectal cancer in New South Wales: a population‐based study. Med J Aust 205, 465–470. [DOI] [PubMed] [Google Scholar]
- Brenner DR, Ruan Y, Shaw E, De P, Heitman SJ and Hilsden RJ (2017) Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med 105, 345–349. [DOI] [PubMed] [Google Scholar]
- Burn J, Gerdes A‐M, Macrae F, Mecklin J‐P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L et al (2011) Long‐term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378, 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Xu Y, Lu H, Shi Y, Lian P, Peng J, Du X, Zhou X, Guan Z, Shi D et al (2008) Clinicopathologic and molecular features of sporadic microsatellite‐ and chromosomal‐stable colorectal cancers. Int J Colorectal Dis 23, 365–373. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari R, Friedl W, Mandl M, Möslein G, Kadmon M, Knapp M, Jacobasch KH, Ecker KW, Kreissler‐Haag D, Timmermanns G et al (1994) Familial adenomatous polyposis: mutation at codon 1309 and early onset of colon cancer. Lancet 343, 629–632. [DOI] [PubMed] [Google Scholar]
- Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, Castaño‐Vinyals G, Pérez‐Gómez B, Olmedo‐Requena R, Guevara M, Fernandez‐Tardon G et al (2018) Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr [Epub ahead of print]. 10.1007/s00394-018-1674-5. [DOI] [PubMed] [Google Scholar]
- Chan TL, Curtis LC, Leung SY, Farrington SM, Ho JW, Chan AS, Lam PW, Tse CW, Dunlop MG, Wyllie AH et al (2001) Early‐onset colorectal cancer with stable microsatellite DNA and near‐diploid chromosomes. Oncogene 20, 4871–4876. [DOI] [PubMed] [Google Scholar]
- Chandrasinghe PC, Ediriweera DS, Nazar T, Kumarage S, Hewavisenthi J and Deen KI (2017) Overall survival of elderly patients having surgery for colorectal cancer is comparable to younger patients: results from a South Asian population. Gastroenterol Res Pract 2017, 9670512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, Koong AC, Kunz PA, Fisher GA, Ford JM et al (2012) Clinicopathologic and molecular features of sporadic early‐onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 25, 1128–1139. [DOI] [PubMed] [Google Scholar]
- Chou C‐L, Chang S‐C, Lin T‐C, Chen W‐S, Jiang J‐K, Wang H‐S, Yang SH, Liang WY and Lin JK (2011) Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg 202, 574–582. [DOI] [PubMed] [Google Scholar]
- Chou C‐L, Tseng C‐J and Shiue Y‐L (2017) The impact of young age on the prognosis for colorectal cancer: a population‐based study in Taiwan. Jpn J Clin Oncol 47, 1010–1018. [DOI] [PubMed] [Google Scholar]
- Coccia PF, Pappo AS, Beaupin L, Borges VF, Borinstein SC, Chugh R, Dinner S, Folbrecht J, Frazier AL, Goldsby R et al (2018) Adolescent and young adult oncology, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 16, 66–97. [DOI] [PubMed] [Google Scholar]
- Colorectal Cancer ‐ Cancer Stat Facts (2018). (Accessed 19 May 2018). From https://seer.cancer.gov/statfacts/html/colorect.html
- Damodaran D and Seshadri RA (2016) Clinicopathological attributes and outcomes of treatment in young‐onset rectal cancer. Int J Colorectal Dis 31, 757–759. [DOI] [PubMed] [Google Scholar]
- de Gramont A, Van Cutsem E, Schmoll H‐J, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F et al (2012) Bevacizumab plus oxaliplatin‐based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 13, 1225–1233. [DOI] [PubMed] [Google Scholar]
- Douillard J‐Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J et al (2013) Panitumumab‐FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369, 1023–1034. [DOI] [PubMed] [Google Scholar]
- Durno C, Aronson M, Bapat B, Cohen Z, Gallinger S (2005) Family history and molecular features of children, adolescents, and young adults with colorectal carcinoma. Gut 54, 1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durno CA, Sherman PM, Aronson M, Malkin D, Hawkins C, Bakry D, Bouffet E, Gallinger S, Pollett A, Campbell B et al (2015) Phenotypic and genotypic characterisation of biallelic mismatch repair deficiency (BMMR‐D) syndrome. Eur J Cancer 1990, 977–983. [DOI] [PubMed] [Google Scholar]
- Edwards BK, Noone A‐M, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA et al (2014) Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120, 1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DG, Walsh S, Hill J and McMahon RT (2007) Strategies for identifying hereditary nonpolyposis colon cancer. Semin Oncol 34, 411–417. [DOI] [PubMed] [Google Scholar]
- Exarchakou A, Donaldson L, Coleman MP (2018) Increasing colorectal cancer incidence among young adults in England diagnosed during 2001‐2014. Ann Oncol 29, viii562–viii575. 10.1093/annonc/mdy297. [DOI] [Google Scholar]
- Fast Stats (2018). (Accessed 19 August 2018). From https://seer.cancer.gov/faststats/selections.php?#Output
- Ferrari A, Rognone A, Casanova M, Zaffignani E, Piva L, Collini P, Bertario L, Sala P, Leo E, Belli F et al (2008) Colorectal carcinoma in children and adolescents: the experience of the Istituto Nazionale Tumori of Milan, Italy. Pediatr Blood Cancer 50, 588–593. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui‐Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ et al (2009) Inhibition of poly(ADP‐ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361, 123–134. [DOI] [PubMed] [Google Scholar]
- Fu J‐F, Huang Y‐Q, Yang J, Yi C‐H, Chen H‐L and Zheng S (2013) Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol 19, 8078–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang Y, Chen H, Yi C, Zheng S and Yuan Y (2014) Young patients (≤ 35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30‐year retrospective review. Medicine (Baltimore) 93, e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi J, Davidson C, Hall C, Pearson J, Eglinton T, Wakeman C and Frizelle F (2017) Population‐based study demonstrating an increase in colorectal cancer in young patients. Br J Surg 104, 1063–1068. [DOI] [PubMed] [Google Scholar]
- Garre P, Martín L, Sanz J, Romero A, Tosar A, Bando I, Llovet P, Diaque P, García‐Paredes B, Díaz‐Rubio E et al (2015) BRCA2 gene: a candidate for clinical testing in familial colorectal cancer type X. Clin Genet 87, 582–587. [DOI] [PubMed] [Google Scholar]
- Goel A, Nagasaka T, Spiegel J, Meyer R, Lichliter WE and Boland CR (2010) Low frequency of Lynch syndrome among young patients with non‐familial colorectal cancer. Clin Gastroenterol Hepatol 8, 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M‐M, Vreede L, Bemelmans SASA, van der Looij E, van Kessel AG, Schackert HK, Ligtenberg MJ, Hoogerbrugge N, Kuiper RP and de Voer RM (2016) Prevalence of germline mutations in the spindle assembly checkpoint gene BUB1B in individuals with early‐onset colorectal cancer. Genes Chromosom Cancer 55, 855–863. [DOI] [PubMed] [Google Scholar]
- Haleshappa RA, Rao SA, Garg S, Kuntegowdanahalli CL, Kanakasetty GB and Dasappa L (2017) Is colorectal cancer in young (<40 Years) different from those in the elderly (>40 Years): experience from a regional care center. Indian J Med Paediatr Oncol 38, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW and Westman J et al. (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352, 1851–1860. [DOI] [PubMed] [Google Scholar]
- Hawk NN, Long T‐E, Imam MH, Mathew BM, Kim S, Chen Z, Goodman M, Sullivan P, Brutcher E, Kauh J et al (2015) Clinicopathologic features and outcome of young adults with stage IV colorectal cancer. Am J Clin Oncol 38, 543–549. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR and Flegal KM (2004) Prevalence of overweight and obesity among US children, adolescents, and adults, 1999‐2002. JAMA 291, 2847–2850. [DOI] [PubMed] [Google Scholar]
- Hessami Arani S and Kerachian MA (2017) Rising rates of colorectal cancer among younger Iranians: is diet to blame? Curr Oncol 24, e131–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Furman WL, Billups CA, Riedley SE, Cain AM, Rao BN, Pratt CB and Spunt SL (2007) Colorectal carcinoma in childhood and adolescence: a clinicopathologic review. J Clin Oncol 25, 5808–5814. [DOI] [PubMed] [Google Scholar]
- Hofheinz R‐D, Arnold D, Fokas E, Kaufmann M, Hothorn T, Folprecht G, Fietkau R, Hohenberger W, Ghadimi M, Liersch T et al (2018) Impact of age on the efficacy of oxaliplatin in the preoperative chemoradiotherapy and adjuvant chemotherapy of rectal cancer: A post hoc analysis of the CAO/ARO/AIO‐04 phase 3 trial. Ann Oncol 29, 1793–1799. 10.1093/annonc/mdy205. [DOI] [PubMed] [Google Scholar]
- Holch JW, Ricard I, Stintzing S, Modest DP and Heinemann V (2017) The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta‐analysis of first‐line clinical trials. Eur J Cancer 1990, 87–98. [DOI] [PubMed] [Google Scholar]
- Holme Ø, Bretthauer M, Fretheim A, Odgaard‐Jensen J, Hoff G (2013) Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 9, CD009259 10.1002/14651858.CD009259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Ho KS, Eu KW and Cheah PY (2007) A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clin Cancer Res 13, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Hubbard J, Thomas DM, Yothers G, Green E, Blanke C, O'Connell MJ, Labianca R, Shi Q, Bleyer A, de Gramont A et al (2012) Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set. J Clin Oncol 30, 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indini A, Bisogno G, Cecchetto G, Vitellaro M, Signoroni S, Massimino M, Riccipetitoni G, Zecca M, Dall'Igna P, De Pasquale MD et al (2017) Gastrointestinal tract carcinoma in pediatric and adolescent age: the Italian TREP project experience. Pediatr Blood Cancer 64, 10.1002/pbc.26658. [DOI] [PubMed] [Google Scholar]
- Järvinen HJ, Aarnio M, Mustonen H, Aktan‐Collan K, Aaltonen LA, Peltomäki P, De La Chapelle A and Mecklin JP (2000) Controlled 15‐year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118, 829–834. [DOI] [PubMed] [Google Scholar]
- Jasperson KW, Tuohy TM, Neklason DW and Burt RW (2010) Hereditary and familial colon cancer. Gastroenterology 138, 2044–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josifovski J, Stojanović S, Radocević‐Jelić L and Josifovski T (2004) Localization, clinical and pathological characteristics and survival in sporadic colon cancer patients younger than 40 and over 65 years of age. J BUON 9, 403–408. [PubMed] [Google Scholar]
- Kam MH, Eu KW, Barben CP and Seow‐Choen F (2004) Colorectal cancer in the young: a 12‐year review of patients 30 years or less. Colorectal Dis 6, 191–194. [DOI] [PubMed] [Google Scholar]
- Karnak I, Ciftci AO, Senocak ME and Büyükpamukçu N (1999) Colorectal carcinoma in children. J Pediatr Surg 34, 1499–1504. [DOI] [PubMed] [Google Scholar]
- Kempers MJE, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, Schackert HK, Steinke V, Holinski‐Feder E, Morak M et al (2011) Risk of colorectal and endometrial cancers in EPCAM deletion‐positive Lynch syndrome: a cohort study. Lancet Oncol 12, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Morris M, Idrees K, Gimbel MI, Rosenberg S, Zeng Z, Li F, Gan G, Shia J, LaQuaglia MP et al (2016) Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg 51, 1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Kim ER, Hong SN, Chang DK and Kim Y‐H (2016) Long‐term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional‐based retrospective study. Medicine (Baltimore) 95, e3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SB, Lee HJ, Park SJ, Hong SP, Cheon JH, Kim WH and Kim TI (2017) Comparison of colonoscopy surveillance outcomes between young and older colorectal cancer patients. J Cancer Prev 22, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Jung YS, Yang H‐J, Park S‐K, Park JH, Park DI and Sohn CI (2018) Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20‐39 years old). Clin Gastroenterol Hepatol. pii: S1542‐3565(18)30711‐0. 10.1016/j.cgh.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Kirzin S, Marisa L, Guimbaud R, De Reynies A, Legrain M, Laurent‐Puig P, Cordelier P, Pradère B, Bonnet D, Meggetto F et al (2014) Sporadic early‐onset colorectal cancer is a specific sub‐type of cancer: a morphological, molecular and genetics study. PLoS ONE 9, e103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneuertz PJ, Chang GJ, Hu C‐Y, Rodriguez‐Bigas MA, Eng C, Vilar E, Skibber JM, Feig BW, Cormier JN and You YN (2015) Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 150, 402–409. [DOI] [PubMed] [Google Scholar]
- Kolarich A, George TJ, Hughes SJ, Delitto D, Allegra CJ, Hall WA, Chang GJ, Tan SA, Shaw CM and Iqbal A (2018) Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline‐directed treatment for stage II and III disease. Cancer 124, 3510–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G, Gafà R, Santini A, Maestri I, Guerzoni L and Cavazzini L (2006) Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol 24, 2359–2367. [DOI] [PubMed] [Google Scholar]
- Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T et al. (2012) Olaparib maintenance therapy in platinum‐sensitive relapsed ovarian cancer. N Engl J Med 366, 1382–1392. [DOI] [PubMed] [Google Scholar]
- Li Q, Cai G, Li D, Wang Y, Zhuo C and Cai S (2014) Better long‐term survival in young patients with non‐metastatic colorectal cancer after surgery, an analysis of 69 835 patients in SEER database. PLoS ONE 9, e93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS and Wang SM (2003) Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg 90, 205–214. [DOI] [PubMed] [Google Scholar]
- Lieu CH, Renfro LA, de Gramont A, Meyers JP, Maughan TS, Seymour MT, Saltz L, Goldberg RM, Sargent DJ, Eckhardt SG et al (2014) Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol 32, 2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg MJL, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, Lee TY, Bodmer D, Hoenselaar E, Hendriks‐Cornelissen SJ et al (2009) Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet 41, 112–117. [DOI] [PubMed] [Google Scholar]
- Losi L, Di Gregorio C, Pedroni M, Ponti G, Roncucci L, Scarselli A, Genuardi M, Baglioni S, Marino M, Rossi G et al (2005) Molecular genetic alterations and clinical features in early‐onset colorectal carcinomas and their role for the recognition of hereditary cancer syndromes. Am J Gastroenterol 100, 2280–2287. [DOI] [PubMed] [Google Scholar]
- Lubbe SJ, Di Bernardo MC, Chandler IP and Houlston RS (2009) Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 27, 3975–3980. [DOI] [PubMed] [Google Scholar]
- Luzzatto L and Pandolfi PP (2015) Causality and chance in the development of cancer. N Engl J Med 373, 84–88. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Lynch JF, Lynch PM and Attard T (2008) Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis and management. Fam Cancer 7, 27–39. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Snyder CL, Shaw TG, Heinen CD and Hitchins MP (2015) Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer 15, 181–194. [DOI] [PubMed] [Google Scholar]
- Magnani G, Furlan D, Sahnane N, Reggiani Bonetti L, Domati F and Pedroni M (2015) Molecular features and methylation status in early onset (≤40 Years) colorectal cancer: a population based case‐control study. Gastroenterol Res Pract 2015, 132190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekzadeh R, Bishehsari F, Mahdavinia M and Ansari R (2009) Epidemiology and molecular genetics of colorectal cancer in iran: a review. Arch Iran Med 12, 161–169. [PubMed] [Google Scholar]
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C and Negri E (2018) European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol 29, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Manjelievskaia J, Brown D, McGlynn KA, Anderson W, Shriver CD and Zhu K (2017) Chemotherapy use and survival among young and middle‐aged patients with colon cancer. JAMA Surg 152, 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz SD and Bertagnolli MM (2009) Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 361, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez‐Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N et al (2015) DNA‐repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373, 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DC and McArdle CS (2009) The impact of young age on cancer‐specific and non‐cancer‐related survival after surgery for colorectal cancer: 10‐year follow‐up. Br J Cancer 101, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JE, Narang T, Schnoll‐Sussman FH, Pochapin MB, Christos PJ and Sherr DL (2010) Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer 116, 4354–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguchi M, Hinoi T, Tanakaya K, Yamaguchi T, Furukawa Y, Yoshida T, Tamura K, Sugano K, Ishioka C, Matsubara N et al (2018) Alcohol consumption and early‐onset risk of colorectal cancer in Japanese patients with Lynch syndrome: a cross‐sectional study conducted by the Japanese Society for Cancer of the Colon and Rectum. Surg Today 48, 810–814. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J and Altman DG, and PRISMA Group (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–1012. [DOI] [PubMed] [Google Scholar]
- Mork ME, You YN, Ying J, Bannon SA, Lynch PM, Rodriguez‐Bigas MA and Vilar E (2015) High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J Clin Oncol 33, 3544–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur P, De Voer RM, Olivera‐Salguero R, Rodríguez‐Perales S, Pons T, Setién F, Aiza G, Valdés‐Mas R, Bertini A, Pineda M et al (2018) Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are infrequent in familial colorectal cancer and polyposis. Mol Cancer 17, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Nagasaki T, Nagata J, Ohno R et al (2016) Clinicopathological characteristics of young patients with sporadic colorectal cancer. Surg Today 46, 1166–1175. [DOI] [PubMed] [Google Scholar]
- Mylavarapu S, Das A and Roy M (2018) Role of BRCA mutations in the modulation of response to platinum therapy. Front Oncol 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejadtaghi M, Jafari H, Farrokhi E and Samani KG (2017) Familial colorectal cancer type X (FCCTX) and the correlation with various genes‐A systematic review. Curr Probl Cancer 41, 388–397. [DOI] [PubMed] [Google Scholar]
- O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH and Ko CY (2003) Rates of colon and rectal cancers are increasing in young adults. Am Surg 69, 866–872. [PubMed] [Google Scholar]
- O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH and Ko CY (2004a) Do young colon cancer patients have worse outcomes? World J Surg 28, 558–562. [DOI] [PubMed] [Google Scholar]
- O'Connell JB, Maggard MA, Livingston EH and Yo CK (2004b) Colorectal cancer in the young. Am J Surg 187, 343–348. [DOI] [PubMed] [Google Scholar]
- Orsini RG, Verhoeven RHA, Lemmens VEPP, van Steenbergen LN, de Hingh IHJT, Nieuwenhuijzen GAP, Rutten HJT (2015) Comparable survival for young rectal cancer patients, despite unfavourable morphology and more advanced‐stage disease. Eur J Cancer 1990, 1675–1682. [DOI] [PubMed] [Google Scholar]
- Pai SG, Carneiro BA, Chae YK, Costa RL, Kalyan A, Shah HA, Helenowski I, Rademaker AW, Mahalingam D and Giles FJ (2017) Correlation of tumor mutational burden and treatment outcomes in patients with colorectal cancer. J Gastrointest Oncol 8, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palles C, Cazier J‐B, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I et al (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomaki GE, McClain MR, Melillo S, Hampel HL and Thibodeau SN (2009) EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med 11, 42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, Bigley C, Nelsen L, Goodfellow PJ et al (2017) Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early‐onset colorectal cancer. JAMA Oncol 3, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea J, Arriba M, Rodríguez Y, Rueda D, García JL, Pérez J, González‐Sarmiento R and Urioste M (2017) Frequency and impact of KRAS mutation in early onset colorectal cancer. Hum Pathol 61, 221–222. [DOI] [PubMed] [Google Scholar]
- Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, Smith RA, Zauber AG and Lansdorp‐Vogelaar I (2018) The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 124, 2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñol V, Castells A, Andreu M, Castellví‐Bel S, Alenda C, Llor X, Xicola RM, Rodríguez‐Moranta F, Payá A, Jover R et al (2005) Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 293, 1986–1994. [DOI] [PubMed] [Google Scholar]
- Pokharkar AB, Bhandare M, Patil P, Mehta S, Engineer R and Saklani AP (2017) Young Vs Old colorectal cancer in indian subcontinent: a tertiary care center experience. Indian J Surg Oncol 8, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccini A, Lenz H‐J, Marshall JL, Arguello D, Raghavan D, Korn WM, Weinberg BA, Poorman K, Heeke AL, Philip PA et al (2018) Impact of patient age on molecular alterations of left‐sided colorectal tumors. Oncologist. https://doi:10.1634/theoncologist.2018-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah HM, Joseph R, Schrag D, Shia J, Guillem JG, Paty PB, Temple LK, Wong WD and Weiser MR (2007) Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol 14, 2759–2765. [DOI] [PubMed] [Google Scholar]
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF et al (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 107, 1315–1329; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho YS, Gilabert M, Polom K, Aladashvili A, Kopeckova K, Megdanova V, Coleman N, Greally M, Marrelli D, Roviello F et al (2017) Comparing clinical characteristics and outcomes of young‐onset and late‐onset colorectal cancer: an international collaborative study. Clin Colorectal Cancer 16, 334–342. [DOI] [PubMed] [Google Scholar]
- Riaz R, Masood N and Benish A (2017) Red flag symptoms: detailed account of clinicopathological features in young‐onset colorectal cancer. Intest Res 15, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M, Im S‐A, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377, 523–533. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Brennan K, Karim S, Nanji S, Patel SV and Booth CM (2018) Disease characteristics, clinical management, and outcomes of young patients with colon cancer: a population‐based study. Clin Colorectal Cancer 17, e651–e661. [DOI] [PubMed] [Google Scholar]
- Shaikh T, Handorf EA, Meyer JE, Hall MJ and Esnaola NF (2018) Mismatch repair deficiency testing in patients with colorectal cancer and nonadherence to testing guidelines in young adults. JAMA Oncol 4, e173580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Mo M, Jia L, Jia H, Li Q, Liang L, Shi D, Zhang Z, Cai S, Li X et al (2018) Poorer prognosis in young female patients with non‐metastatic colorectal cancer: a hospital‐based analysis of 5047 patients in China. Cancer Manag Res 10, 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Ahiko Y, Tanabe T, Yoshida T, Tsukamoto S, Ochiai H, Takashima A, Boku N and Kanemitsu Y (2018) Shorter survival in adolescent and young adult patients, compared to adult patients, with stage IV colorectal cancer in Japan. BMC Cancer 18, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Cho H‐S, Kim D‐W, Ahn SY, Ihn MH, Park HJ, Oh HK and Kang SB (2017) Does routine colonoscopy help diagnose familial adenomatous polyposis in patients presenting with desmoid tumors but no gastrointestinal symptoms? Int J Colorectal Dis 32, 151–154. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD and Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Silla IO, Rueda D, Rodríguez Y, García JL, de la Cruz Vigo F and Perea J (2014) Early‐onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol 20, 17288–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver A, Sengupta N, Propper D, Wilson P, Hagemann T, Patel A, Parker A, Ghosh A, Feakins R, Dorudi S et al (2012) A distinct DNA methylation profile associated with microsatellite and chromosomal stable sporadic colorectal cancers. Int J Cancer 130, 1082–1092. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA (2018) Lynch syndrome‐associated colorectal cancer. N Engl J Med 379, 764–773. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Halling KC, Foster N, Sargent DJ, La Plant B, French AJ, Laurie JA, Goldberg RM, Thibodeau SN et al (2006) Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 131, 729–737. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Boucher KM, Caan BJ, Potter JD and Ma KN (1998) Eating patterns and risk of colon cancer. Am J Epidemiol 148, 4–16. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R and Schaffer D (2003) Physical activity and colorectal cancer. Am J Epidemiol 158, 214–224. [DOI] [PubMed] [Google Scholar]
- Snover DC (2011) Update on the serrated pathway to colorectal carcinoma. Hum Pathol 42, 1–10. [DOI] [PubMed] [Google Scholar]
- Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, Osborne J, Williams L, Hanson K, Gruber SB and Rozek LS (2018) Germline genetic features of young individuals with colorectal cancer. Gastroenterology 154, 897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan I, Rodriguez‐Galindo C, El‐Taani H, Pastore G, Casanova M, Gallino G and Ferrari A (2010) Distinct features of colorectal cancer in children and adolescents: a population‐based study of 159 cases. Cancer 116, 758–765. [DOI] [PubMed] [Google Scholar]
- Taieb J, Balogoun R, Le Malicot K, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Emile JF, Mulot C, Fratté S et al (2017) Adjuvant FOLFOX ± cetuximab in full RAS and BRAF wildtype stage III colon cancer patients. Ann Oncol 28, 824–830. [DOI] [PubMed] [Google Scholar]
- Tang R, Changchien CR, Wu M‐C, Fan C‐W, Liu K‐W, Chen J‐S, Chien HT and Hsieh LL (2004) Colorectal cancer without high microsatellite instability and chromosomal instability–an alternative genetic pathway to human colorectal cancer. Carcinogenesis 25, 841–846. [DOI] [PubMed] [Google Scholar]
- Thune I and Lund E (1996) Physical activity and risk of colorectal cancer in men and women. Br J Cancer 73, 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C and Vogelstein B (2015) Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran B, Kopetz S, Tie J, Gibbs P, Jiang Z‐Q, Lieu CH, Agarwal A, Maru DM, Sieber O and Desai J (2011) Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117, 4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafillidis JK, Nasioulas G and Kosmidis PA (2009) Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 29, 2727–2737. [PubMed] [Google Scholar]
- Tricoli JV, Blair DG, Anders CK, Bleyer WA, Boardman LA, Khan J, Kummar S, Hayes‐Lattin B, Hunger SP, Merchant M et al (2016) Biologic and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer 122, 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeung L, Sodhi‐Berry N, Martini A, Malacova E, Ee H, O'Leary P, Lansdorp‐Vogelaar I and Preen DB (2017) Increasing incidence of colorectal cancer in adolescents and young adults aged 15‐39 years in Western Australia 1982‐2007: examination of colonoscopy history. Front Public Health 5, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J‐H, Liau J‐Y, Lin Y‐L, Tseng L‐H, Lin L‐I, Yeh K‐H and Jeng Y‐M (2016) Frequent BRAF mutation in early‐onset colorectal cancer in Taiwan: association with distinct clinicopathological and molecular features and poor clinical outcome. J Clin Pathol 69, 319–325. [DOI] [PubMed] [Google Scholar]
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R et al (2004) Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force (2002) Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 137, 129–131. [DOI] [PubMed] [Google Scholar]
- Vale Rodrigues R, Claro I, Lage P, Rosa I, Ferreira S, Pereira da Silva J and Dias Pereira A (2018) Colorectal cancer surveillance in Portuguese families with lynch syndrome: a cohort study. Int J Colorectal Dis 33, 695–702. [DOI] [PubMed] [Google Scholar]
- Valle L, Hernández‐Illán E, Bellido F, Aiza G, Castillejo A, Castillejo M‐I, Navarro M, Seguí N, Vargas G, Guarinos C et al (2014) New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum Mol Genet 23, 3506–3512. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Köhne C‐H, Hitre E, Zaluski J, Chang Chien C‐R, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360, 1408–1417. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, and ESMO Guidelines Working Group (2014) Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 25(Suppl 3), iii1–iii9. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken HJ, Aderka D, Aranda Aguilar E, Bardelli A, Benson A et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27, 1386–1422. [DOI] [PubMed] [Google Scholar]
- Vasen HF, Mecklin JP, Khan PM and Lynch HT (1991) The International Collaborative Group On Hereditary Non‐Polyposis Colorectal Cancer (ICG‐HNPCC). Dis Colon Rectum 34, 424–425. [DOI] [PubMed] [Google Scholar]
- Vasen HF, Watson P, Mecklin JP and Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 116, 1453–1456. [DOI] [PubMed] [Google Scholar]
- Vatandoust S, Price TJ, Ullah S, Roy AC, Beeke C, Young JP, Townsend A, Padbury R, Roder D and Karapetis CS (2016) Metastatic colorectal cancer in young adults: a study from the South Australian population‐based registry. Clin Colorectal Cancer 15, 32–36. [DOI] [PubMed] [Google Scholar]
- Vuik FER, Nieuwenburg SAV, Bardou M, Dinis‐Ribeiro M, Bento MJ, Zadnik V, Esteban L, Urquiza MP, Kaminski MF and Suchanek S et al (2018) Increasing incidence of colorectal cancer in young adults in Europe. United European Gastroenterol J 6(Suppl 1). [Google Scholar]
- Wang M‐J, Ping J, Li Y, Adell G, Arbman G, Nodin B, Meng WJ, Zhang H, Yu YY, Wang C et al (2015a) The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep 5, 10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wang M‐J and Ping J (2015b) Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: a population‐based cohort study of SEER 9 registries data (1988‐2011). Medicine (Baltimore) 94, e1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier SK and Kalady MF (2012) Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin Colon Rectal Surg 25, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Liu T‐C and Ruzinova MB (2016) High frequency of KRAS mutation in early onset colorectal adenocarcinoma: implications for pathogenesis. Hum Pathol 56, 163–170. [DOI] [PubMed] [Google Scholar]
- Welch HG and Robertson DJ (2016) Colorectal cancer on the decline – why screening can't explain it all. N Engl J Med 374, 1605–1607. [DOI] [PubMed] [Google Scholar]
- Weren RDA, Ligtenberg MJL, Kets CM, de Voer RM, Verwiel ETP, Spruijt L, van Zelst‐Stams WA, Jongmans MC, Gilissen C, Hehir‐Kwa JY et al (2015) A germline homozygous mutation in the base‐excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet 47, 668–671. [DOI] [PubMed] [Google Scholar]
- Weren RD, Ligtenberg MJ, Geurts van Kessel A, De Voer RM, Hoogerbrugge N and Kuiper RP (2018) NTHL1 and MUTYH polyposis syndromes: two sides of the same coin? J Pathol 244, 135–142. [DOI] [PubMed] [Google Scholar]
- Wimmer K and Etzler J (2008) Constitutional mismatch repair‐deficiency syndrome: have we so far seen only the tip of an iceberg? Hum Genet 124, 105–122. [DOI] [PubMed] [Google Scholar]
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D et al (2003) Colorectal cancer screening and surveillance: clinical guidelines and rationale‐Update based on new evidence. Gastroenterology 124, 544–560. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kang L, Wang L, Xiang J, Cai G, Cui J, Peng J, Lan P and Wang J (2012) Characteristics and long‐term survival of colorectal cancer patients aged 44 years and younger. Clin Transl Oncol 14, 896–904. [DOI] [PubMed] [Google Scholar]
- Yantiss RK, Goodarzi M, Zhou XK, Rennert H, Pirog EC, Banner BF and Chen Y‐T (2009) Clinical, pathologic, and molecular features of early‐onset colorectal carcinoma. Am J Surg Pathol 33, 572–582. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Mizuno N, Hara K, Hijioka S, Imaoka H, Hieda N et al (2017) The features of colorectal tumors in a patient with Li‐Fraumeni syndrome. Intern Med 56, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu R‐H, Kim TW, Ismail F, Tan IB, Yeh KH et al (2018) Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 29, 44–70. [DOI] [PubMed] [Google Scholar]
- You YN, Dozois EJ, Boardman LA, Aakre J, Huebner M and Larson DW (2011) Young‐onset rectal cancer: presentation, pattern of care and long‐term oncologic outcomes compared to a matched older‐onset cohort. Ann Surg Oncol 18, 2469–2476. [DOI] [PubMed] [Google Scholar]
- You YN, Xing Y, Feig BW, Chang GJ and Cormier JN (2012) Young‐onset colorectal cancer: is it time to pay attention? Arch Intern Med 172, 287–289. [DOI] [PubMed] [Google Scholar]
- Yurgelun MB, Masciari S, Joshi VA, Mercado RC, Lindor NM, Gallinger S, Hopper JL, Jenkins MA, Buchanan DD, Newcomb PA et al (2015) Germline TP53 mutations in patients with early‐onset colorectal cancer in the colon cancer family registry. JAMA Oncol 1, 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaanan A, Shi Q, Taieb J, Alberts SR, Meyers JP, Smyrk TC, Julie C, Zawadi A, Tabernero J, Mini E et al (2018) Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol 4, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbuk K, Sidebotham EL, Bleyer A and La Quaglia MP (2009) Colorectal cancer in young adults. Semin Oncol 36, 439–450. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bao F, Yan J, Liu H, Li T, Chen H and Li G (2017) Poor prognosis of young patients with colorectal cancer: a retrospective study. Int J Colorectal Dis 32, 1147–1156. [DOI] [PubMed] [Google Scholar]


