Abstract
Androgen deprivation therapy is frequently used to treat prostate cancer (PCa), but resistance can occur, a condition known as castration‐resistant prostate cancer (CRPC). Thus, novel approaches for identification of CRPC are important for designing effective PCa treatments. Analysis of microRNA (miRNA) expression signatures by RNA sequencing showed that both passenger and guide strands of the miR‐455‐duplex (miR‐455‐5p and miR‐455‐3p, respectively) acted as antitumor miRNAs in PCa cells. The involvement of miRNA passenger strands in cancer pathogenesis is a novel concept for miRNA functionality. Based on a large patient cohort in The Cancer Genome Atlas, expression of eight miR‐455‐5p/‐3p target genes (PIR: P = 0.0137, LRP8: P = 0.0495, IGFBP3: P = 0.0172, DMBX1: P = 0.0175, CCDC64: P = 0.0446, TUBB1: P = 0.0149, KIF21B: P = 0.0336, and NFAM1: P = 0.0013) was significantly associated with poor prognosis of PCa patients. Here, we focused on PIR (pirin), a highly conserved member of the cupin superfamily. PIR expression was directly regulated by miR‐455‐5p, and PIR overexpression was detected in hormone‐sensitive prostate cancer (HSPC) surgical specimens and CRPC autopsy specimens. Loss‐of‐function assays using siRNA or an inhibitor (bisamide) showed that downregulation of PIR expression blocked cancer cell migration and invasion. Moreover, the miR‐455‐5p/PIR axis contributed to cancer cell aggressiveness. These results suggest that PIR might be a promising diagnostic marker for HSPC and CRPC. Furthermore, CRPC treatment strategies targeting PIR may be possible in the future. Identification of antitumor miRNAs, including miRNA passenger strands, may contribute to the development of new diagnostic markers and therapeutic strategies for CRPC.
Keywords: bisamide, castration‐resistant prostate cancer, miR‐455‐5p, pirin
Abbreviations
- ADT
androgen deprivation therapy
- AR
androgen receptor
- CRPC
castration‐resistant prostate cancer
- DFS
disease‐free survival
- HSF1
heat shock transcriptional factor 1
- HSPC
hormone‐sensitive prostate cancer
- miRNA
microRNA
- PCa
prostate cancer
- PIR
pirin
- TCGA
The Cancer Genome Atlas
1. Introduction
Due to the expansion of prostate‐specific antigen (PSA) screening, prostate cancer (PCa) is now the most frequently diagnosed cancer among men in developed countries. In 2017, PCa was the third leading cause of cancer‐related death among men (approximately 320 000 individuals) in the United States (Siegel et al., 2017). Activation of androgen signaling through the androgen receptor (AR) is essential for development, proliferation, and survival of PCa cells. Therefore, AR is considered to be the most relevant therapeutic target for both hormone‐sensitive prostate cancer (HSPC) and advanced PCa (Crawford et al., 2015; Crona and Whang, 2017). Most HSPC patients initially respond to androgen deprivation therapy (ADT), but eventually PCa cells acquire resistance to ADT and gain increased proliferative and metastatic potential, a condition referred to as castration‐resistant prostate cancer (CRPC). Recently approved agents such as second generation AR‐targeted agents, bone‐targeted agents and chemotherapeutic agents increase survival rates of CRPC patients, but do not provide a cure (Crawford et al., 2015; Crona et al., 2015). Thus, increasing the likelihood of survival for CRPC patients is an important consideration for successful PCa treatments.
Many studies showed that microRNAs (miRNAs), a type of small noncoding RNA, are associated with cancer pathogenesis (Esquela‐Kerscher and Slack, 2006; Iorio and Croce, 2009; Nelson and Weiss, 2008; Wiemer, 2007). A single miRNA species can regulate many protein‐coding or noncoding RNA transcripts in physiological and pathological conditions (Friedman et al., 2009). Therefore, novel RNA networks in cancer cells can be searched beginning with aberrantly expressed miRNAs.
Based on HSPC and CRPC miRNA signatures, we sequentially identified antitumor miRNAs and the oncogenic targets they control, including FSCN1, GOLM1, PNP, WWP1, and Ecm29 (Fuse et al., 2011; Goto et al., 2015, 2016; Kojima et al., 2012, 2014). Our recent RNA sequencing analyses of CRPC miRNA signatures revealed that expression of both strands of pre‐miR‐145 (miR‐145‐5p and miR‐145‐3p, the passenger and guide strand, respectively) was significantly reduced in HSPC and CRPC clinical specimens and that miR‐145‐5p/‐3p acted as an antitumor miRNA in PCa cells (Goto et al., 2017). Interestingly, ectopic expression of miR‐145‐3p markedly blocked cancer cell aggressiveness through direct regulation of several oncogenic genes, including MELK, NCAPG, BUB1, and CDK1 (Goto et al., 2017). Recent studies of miRNA biogenesis showed that some miRNA passenger strands, which were previously thought to be degraded and not functioning, also had important roles in human cells, including cancer cells (Marzi et al., 2016; McCall et al., 2017).
In this study, our aim was to identify novel therapeutic targets and prognostic markers for CRPC. We demonstrated that both miR‐455‐duplex strands (miR‐455‐5p, the passenger strand; and miR‐455‐3p, the guide strand) possess antitumor functions. We also found some molecular targets of miR‐455‐5p/‐3p to reveal new characteristics of PCa pathogenesis. Expression of eight genes (PIR, LRP8, IGFBP3, DMBX1, CCDC64, TUBB1, KIF21B, and NFAM1) was regulated by miR‐455‐5p/‐3p, and high expression of these genes was significantly predictive of survival in PCa patients. Moreover, we validated the functional significance of PIR as a promising therapeutic target for CRPC.
2. Materials and methods
2.1. Collection of clinical prostate specimens and cell lines
Clinical specimens were provided by the Teikyo University Chiba Medical Center between 2013 and 2017. Table S1 lists the clinical characteristics of these patients. The research protocol was approved by the Teikyo University Institutional Review Committee. The experiments were undertaken with the understanding and written consent of each subject, and the study methodologies conformed to the standards set by the Declaration of Helsinki.
We used human PCa cell lines (PC3, DU145, and C4‐2) obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer Tohoku University (Sendai, Japan), and the American Type Culture Collection (Manassas, VA, USA). The cells were maintained as described in our previous reports (Arai et al., 2018b; Goto et al., 2017; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b).
2.2. Quantitative real‐time reverse transcription polymerase chain reaction
Expression levels of miR‐455‐5p and miR‐455‐3p normalized to expression of RNU48 were analyzed by TaqMan quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR). PIR expression levels were normalized to GAPDH or GUSB. The procedure for qRT‐PCR quantification was described in our previous reports (Arai et al., 2017, 2018b; Goto et al., 2017; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b). Details of the reagents used are shown in Table S2.
2.3. Transfection with mature miRNA, small‐interfering RNA, or plasmid vectors
For experiments involving mature miRNAs, small‐interfering RNAs (siRNAs), and plasmid vectors, we used pre‐miR miRNA precursors (hsa‐miR‐455‐5p and hsa‐miR‐455‐3p), Stealth Select RNAi siRNAs (si‐PIR‐1 and si‐PIR‐2), and negative control miRNA/siRNA as well as a PIR plasmid vector designed by Kazusa DNA Research (Product ID: FHC03682; Kisarazu, Japan). miRNAs or siRNAs were introduced into cells at a concentration of 10 nm by reverse transfection, and a vector plasmid was introduced into cells by forward transfection. The procedures were as previously reported (Arai et al., 2017, 2018b; Goto et al., 2017; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b), and the reagent details are given in Table S2.
2.4. Small‐molecule PIR inhibitor bisamide for in vitro studies
Bisamide, which was previously reported to be a small‐molecule PIR inhibitor, was used to inhibit PIR in in vitro assays (CCT251236; MedChem Express Monmouth Junction, NJ, USA; Cat No. 1693731‐40‐6; Cheeseman et al., 2017). Bisamide was dissolved in DMSO, and the final DMSO concentration in experiments involving bisamide was ≤ 0.1%.
2.5. Cell proliferation, migration, and invasion assays
To investigate the functional roles of miRNAs or siRNAs in PCa cells, cell proliferation (XTT assay), migration (wound‐healing assay), and invasion (Matrigel invasion assay) assays were carried out as previously described (Arai et al., 2017, 2018b; Goto et al., 2017; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b).
2.6. Validation of miRNAs incorporated into the RNA‐induced silencing complex
To explore whether both exogenous strands of pre‐miR‐455 (miR‐455‐5p and miR‐455‐3p) were incorporated into the RNA‐induced silencing complex (RISC), we performed Ago2 immunoprecipitation assays using a miRNA isolation kit for human Ago2 (Wako, Osaka, Japan). The procedure was described previously (Goto et al., 2017; Okato et al., 2017a). Quantification of miRNAs bound to Ago2 was achieved by qRT‐PCR, and normalization was performed relative to the expression of miR‐26a that is unaffected by transfection with miR‐455‐5p/‐3p. Details of the reagents used are shown in Table S2.
2.7. Strategy for exploring genes regulated by miR‐455‐5p, miR‐455‐3p si‐PIR, and PIR inhibitor in PCa cells
We combined in silico database analyses and comprehensive gene expression analyses using an oligo microarray (Agilent Technologies, Tokyo, Japan; Human Ge 60K) to focus on target gene candidates as previously described (Arai et al., 2017, 2018a,b; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b). For in silico analyses, we used the TargetScanHuman 7.1 database (June, 2016 release, http://www.targetscan.org/vert_71). The microarray data were deposited into the GEO database (https://www.ncbi.nlm.nih.gov/geo/).
2.8. Western blotting
Western blotting was carried out as previously described with anti‐PIR antibodies (diluted to 1 : 400) and anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibodies (diluted to 1 : 10 000) as an internal loading control (Arai et al., 2017, 2018b; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b). Antibody details are described in Table S2.
2.9. Plasmid construction and dual‐luciferase reporter assays
A partial wild‐type sequence of the PIR 3′‐untranslated region (UTR) or a sequence having a mutation in the miR‐455‐5p target site was inserted into the psiCHECK‐2 vector (C8021; Promega, Madison, WI, USA). The assay procedure was reported previously (Arai et al., 2017, 2018b; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b).
2.10. Immunohistochemistry
Tissue specimens were incubated overnight at 4 °C with anti‐PIR antibodies (diluted to 1 : 80). Tissue microarrays were obtained from provitro (Cat. no. 4012209, Berlin, Germany). Details of the tissue microarray are given in http://www.provitro.com/fileadmin/provitro-data/TMA/4012209.pdf. The reagents used are described in Table S2, and the procedure was described previously (Arai et al., 2017, 2018a,b; Goto et al., 2017; Kurozumi et al., 2016; Okato et al., 2017a; Yamada et al., 2018b). IHC score was evaluated by weighted intensity. The intensity of staining was graded from 0 to 3 as follows: 0, negative staining; 1, mild staining; 2, moderate staining; and 3, intense staining. The procedure was performed as described previously (Okato et al., 2016).
2.11. The Cancer Genome Atlas database analyses of PCa
To investigate the clinical significance of miRNAs and genes in PCa patients, we utilized the The Cancer Genome Atlas (TCGA) database. Gene expression data and clinical information for PCa patients were analyzed using cBioPortal (http://www.cbioportal.org/; Gao et al., 2013). The data were downloaded on August 29, 2017. The expression of each gene was divided into two groups, high and low, and the period preceding postoperative recurrence (disease‐free survival) was compared and examined.
2.12. Statistical analysis
The relationship between two groups was analyzed using the Mann–Whitney U‐test. The relationship of three or more variables was analyzed using Bonferroni‐adjusted Mann–Whitney U‐tests. The correlation between two groups was evaluated by Spearman's rank test. Survival analyses by Kaplan–Meier method and log‐rank test were performed using jmp software (version 13; SAS Institute Inc., Cary, NC, USA). For all other analyses, expert statview (version 5; SAS Institute, Inc.) was used.
3. Results
3.1. Expression levels of both pre‐miR‐455 strands in PCa specimens and cell lines
In the human genome, pre‐miR‐455 is located on chromosome 9q32 and the mature sequences of miR‐455‐5p and miR‐455‐3p are 5′‐UAUGUGCCUUUGGACUACAUCG‐3′ and 5′‐GCAGUCCAUGGGCAUAUACAC‐3′, respectively (Fig. S1). miR‐455‐5p is the passenger strand (minor strand), and miR‐455‐3p is the guide strand (major strand). We validated miR‐455‐5p and miR‐455‐3p expression levels in clinical prostate specimens [benign prostate tissues: n = 17; HSPC: n = 17; and CRPC: n = 5; Table S1] and PCa cell lines (PC3, DU145, and C4‐2). qRT‐PCR indicated that miR‐455‐5p and miR‐455‐3p expression was markedly downregulated in HSPC and CRPC tissues compared with benign prostate tissues (miR‐455‐5p: P < 0.0001 and P = 0.0001; miR‐455‐3p: P < 0.0001 and P = 0.0002; Fig. 1A,B). PCa cell lines also had very low expression levels of both miR‐455‐5p and miR‐455‐3p (Fig. 1A,B). Moreover, miR‐455‐5p and miR‐455‐3p expression was positively correlated in prostate tissues (r = 0.938, P < 0.0001; Fig. 1C). In general, the expression levels of miRNA passenger strands in clinical specimens are quite low, although here the expression levels of miR‐455‐5p and miR‐455‐3p were comparable. This result suggests that both the passenger strand and the guide strand may play important functional roles in PCa.
Figure 1.
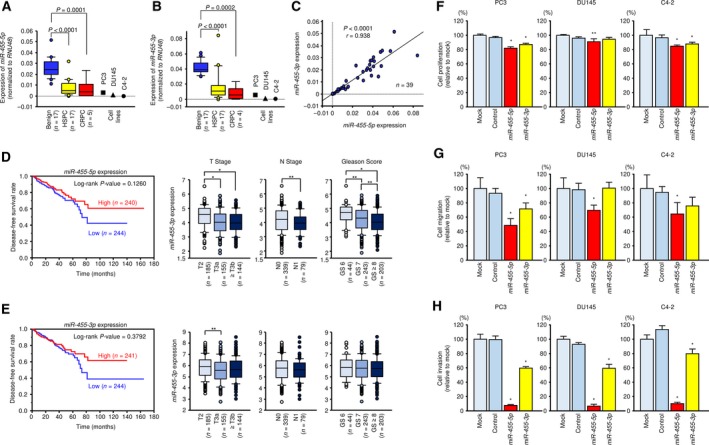
Expression of miR‐455‐5p/‐3p in clinical PCa specimens and functional analysis of miR‐455‐5p/‐3p in PCa cell lines. Expression levels of (A) miR‐455‐5p and (B) miR‐455‐3p in PCa clinical specimens and cell lines determined using qRT‐PCR. RNU48 was used as an internal control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (C) Correlations between the relative expression levels of miR‐455‐5p and miR‐455‐3p analyzed by using Spearman's rank test. (D) Kaplan–Meier patient survival curves for DFS rates based on miR‐455‐5p expression (left) and relationships between miR‐455‐5p expression and T stage, N stage, and Gleason score (right) in PCa patients from the TCGA database. (E) Kaplan–Meier patient survival curves for DFS rates based on miR‐455‐3p expression (left) and relationships between expression of miR‐455‐3p and T stage, N stage, and Gleason score (right). *P < 0.0001 and **P < 0.05. P‐values were calculated using Mann–Whitney U‐test or Bonferroni‐adjusted Mann–Whitney U‐test. (F–H) Cell proliferation, migration, and invasion assays in cells transfected with miR‐455‐5p/‐3p. Error bars are represented as mean ± SD (n = 5, n = 8, and n = 8, respectively). *P < 0.0001 and **P < 0.05, relative to both mock and control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test.
3.2. Binding of both pre‐miR‐455 strands to Ago2
To confirm that both miR‐455‐5p and miR‐455‐3p function by incorporating into the RISC, we performed immunoprecipitation with antibodies targeting Ago2, a central player for miRNA incorporation into the RISC (Fig. S2A). qRT‐PCR showed that the amount of miR‐455‐5p bound to Ago2 was significantly higher in miR‐455‐5p transfectants compared to mock‐transfected cells and cells transfected with miR‐control and miR‐455‐3p (P < 0.0001; Fig. S2B). Similarly, the amount of miR‐455‐3p bound to Ago2 was markedly higher in miR‐455‐3p transfectants than that of mock‐, miR‐control‐, and miR‐455‐5p‐transfected cells (P < 0.0001; Fig. S2B).
3.3. The clinical significance of miR‐455‐5p/‐3p in PCa patients and functional effects of restoring miR‐455‐5p/‐3p in PCa cells
To investigate the clinical significance of miR‐455‐5p and miR‐455‐3p in PCa patients, we carried out several analyses using the TCGA database. There was no significance in disease‐free survival (DFS) rate in the expression difference of miR‐455‐5p, but the prognosis tended to be poor in PCa patients with lower levels of miR‐455‐5p expression (Fig. 1D). Additionally, analyses of T stage, N stage, and Gleason score showed that miR‐455‐5p expression was markedly decreased in advanced PCa cases or cases with high malignancy (Fig. 1D). On the other hand, no clinically significant differences were seen for miR‐455‐3p expression in terms of survival or stage (Fig. 1E).
To confirm the functional roles of miR‐455‐5p and miR‐455‐3p, we performed ectopic expression assays and evaluated the cell proliferation, migration, and invasion activity in PCa cells (PC3, DU145, and C4‐2). Our ectopic expression assays showed that miR‐455‐5p inhibited cancer cell migration and invasive abilities in all PCa cells (Fig. 1G,H). In contrast to miR‐455‐5p, the inhibitory effects of the migration and invasive abilities of miR‐455‐3p were inferior in all PCa cells (Fig. 1G,H). In addition, both miRNAs had only slight suppression effects on cell proliferation in PCa cells (Fig. 1F).
These results suggested that miR‐455‐5p is clinically important in PCa and acts as an antitumor miRNA to suppress cancer cell migration and invasive abilities in vitro.
3.4. Search for putative oncogenes regulated by miR‐455‐5p/‐3p in PCa cells
To identify oncogenes regulated by miR‐455‐5p, we searched the TargetScanHuman 7.1 database and compiled a list of 3032 candidate genes having sites in the 3′‐UTR that are bound by miR‐455‐5p. From these candidate genes, we selected 70 genes that also showed downregulated expression (fold change log2 < −1.5) in PCa cells transfected with miR‐455‐5p (GEO accession number: GSE100746; Table 1A). Then, we analyzed the patient's DFS in the TCGA database and selected genes significantly associated with poor prognosis when expressed at high level. The flowchart of the search is shown in Fig. 2A. These analyses identified four genes: PIR, LRP8, IGFBP3, and DMBX1 (Fig. 2B). We also used the same method for miR‐455‐3p and identified four candidate oncogenes: CCDC64, TUBB1, KIF21B, and NFAM1 (Fig. 2C,D and Table 1B). The results of clinicopathological analyses in PCa patients (T stage, N stage, and Gleason score) for the expression of these eight genes are shown in Fig. 3C and Fig. S3.
Table 1.
Putative target genes regulated by (A) miR‐455‐5p and (B) miR‐455‐3p in PCa cells
| (A) | ||||||
|---|---|---|---|---|---|---|
| Entrez gene ID | Gene symbol | Gene name | Location | No. conserved sites | No. poorly conserved sites | PC3 miR‐455‐5p transfectant (Log2 ratio) |
| 5738 | PTGFRN | Prostaglandin F2 receptor inhibitor | 1p13.1 | 1 | 0 | −2.78 |
| 137075 | CLDN23 | Claudin 23 | 8p23.1 | 0 | 1 | −2.73 |
| 11031 | RAB31 | RAB31, member RAS oncogene family | 18p11.22 | 0 | 3 | −2.63 |
| 66008 | TRAK2 | Trafficking protein, kinesin binding 2 | 2q33.1 | 1 | 1 | −2.63 |
| 7084 | TK2 | Thymidine kinase 2, mitochondrial | 16q21 | 0 | 2 | −2.56 |
| 10959 | TMED2 | Transmembrane emp24 domain trafficking protein 2 | 12q24.31 | 1 | 1 | −2.45 |
| 3964 | LGALS8 | Lectin, galactoside‐binding, soluble, 8 | 1q43 | 0 | 1 | −2.44 |
| 4201 | MEA1 | Male‐enhanced antigen 1 | 6p21.1 | 0 | 1 | −2.41 |
| 9563 | H6PD | Hexose‐6‐phosphate dehydrogenase (glucose 1‐dehydrogenase) | 1p36.22 | 0 | 1 | −2.38 |
| 141 | ADPRH | ADP‐ribosylarginine hydrolase | 3q13.33 | 0 | 2 | −2.37 |
| 2820 | GPD2 | Glycerol‐3‐phosphate dehydrogenase 2 (mitochondrial) | 2q24.1 | 0 | 2 | −2.33 |
| 8544 | PIR | Pirin (iron‐binding nuclear protein) | Xp22.2 | 0 | 1 | −2.29 |
| 29943 | PADI1 | Peptidyl arginine deiminase, type I | 1p36.13 | 0 | 1 | −2.29 |
| 7804 | LRP8 | Low‐density lipoprotein receptor‐related protein 8, apolipoprotein e receptor | 1p32.3 | 0 | 2 | −2.28 |
| 314 | AOC2 | Amine oxidase, copper containing 2 (retina‐specific) | 17q21.31 | 0 | 1 | −2.25 |
| 4038 | LRP4 | Low‐density lipoprotein receptor‐related protein 4 | 11p11.2 | 0 | 1 | −2.17 |
| 64506 | CPEB1 | Cytoplasmic polyadenylation element binding protein 1 | 15q25.2 | 1 | 0 | −2.15 |
| 8000 | PSCA | Prostate stem cell antigen | 8q24.3 | 0 | 1 | −2.14 |
| 8895 | CPNE3 | Copine III | 8q21.3 | 0 | 1 | −2.11 |
| 8228 | PNPLA4 | Patatin‐like phospholipase domain containing 4 | Xp22.31 | 0 | 1 | −2.08 |
| 127343 | DMBX1 | Diencephalon/mesencephalon homeobox 1 | 1p33 | 0 | 2 | −2.05 |
| 54749 | EPDR1 | Ependymin related 1 | 7p14.1 | 0 | 2 | −1.98 |
| 3992 | FADS1 | Fatty acid desaturase 1 | 11q12.2 | 0 | 2 | −1.97 |
| 659 | BMPR2 | Bone morphogenetic protein receptor, type II (serine/threonine kinase) | 2q33.2 | 0 | 2 | −1.96 |
| 1047 | CLGN | Calmegin | 4q31.1 | 0 | 1 | −1.94 |
| 100128927 | ZBTB42 | Zinc finger and BTB domain containing 42 | 14q32.33 | 0 | 2 | −1.94 |
| 10584 | COLEC10 | Collectin subfamily member 10 (C‐type lectin) | 8q24.12 | 0 | 2 | −1.90 |
| 143903 | LAYN | Layilin | 11q23.1 | 1 | 1 | −1.87 |
| 22873 | DZIP1 | DAZ interacting zinc finger protein 1 | 13q32.1 | 0 | 1 | −1.84 |
| 79718 | TBL1XR1 | Transducin (beta)‐like 1 X‐linked receptor 1 | 3q26.32 | 1 | 0 | −1.84 |
| 56180 | MOSPD1 | Motile sperm domain containing 1 | Xq26.3 | 0 | 1 | −1.84 |
| 3486 | IGFBP3 | Insulin‐like growth factor binding protein 3 | 7p12.3 | 0 | 1 | −1.82 |
| 84327 | ZBED3 | Zinc finger, BED‐type containing 3 | 5q13.3 | 0 | 2 | −1.80 |
| 203447 | NRK | Nik‐related kinase | Xq22.3 | 0 | 1 | −1.78 |
| 1238 | ACKR2 | Atypical chemokine receptor 2 | 3p22.1 | 0 | 1 | −1.77 |
| 9194 | SLC16A7 | Solute carrier family 16 (monocarboxylate transporter), member 7 | 12q14.1 | 0 | 3 | −1.76 |
| 128989 | TANGO2 | Transport and golgi organization 2 homolog (Drosophila) | 22q11.21 | 0 | 1 | −1.74 |
| 9870 | AREL1 | Apoptosis‐resistant E3 ubiquitin protein ligase 1 | 14q24.3 | 0 | 1 | −1.72 |
| 5500 | PPP1CB | Protein phosphatase 1, catalytic subunit, beta isozyme | 2p23.2 | 0 | 1 | −1.71 |
| 6444 | SGCD | Sarcoglycan, delta (35 kDa dystrophin‐associated glycoprotein) | 5q33.3 | 0 | 1 | −1.70 |
| 4942 | OAT | Ornithine aminotransferase | 10q26.13 | 0 | 1 | −1.66 |
| 116071 | BATF2 | Basic leucine zipper transcription factor, ATF‐like 2 | 11q13.1 | 0 | 1 | −1.65 |
| 286077 | FAM83H | Family with sequence similarity 83, member H | 8q24.3 | 0 | 1 | −1.64 |
| 414149 | ACBD7 | Acyl‐CoA binding domain containing 7 | 10p13 | 0 | 2 | −1.64 |
| 100132386 | KRTAP4‐9 | Keratin associated protein 4‐9 | 17q21.2 | 0 | 2 | −1.63 |
| 55163 | PNPO | Pyridoxamine 5′‐phosphate oxidase | 17q21.32 | 0 | 1 | −1.63 |
| 23414 | ZFPM2 | Zinc finger protein, FOG family member 2 | 8q22.3 | 1 | 0 | −1.63 |
| 26049 | FAM169A | Family with sequence similarity 169, member A | 5q13.3 | 0 | 2 | −1.63 |
| 83394 | PITPNM3 | PITPNM family member 3 | 17p13.2 | 0 | 1 | −1.63 |
| 132228 | LSMEM2 | Leucine‐rich single‐pass membrane protein 2 | 3p21.31 | 0 | 1 | −1.61 |
| 8428 | STK24 | Serine/threonine kinase 24 | 13q32.2 | 1 | 0 | −1.60 |
| 4352 | MPL | Myeloproliferative leukemia virus oncogene | 1p34.2 | 0 | 1 | −1.60 |
| 1901 | S1PR1 | Sphingosine‐1‐phosphate receptor 1 | 1p21.2 | 1 | 1 | −1.60 |
| 9258 | MFHAS1 | Malignant fibrous histiocytoma amplified sequence 1 | 8p23.1 | 1 | 0 | −1.59 |
| 9779 | TBC1D5 | TBC1 domain family, member 5 | 3p24.3 | 0 | 1 | −1.58 |
| 57722 | IGDCC4 | Immunoglobulin superfamily, DCC subclass, member 4 | 15q22.31 | 0 | 1 | −1.57 |
| 144110 | TMEM86A | Transmembrane protein 86A | 11p15.1 | 1 | 1 | −1.57 |
| 2782 | GNB1 | Guanine nucleotide binding protein (G protein), beta polypeptide 1 | 1p36.33 | 0 | 1 | −1.56 |
| 255027 | MPV17L | MPV17 mitochondrial membrane protein‐like | 16p13.11 | 0 | 1 | −1.56 |
| 81031 | SLC2A10 | Solute carrier family 2 (facilitated glucose transporter), member 10 | 20q13.12 | 0 | 1 | −1.55 |
| 9214 | FAIM3 | Fas apoptotic inhibitory molecule 3 | 1q32.1 | 0 | 1 | −1.55 |
| 153339 | TMEM167A | Transmembrane protein 167A | 5q14.2 | 1 | 0 | −1.54 |
| 8498 | RANBP3 | RAN binding protein 3 | 19p13.3 | 1 | 0 | −1.54 |
| 11337 | GABARAP | GABA(A) receptor‐associated protein | 17p13.1 | 0 | 1 | −1.54 |
| 83445 | GSG1 | Germ cell associated 1 | 12p13.1 | 0 | 2 | −1.53 |
| 142940 | TRUB1 | TruB pseudouridine (psi) synthase family member 1 | 10q25.3 | 0 | 1 | −1.53 |
| 10423 | CDIPT | CDP‐diacylglycerol‐inositol 3‐phosphatidyltransferase | 16p11.2 | 0 | 1 | −1.53 |
| 4155 | MBP | Myelin basic protein | 18q23 | 0 | 1 | −1.52 |
| 374900 | ZNF568 | Zinc finger protein 568 | 19q13.12 | 0 | 1 | −1.52 |
| 9658 | ZNF516 | Zinc finger protein 516 | 18q23 | 1 | 1 | −1.51 |
| (B) | ||||||
|---|---|---|---|---|---|---|
| Entrez gene ID | Gene symbol | Gene name | Location | No. conserved sites | No. poorly conserved sites | PC3 miR‐455‐3p transfectant (Log2 ratio) |
| 9887 | SMG7 | SMG7 nonsense‐mediated mRNA decay factor | 1q25.3 | 0 | 1 | −2.24 |
| 81033 | KCNH6 | Potassium voltage‐gated channel, subfamily H (eag‐related), member 6 | 17q23.3 | 0 | 1 | −2.08 |
| 8778 | SIGLEC5 | Sialic acid binding Ig‐like lectin 5 | 19q13.41 | 0 | 1 | −2.02 |
| 92558 | CCDC64 | Coiled‐coil domain containing 64 | 12q24.23 | 0 | 1 | −1.88 |
| 23201 | FAM168A | Family with sequence similarity 168, member A | 11q13.4 | 0 | 2 | −1.86 |
| 50615 | IL21R | Interleukin‐21 receptor | 16p12.1 | 0 | 1 | −1.81 |
| 56479 | KCNQ5 | Potassium voltage‐gated channel, KQT‐like subfamily, member 5 | 6q13 | 0 | 3 | −1.81 |
| 7099 | TLR4 | Toll‐like receptor 4 | 9q33.1 | 0 | 2 | −1.77 |
| 85441 | HELZ2 | Helicase with zinc finger 2, transcriptional coactivator | 20q13.33 | 0 | 1 | −1.76 |
| 339327 | ZNF546 | Zinc finger protein 546 | 19q13.2 | 0 | 1 | −1.76 |
| 79663 | HSPBAP1 | HSPB (heat shock 27 kDa) associated protein 1 | 3q21.1 | 1 | 0 | −1.75 |
| 81027 | TUBB1 | Tubulin, beta 1 class VI | 20q13.32 | 0 | 1 | −1.71 |
| 23046 | KIF21B | Kinesin family member 21B | 1q32.1 | 0 | 2 | −1.71 |
| 1236 | CCR7 | Chemokine (C‐C motif) receptor 7 | 17q21.2 | 0 | 1 | −1.66 |
| 1002 | CDH4 | Cadherin 4, type 1, R‐cadherin (retinal) | 20q13.33 | 0 | 1 | −1.61 |
| 1238 | ACKR2 | Atypical chemokine receptor 2 | 3p22.1 | 0 | 1 | −1.59 |
| 150372 | NFAM1 | NFAT activating protein with ITAM motif 1 | 22q13.2 | 0 | 3 | −1.53 |
| 283078 | MKX | Mohawk homeobox | 10p12.1 | 0 | 1 | −1.51 |
Figure 2.
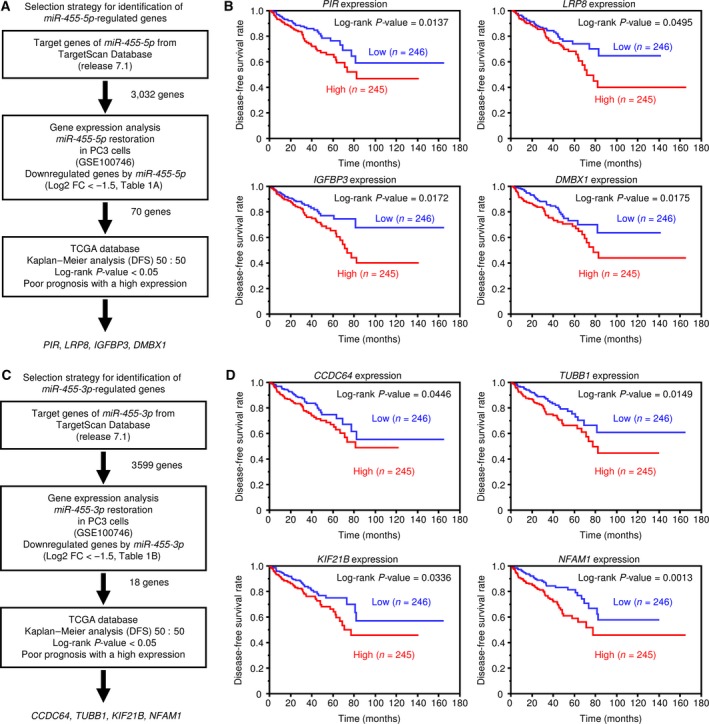
Identification of miR‐455‐5p/‐3p target genes and relationship between putative target genes and DFS rates. (A) Flowchart of the strategy used to identify miR‐455‐5p target genes. (B) Kaplan–Meier patient survival curves for DFS rates based on PIR, LRP8, IGFBP3, and DMBX1 expression in PCa patients from the TCGA database. (C) Flowchart of the strategy to identify miR‐455‐3p target genes. (D) Kaplan–Meier patient survival curves for DFS rates based on CDC64, TUBB1, KIF21B, and NFAM1 expression.
Figure 3.
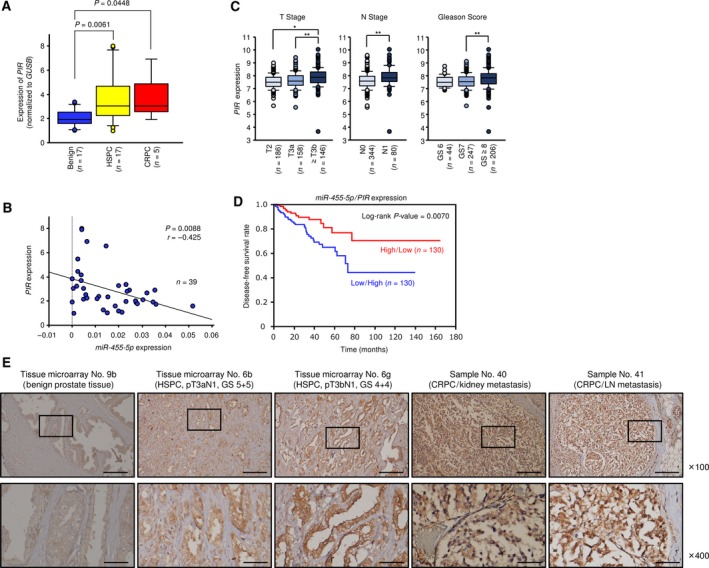
Expression of PIR in clinical PCa specimens and relationship between PIR and clinicopathological factors. (A) Expression levels of PIR in PCa clinical specimens and cell lines. GUSB was used as an internal control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (B) Negative correlation between miR‐455‐5p and PIR expression analyzed by using Spearman's rank test. (C) Relationships between PIR expression and T stage, N stage, and Gleason score in PCa patients from the TCGA database. *P < 0.0001, **P < 0.001. P‐values were calculated using Mann–Whitney U‐test or Bonferroni‐adjusted Mann–Whitney U‐test. (D) Kaplan–Meier patient survival curves for DFS rates by a combination of miR‐455‐5p and PIR expression (miR‐455‐5p high/PIR low versus miR‐455‐5p low/PIR high). (E) Immunochemical staining of PIR in prostate specimens. Scale bars of ×100 and ×400 represent 200 and 50 μm, respectively.
3.5. Prognostic application of genes regulated by miRNAs
We then attempted to determine whether useful prognostic evaluations could be made by examining the expression levels of each gene targeted by miR‐455‐5p or miR‐455‐3p. To evaluate gene expression, we created heatmaps by cluster analyses using R2: Genomics Analysis and Visualization Platform (https://r2.amc.nl/; Fig. S4A,C). For each of the four candidate genes for miR‐455‐5p and miR‐455‐3p regulation, we determined the averages of the Z‐scores and analyzed the DFS based on the resulting value (Z‐score < 0 versus ≥ 0). The candidate genes regulated by miR‐455‐5p and miR‐455‐3p showed significant differences in expression (P < 0.0001 and P < 0.0001, respectively; Fig. S4B,D). In particular, the Kaplan–Meier curve for DFS generated from a combination of the four candidate target genes for miR‐455‐5p showed a great impact.
Here, we focused on genes that were regulated by miR‐455‐5p and are thought to be important in controlling PCa progression. Among the four final candidate oncogenes, PIR showed the highest miR‐455‐5p‐mediated downregulation of expression and had the most significant effect on prognosis according to a log‐rank test.
3.6. PIR expression was directly regulated by miR‐455‐5p in PCa cells
To confirm the control of PIR expression by miR‐455‐5p, we performed qRT‐PCR and western blotting. Both PIR mRNA and protein expression levels were significantly reduced by miR‐455‐5p transfection compared to those of mock‐ or miR‐control‐transfected cells (Fig. S5A,B).
According to the TargetScan database, miR‐455‐5p binds to position 167–173 in the 3′‐UTR of PIR. To validate the direct binding of miR‐455‐5p to PIR mRNA, we performed luciferase reporter assays, which showed that the luminescence intensity was markedly decreased by cotransfection with miR‐455‐5p and a vector carrying wild‐type PIR 3′‐UTR. On the other hand, cells cotransfected with a vector carrying a mutated miR‐455‐5p target site showed no change in luminescence intensity (Fig. S5C).
3.7. PIR expression in PCa clinical specimens
We evaluated PIR expression in PCa clinical specimens (benign prostate tissues: n = 17; HSPC tissues: n = 17; and CRPC tissues: n = 5; Table S1). PIR expression was significantly upregulated in HSPC as compared to normal tissues and tended to be increased in CRPC tissues (P = 0.0061 and P = 0.0448, respectively; Fig. 3A). A Spearman's rank test showed a significant inverse correlation between PIR and miR‐455‐5p expression (P = 0.0088, r = −0.425; Fig. 3B).
Immunohistochemistry analysis of PIR protein expression in PCa clinical specimens (tissue microarray and Table S1) indicated strong expression of the PIR protein in the cytoplasm of PCa cells (Fig. 3E). According to evaluation using tissue microarray, the expression score for PIR protein was significantly higher in PCa tissues than in noncancerous tissues (Fig. S6A). The patient backgrounds and IHC scores of PIR are summarized in Table S3, and representative immunostaining pictures are shown in Fig. S6B–D.
3.8. Clinical significance of PIR in PCa
As mentioned above, high expression of PIR was associated with poor prognosis in DFS (Fig. 2B). According to the TCGA database, PIR expression was enhanced in cases with advanced T stage, advanced N stage, and high Gleason score (Fig. 3C). Patients having low miR‐455‐5p/high PIR expression had significantly shorter DFS than the high‐miR‐455‐5p/low‐PIR group (Fig. 3D), suggesting that by combination of these two factors, it would be possible to predict the prognosis of PCa patients more effectively than to evaluate by PIR alone.
3.9. Effects of PIR silencing in PCa cell lines
We examined the effects of PIR knockdown in PC3, DU145, and C4‐2 cells using two types of si‐PIR: si‐PIR‐1 and si‐PIR‐2. Both siRNAs effectively downregulated PIR mRNA and PIR protein expression (Fig. S7A,B). Additionally, functional assays showed antitumor effects, particularly reduced cell migration and invasion, upon PIR knockdown (Fig. 4A–C).
Figure 4.
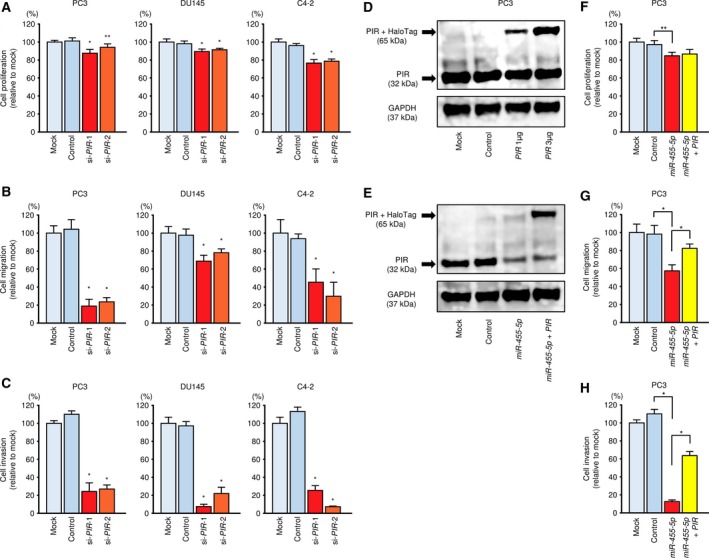
Functional analysis of PIR in PCa cells. (A–C) Knockdown assay with si‐PIR. Cell proliferation, migration, and invasion assays following transfection with si‐PIR‐1 and si‐PIR‐2. Error bars are represented as mean ± SD (n = 5, n = 8, and n = 8, respectively). *P < 0.0001 and **P < 0.001, relative to both mock and control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (D) PIR protein expression was evaluated by western blot analysis of PC3 cells 48 h after forward transfection with the PIR vector. GAPDH was used as a loading control. (E) PIR protein expression was evaluated by western blot analysis of PC3 cells 72 h after reverse transfection with miR‐455‐5p and 48 h after forward transfection with the PIR vector. (F–H) Rescue experiments with miR‐455‐5p and PIR vector. Cell proliferation, migration, and invasion assays following transfection with miR‐455‐5p and PIR vector. Error bars are represented as mean ± SD (n = 5, n = 8, and n = 8, respectively). *P < 0.0001, **P < 0.001. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test.
3.10. Effects of PIR/miR‐455‐5p cotransfection of PC3 cells
We performed PIR rescue experiments by cotransfection with PIR and miR‐455‐5p in PC3 cells. Western blot was used to confirm the restoration of PIR protein levels (Fig. 4D,E). Although proliferation rates were not noticeably affected by PIR rescue (Fig. 4F), the migration and invasion capacities of PC3 cells were recovered by cotransfection with PIR and miR‐455‐5p (Fig. 4G,H), suggesting that the miR‐455‐5p/PIR axis may play an important role in PCa progression.
3.11. Antitumor effects using a small‐molecule PIR inhibitor in PC3 cells
We examined the tumor‐suppressive effects in vitro using the recently described small‐molecule PIR inhibitor bisamide (CCT251236; Cheeseman et al., 2017). In PC3, DU145, and C4‐2 cells using bisamide, the expression of PIR at the mRNA level was decreased in a concentration‐dependent manner (Fig. S8). We evaluated cell proliferation, migration (wound‐healing), and invasion activities at different bisamide concentrations ranging from 1 to 100 nm in PC3, DU145, and C4‐2 cells. In the wound‐healing assay, the cells were plated at high confluency to reduce the influence of cell proliferation suppression and bisamide was administered after scratching.
First, the result of the XTT assay over time is shown in Fig. 5A. In all cell lines, bisamide suppressed cell proliferation in a concentration‐dependent manner, and its ability was almost lost at concentrations above 100 nm. A dose‐dependent curve in each cell line at 72 h is shown in Fig. 5B. Furthermore, as a result of evaluating migration and invasive abilities using PC3 cells, they were similarly suppressed by concentration dependence of bisamide (migration; Fig. 5C,D; and invasion; Fig. 5E,F). The aggressiveness of cancer cells disappeared under 100 nm bisamide or more. The dramatic effects on migration and invasion were thought to be due to the failure of the cells to proliferate in the presence of high concentrations of bisamide.
Figure 5.
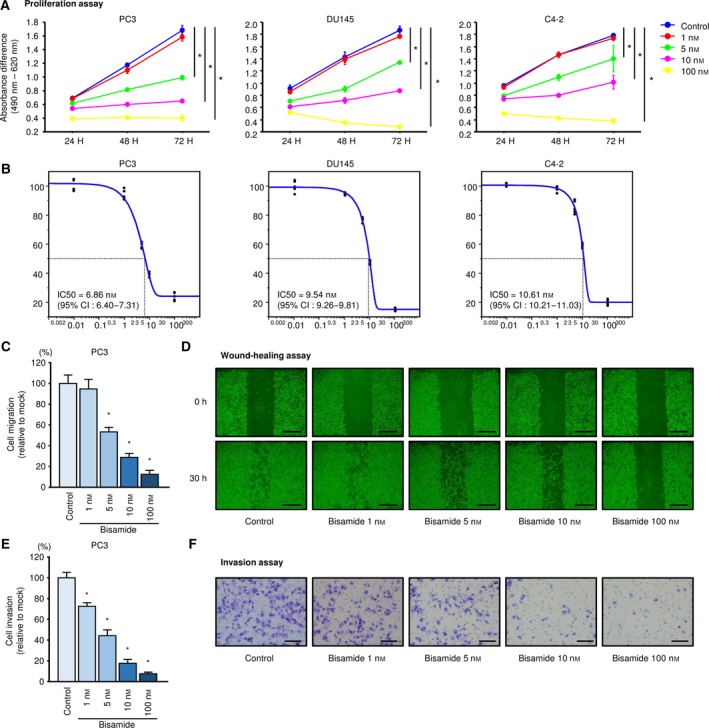
Effects of the small‐molecule PIR inhibitor bisamide (CCT251236) on PCa proliferation, migration, and invasion. (A) Proliferation curves over time according to the results of XTT assays following bisamide treatment in PC3, DU145, and C4‐2 were generated using the absorbance difference between 490 and 620 nm. Error bars are represented as mean ± SD (n = 5). *P < 0.0001, relative to control at 72 h. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (B) Dose‐dependent curves of bisamide on cell proliferation at 72 h in PC3, DU145, and C4‐2. The IC50 was calculated using jmp software. (C) Cell migration assays using bisamide in PC3. Error bars are represented as mean ± SD (n = 8). *P < 0.0001, relative to control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (D) Phase micrographs of wound‐healing assays using bisamide in PC3. Scale bars represent 500 μm. (E) Cell invasion assays using bisamide in PC3. Error bars are represented as mean ± SD (n = 8). *P < 0.0001, relative to control. P‐values were calculated using Bonferroni‐adjusted Mann–Whitney U‐test. (F) Phase micrographs of invasion assays using bisamide in PC3. Scale bars represent 200 μm.
3.12. Search for genes downstream of PIR
We next used microarray analyses with si‐PIR or bisamide (GEO accession number: GSE115801 and GSE115800, respectively) to identify downstream genes affected by PIR in PCa cells (Fig. S9A; Tables [Link], [Link]–S6). Interestingly, ACTL8 was detected by si‐PIR and bisamide mediated downstream gene in PCa cells (Tables [Link], [Link]–S6). ACTL8 belongs to the cancer testis antigen (CTA) family (Yao et al., 2014). Although biological roles of CTAs are hardly elucidated, it is known as a multifunctional protein group having a specific expression pattern in various types of cancer cells (Gordeeva, 2018). At this time, functional significance of ACTL8 for PCa cells is unknown. In the future, detailed functional analysis of ACTL8 in PCa cells is necessary.
Given that bisamide is an inhibitor of heat shock transcriptional factor 1 (HSF1) stress pathways (Cheeseman et al., 2017), we investigated its effect on HSF1‐mediated genes, particularly for those genes registered in the Reactome pathway ‘Cellular response to heat stress’ (https://reactome.org/). The expression of most HSF1‐mediated genes was indeed downregulated in the presence of bisamide (Fig. S9B and Table S7).
4. Discussion
Specific features of miRNAs allow a single miRNA to regulate many different RNA transcripts under various physiological or pathological conditions. Based on this broad regulation, novel RNA networks can be identified from relevant miRNAs in cancer cells. To determine which miRNAs are dysregulated in cancer cells, we used RNA sequencing technologies to identify miRNA expression signatures in drug‐resistant clinical specimens and autopsy specimens. Analyses of our previously identified miRNA signatures highlighted novel RNA networks that are controlled by novel miRNAs in several cancers (Goto et al., 2017; Koshizuka et al., 2017b; Mizuno et al., 2017).
In earlier studies, we made the unexpected discovery that some miRNA passenger strands have antitumor activity, for example, miR‐145‐3p, miR‐150‐3p, miR‐149‐3p, and miR‐99a‐3p, in several cancer cells (Arai et al., 2018b; Goto et al., 2017; Koshizuka et al., 2017b; Okato et al., 2017b). The traditional view of miRNA function was that only one strand of the miRNA‐duplex is incorporated into the RISC to become the active strand (guide strand). In contrast, the other strand, the passenger strand or miRNA*, was thought to be degraded and to have no function (Chendrimada et al., 2005; Hutvagner and Zamore, 2002; Matranga et al., 2005). Our recent studies showed that both strands of the miRNA‐duplex, for example, miR‐145, miR‐223, miR‐150, and miR‐199, have antitumor functions and their guide and passenger strands control several oncogenes independently or jointly (Goto et al., 2017; Koshizuka et al., 2017a,b; Sugawara et al., 2018).
Here, we focused on the miR‐455‐duplex (miR‐455‐5p, the passenger strand; and miR‐455‐3p, the guide strand) based on our original CRPC signature (Goto et al., 2017). The functional assays in this study showed that both miR‐455 strands had tumor‐suppressive functions in PCa cells. In particular, miR‐455‐5p, the passenger strand, strongly suppressed the migratory and invasive ability of PCa cells. Previous studies showed that downregulation of miR‐455‐3p (the guide strand) expression is frequently seen in several cancers, for example, colon cancer, non‐small‐cell lung cancer, and esophageal squamous cell carcinoma, and that ectopic expression of miR‐455‐3p inhibited the metastatic activity of cancer cells (Gao et al., 2018; Yang et al., 2017; Zheng et al., 2016). In PCa cells, miR‐455‐3p inhibits cancer cell proliferation by targeting the transcription factor eIF4E (Zhao et al., 2017). Interestingly, miR‐455‐5p had an opposite function depending on cancer type. For example, miR‐455‐5p expression is upregulated in NSCLC and could enhance cancer proliferation and metastasis by suppressing SOCO3 expression (Wang et al., 2017). In oral squamous cell carcinoma, miR‐455‐5p expression was promoted by the regulation of the TGF‐β‐dependent pathway and contributed to cancer tumorigenesis by downregulating UBE2B expression (Cheng et al., 2016). However, miR‐455‐5p expression in gastric cancer was lower, and thus, in cancer miR‐455‐5p functions as a tumor suppressor through regulation of RAB18 (Liu et al., 2016). Our recent study demonstrated that expression of both strands of the miR‐455‐duplex was downregulated in renal cell carcinoma tissues and that ectopic expression of both miRNAs inhibited cancer cell migration and invasive abilities (Yamada et al., 2018a). The best part of miRNAs study is to identify responsible target genes and pathways controlled by miRNAs in cells.
In this study, exploration of the RNA network controlled by antitumor miR‐455‐5p and miR‐455‐3p expands our understanding of the novel molecular pathogenesis of HSPC and CRPC. Through our search strategies, we identified PIR, LRP8, IGFBP3, and DMBX1 as genes controlled by miR‐455‐5p, and CCDC64, TUBB1, KIF21B, and NFAM1 as genes controlled by miR‐455‐3p. According to analyses based on the TCGA database, these genes may have cancer‐promoting activity because their high expression correlates with poor prognosis of PCa patients. Interestingly, by combining the expression of these genes, survival curves for DFS showed more impressive results. In particular, the combination of the four target genes of miR‐455‐5p had a great impact. This may lead to the establishment of a strong prognostic biomarker by analyzing molecules originating from miRNAs. In this study, we focused on PIR, which was most strongly regulated by miR‐455‐5p, and proceeded the analyses.
PIR (pirin) is a member of the cupin superfamily and is a highly conserved gene among mammals, plants, fungi, and prokaryotes (Wendler et al., 1997). Previous reports suggested that PIR contributes to malignant transformation of cancer cells by acting as a transcriptional cofactor that interacts with the Bcl3–NF‐қB complex in several cancers (Adeniran and Hamelberg, 2017; Komai et al., 2015; Qiao et al., 2014). Additionally, a previous study showed that knockdown of PIR or treatment with the small molecule markedly suppressed migration of melanoma cells (Miyazaki et al., 2010). Furthermore, PIR inhibited the expression of E‐cadherin and induced the epithelial to mesenchymal transition phenotype in HeLa cells (Komai et al., 2015). According to the TCGA database, high expressions of PIR were significantly associated with short overall survival in glioblastoma, low‐grade glioma, and melanoma patients (Fig. S10). In PCa, PIR was reported to enhance cancer cell proliferation by negatively regulating apoptosis induced by EAF2/U19 (Qiao et al., 2014). However, the functions of PIR, including the mechanisms by which PIR controls metastasis in PCa, remain unclear. Our present data showed that siRNA‐mediated PIR knockdown significantly inhibited cancer cell aggressiveness. Surprisingly, transfection with miR‐455‐5p or si‐PIR produced a similar phenotype in cells lines that did (e.g., C4‐2) and did not express AR (e.g., PC3, DU145). Additionally, rescue experiments showed that the migration and invasive abilities of PCa cells that were suppressed by transfection with miR‐455‐5p were remarkably recovered by supplementing with PIR, suggesting that the miR‐455‐5p/PIR axis plays an important role in cancer progression, especially metastasis and invasion of PCa, through AR‐independent pathways. These results also suggest that PIR could be a target molecule for treatment of various cancers, including CRPC. Therefore, strategies to inhibit PIR expression are needed.
The chemical probe bisamide (CCT251236) bound PIR in a functional screen for inhibitors of the HSF1 stress pathways (Cheeseman et al., 2017). HSF1 is a pivotal regulator of the heat shock response under both physiological and pathological conditions (Dai and Sampson, 2016). Aberrant expression and activation of HSF1 is involved in cancer progression and drug resistance (Vydra et al., 2013). Bisamide (CCT251236) is orally bioavailable and successfully inhibited growth of SK‐OV‐3 human ovarian carcinoma xenografts (Cheeseman et al., 2017). Here, we showed that bisamide (CCT251236) dose‐dependently inhibited proliferation, migration, and invasion of PCa cells. Together our results support the potential of PIR as an important therapeutic target for HSPC and CRPC. Toward clinical application of bisamide, more detailed analysis of off‐target effects of si‐PIR or bisamide is necessary. It is important to investigate the molecular mechanism of inhibitory effects of si‐PIR or bisamide in detail with a current genomic approach.
5. Conclusions
Our results showed that expression of both strands of the miR‐455‐duplex (miR‐455‐5p and miR‐455‐3p) was significantly downregulated in HSPC and CRPC specimens, and thus, miR‐455‐duplex could act as an antitumor miRNA in PCa cells. Eight genes were regulated by miR‐455‐5p/‐3p, and the expression of these genes was associated with PCa pathogenesis. Among these genes, aberrant expression of PIR enhanced cancer aggressiveness, suggesting that PIR might be a promising diagnostic marker for HSPC and CRPC. Furthermore, CRPC treatment strategies targeting PIR may be possible in the future. Our approach based on antitumor miRNAs could contribute to the development of new diagnostic markers and therapeutic strategies for CRPC.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
TA, SK, and NS designed the research and managed the study. YY, SS, and MK performed statistical analyses and coordinated the figures. KY, YN, and TI designed the experiments and interpreted the results.
Supporting information
Fig. S1. Schematic representation of the chromosomal location of human miR‐455.
Fig. S2. Incorporation of both miR‐455‐5p and miR‐455‐3p into the RISC.
Fig. S3. Relationship between putative oncogenes regulated by miR‐455‐5p/‐3p and clinicopathological factors in PCa patients.
Fig. S4. Heatmaps of genes regulated by the miR‐455‐duplex and Kaplan–Meier patient survival curves for disease‐free survival rates.
Fig. S5. Direct regulation of PIR expression by miR‐455‐5p in PCa cells.
Fig. S6. IHC score and representative immunostaining pictures.
Fig. S7. Validation of si‐PIR function.
Fig. S8. Evaluation of mRNA expression of PIR using bisamide.
Fig. S9. Search for genes downstream of PIR.
Fig. S10. Kaplan–Meier curves of overall survival in other cancers due to difference in PIR expression based on TCGA database.
Table S1. Patient characteristics.
Table S2. Product numbers for reagents.
Table S3. Patient characteristics and IHC score evaluated using immunohistochemistry.
Table S4. Genes downregulated by si‐PIR in PCa cells.
Table S5. Genes downregulated by PIR inhibitor (bisamide) in PCa cells.
Table S6. Genes downstream of PIR.
Table S7. Trends of HSF1‐mediated gene expression in the presence of the PIR inhibitor bisamide.
Acknowledgements
This study was supported by KAKENHI grants 16H05462(B), 18K09338(C), 18K09160(C), 17K11160(C), 18K16723, 18K16724, and 18K16685.
References
- Adeniran C and Hamelberg D (2017) Redox‐specific allosteric modulation of the conformational dynamics of kappaB DNA by pirin in the NF‐kappaB supramolecular complex. Biochemistry 56, 5002–5010. [DOI] [PubMed] [Google Scholar]
- Arai T, Fuse M, Goto Y, Kaga K, Kurozumi A, Yamada Y, Sugawara S, Okato A, Ichikawa T, Yamanishi T et al (2018a) Molecular pathogenesis of interstitial cystitis based on microRNA expression signature: miR‐320 family‐regulated molecular pathways and targets. J Hum Genet 63, 543–554. [DOI] [PubMed] [Google Scholar]
- Arai T, Okato A, Kojima S, Idichi T, Koshizuka K, Kurozumi A, Kato M, Yamazaki K, Ishida Y, Naya Y et al (2017) Regulation of spindle and kinetochore‐associated protein 1 by antitumor miR‐10a‐5p in renal cell carcinoma. Cancer Sci 108, 2088–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Okato A, Yamada Y, Sugawara S, Kurozumi A, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N (2018b) Regulation of NCAPG by miR‐99a‐3p (passenger strand) inhibits cancer cell aggressiveness and is involved in CRPC. Cancer Med 7, 1988–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman MD, Chessum NE, Rye CS, Pasqua AE, Tucker MJ, Wilding B, Evans LE, Lepri S, Richards M, Sharp SY et al (2017) Discovery of a chemical probe bisamide (CCT251236): an orally bioavailable efficacious pirin ligand from a heat shock transcription factor 1 (HSF1) phenotypic screen. J Med Chem 60, 180–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K and Shiekhattar R (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Shiah SG, Huang CC, Hsiao JR and Chang JY (2016) Up‐regulation of miR‐455‐5p by the TGF‐beta‐SMAD signalling axis promotes the proliferation of oral squamous cancer cells by targeting UBE2B. J Pathol 240, 38–49. [DOI] [PubMed] [Google Scholar]
- Crawford ED, Higano CS, Shore ND, Hussain M and Petrylak DP (2015) Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol 194, 1537–1547. [DOI] [PubMed] [Google Scholar]
- Crona DJ, Milowsky MI and Whang YE (2015) Androgen receptor targeting drugs in castration‐resistant prostate cancer and mechanisms of resistance. Clin Pharmacol Ther 98, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crona DJ and Whang YE (2017) Androgen receptor‐dependent and ‐independent mechanisms involved in prostate cancer therapy resistance. Cancers (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C and Sampson SB (2016) HSF1: guardian of proteostasis in cancer. Trends Cell Biol 26, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela‐Kerscher A and Slack FJ (2006) Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer 6, 259–269. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB and Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T et al (2011) Restoration of miR‐145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol 38, 1093–1101. [DOI] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhao H, Diao C, Wang X, Xie Y, Liu Y, Han J and Zhang M (2018) miR‐455‐3p serves as prognostic factor and regulates the proliferation and migration of non‐small cell lung cancer through targeting HOXB5. Biochem Biophys Res Commun 495, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Gordeeva O (2018) Cancer‐testis antigens: unique cancer stem cell biomarkers and targets for cancer therapy. Semin Cancer Biol. 53, 75–89. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kojima S, Kurozumi A, Kato M, Okato A, Matsushita R, Ichikawa T and Seki N (2016) Regulation of E3 ubiquitin ligase‐1 (WWP1) by microRNA‐452 inhibits cancer cell migration and invasion in prostate cancer. Br J Cancer 114, 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kojima S, Nishikawa R, Kurozumi A, Kato M, Enokida H, Matsushita R, Yamazaki K, Ishida Y, Nakagawa M et al (2015) MicroRNA expression signature of castration‐resistant prostate cancer: the microRNA‐221/222 cluster functions as a tumour suppressor and disease progression marker. Br J Cancer 113, 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kurozumi A, Arai T, Nohata N, Kojima S, Okato A, Kato M, Yamazaki K, Ishida Y, Naya Y et al (2017) Impact of novel miR‐145‐3p regulatory networks on survival in patients with castration‐resistant prostate cancer. Br J Cancer 117, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G and Zamore PD (2002) A microRNA in a multiple‐turnover RNAi enzyme complex. Science 297, 2056–2060. [DOI] [PubMed] [Google Scholar]
- Iorio MV and Croce CM (2009) MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 27, 5848–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M et al (2012) Tumour suppressors miR‐1 and miR‐133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer 106, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Enokida H, Yoshino H, Itesako T, Chiyomaru T, Kinoshita T, Fuse M, Nishikawa R, Goto Y, Naya Y et al (2014) The tumor‐suppressive microRNA‐143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J Hum Genet 59, 78–87. [DOI] [PubMed] [Google Scholar]
- Komai K, Niwa Y, Sasazawa Y and Simizu S (2015) Pirin regulates epithelial to mesenchymal transition independently of Bcl3‐Slug signaling. FEBS Lett 589, 738–743. [DOI] [PubMed] [Google Scholar]
- Koshizuka K, Hanazawa T, Kikkawa N, Arai T, Okato A, Kurozumi A, Kato M, Katada K, Okamoto Y and Seki N (2017a) Regulation of ITGA3 by the anti‐tumor miR‐199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci 108, 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizuka K, Nohata N, Hanazawa T, Kikkawa N, Arai T, Okato A, Fukumoto I, Katada K, Okamoto Y and Seki N (2017b) Deep sequencing‐based microRNA expression signatures in head and neck squamous cell carcinoma: dual strands of pre‐miR‐150 as antitumor miRNAs. Oncotarget 8, 30288–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi A, Goto Y, Matsushita R, Fukumoto I, Kato M, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T et al (2016) Tumor‐suppressive microRNA‐223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci 107, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang J, Li Y, Wang L, Sui B and Dai D (2016) MiR‐455‐5p acts as a novel tumor suppressor in gastric cancer by down‐regulating RAB18. Gene 592, 308–315. [DOI] [PubMed] [Google Scholar]
- Marzi MJ, Ghini F, Cerruti B, de Pretis S, Bonetti P, Giacomelli C, Gorski MM, Kress T, Pelizzola M, Muller H et al (2016) Degradation dynamics of microRNAs revealed by a novel pulse‐chase approach. Genome Res 26, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, Bartel DP and Zamore PD (2005) Passenger‐strand cleavage facilitates assembly of siRNA into Ago2‐containing RNAi enzyme complexes. Cell 123, 607–620. [DOI] [PubMed] [Google Scholar]
- McCall MN, Kim MS, Adil M, Patil AH, Lu Y, Mitchell CJ, Leal‐Rojas P, Xu J, Kumar M, Dawson VL et al (2017) Toward the human cellular microRNAome. Genome Res 27, 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I, Simizu S, Okumura H, Takagi S and Osada H (2010) A small‐molecule inhibitor shows that pirin regulates migration of melanoma cells. Nat Chem Biol 6, 667–673. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Mataki H, Arai T, Okato A, Kamikawaji K, Kumamoto T, Hiraki T, Hatanaka K, Inoue H and Seki N (2017) The microRNA expression signature of small cell lung cancer: tumor suppressors of miR‐27a‐5p and miR‐34b‐3p and their targeted oncogenes. J Hum Genet 62, 671–678. [DOI] [PubMed] [Google Scholar]
- Nelson KM and Weiss GJ (2008) MicroRNAs and cancer: past, present, and potential future. Mol Cancer Ther 7, 3655–3660. [DOI] [PubMed] [Google Scholar]
- Okato A, Arai T, Kojima S, Koshizuka K, Osako Y, Idichi T, Kurozumi A, Goto Y, Kato M, Naya Y et al (2017a) Dual strands of pre‐miR150 (miR1505p and miR1503p) act as antitumor miRNAs targeting SPOCK1 in naive and castration‐resistant prostate cancer. Int J Oncol 51, 245–256. [DOI] [PubMed] [Google Scholar]
- Okato A, Arai T, Yamada Y, Sugawara S, Koshizuka K, Fujimura L, Kurozumi A, Kato M, Kojima S, Naya Y et al (2017b) Dual strands of pre‐miR‐149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int J Mol Sci 18, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okato A, Goto Y, Kurozumi A, Kato M, Kojima S, Matsushita R, Yonemori M, Miyamoto K, Ichikawa T and Seki N (2016) Direct regulation of LAMP1 by tumor‐suppressive microRNA‐320a in prostate cancer. Int J Oncol 49, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z, Wang D, Hahn J, Ai J and Wang Z (2014) Pirin down‐regulates the EAF2/U19 protein and alleviates its growth inhibition in prostate cancer cells. Prostate 74, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD and Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67, 7–30. [DOI] [PubMed] [Google Scholar]
- Sugawara S, Yamada Y, Arai T, Okato A, Idichi T, Kato M, Koshizuka K, Ichikawa T and Seki N (2018) Dual strands of the miR‐223 duplex (miR‐223‐5p and miR‐223‐3p) inhibit cancer cell aggressiveness: targeted genes are involved in bladder cancer pathogenesis. J Hum Genet 63, 657–668. [DOI] [PubMed] [Google Scholar]
- Vydra N, Toma A, Glowala‐Kosinska M, Gogler‐Piglowska A and Widlak W (2013) Overexpression of heat shock transcription factor 1 enhances the resistance of melanoma cells to doxorubicin and paclitaxel. BMC Cancer 13, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Y, Sun D, Bu J, Ren F, Liu B, Zhang S, Xu Z, Pang S and Xu S (2017) miR‐455‐5p promotes cell growth and invasion by targeting SOCO3 in non‐small cell lung cancer. Oncotarget 8, 114956–114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler WM, Kremmer E, Forster R and Winnacker EL (1997) Identification of pirin, a novel highly conserved nuclear protein. J Biol Chem 272, 8482–8489. [DOI] [PubMed] [Google Scholar]
- Wiemer EA (2007) The role of microRNAs in cancer: no small matter. Eur J Cancer 43, 1529–1544. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Arai T, Kojima S, Sugawara S, Kato M, Okato A, Yamazaki K, Naya Y, Ichikawa T and Seki N (2018a) Anti‐tumor roles of both strands of the miR‐455 duplex: their targets SKA1 and SKA3 are involved in the pathogenesis of renal cell carcinoma. Oncotarget 9, 26638–26658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Nishikawa R, Kato M, Okato A, Arai T, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N (2018b) Regulation of HMGB3 by antitumor miR‐205‐5p inhibits cancer cell aggressiveness and is involved in prostate cancer pathogenesis. J Hum Genet 63, 195–205. [DOI] [PubMed] [Google Scholar]
- Yang H, Wei YN, Zhou J, Hao TT and Liu XL (2017) MiR‐455‐3p acts as a prognostic marker and inhibits the proliferation and invasion of esophageal squamous cell carcinoma by targeting FAM83F. Eur Rev Med Pharmacol Sci 21, 3200–3206. [PubMed] [Google Scholar]
- Yao J, Caballero OL, Yung WK, Weinstein JN, Riggins GJ, Strausberg RL and Zhao Q (2014) Tumor subtype‐specific cancer‐testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res 2, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yan M, Yun Y, Zhang J, Zhang R, Li Y, Wu X, Liu Q, Miao W and Jiang H (2017) MicroRNA‐455‐3p functions as a tumor suppressor by targeting eIF4E in prostate cancer. Oncol Rep 37, 2449–2458. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lin Z, Zhang L and Chen H (2016) MicroRNA‐455‐3p inhibits tumor cell proliferation and induces apoptosis in HCT116 human colon cancer cells. Med Sci Monit 22, 4431–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Schematic representation of the chromosomal location of human miR‐455.
Fig. S2. Incorporation of both miR‐455‐5p and miR‐455‐3p into the RISC.
Fig. S3. Relationship between putative oncogenes regulated by miR‐455‐5p/‐3p and clinicopathological factors in PCa patients.
Fig. S4. Heatmaps of genes regulated by the miR‐455‐duplex and Kaplan–Meier patient survival curves for disease‐free survival rates.
Fig. S5. Direct regulation of PIR expression by miR‐455‐5p in PCa cells.
Fig. S6. IHC score and representative immunostaining pictures.
Fig. S7. Validation of si‐PIR function.
Fig. S8. Evaluation of mRNA expression of PIR using bisamide.
Fig. S9. Search for genes downstream of PIR.
Fig. S10. Kaplan–Meier curves of overall survival in other cancers due to difference in PIR expression based on TCGA database.
Table S1. Patient characteristics.
Table S2. Product numbers for reagents.
Table S3. Patient characteristics and IHC score evaluated using immunohistochemistry.
Table S4. Genes downregulated by si‐PIR in PCa cells.
Table S5. Genes downregulated by PIR inhibitor (bisamide) in PCa cells.
Table S6. Genes downstream of PIR.
Table S7. Trends of HSF1‐mediated gene expression in the presence of the PIR inhibitor bisamide.


