Abstract
The aim of the study was to describe the HIV care continuum in emergency department (ED) patients in the Eastern Cape region of South Africa. This is a cross-sectional, identity-unlinked serosurvey, whereby discarded/excess samples from all patients who had blood drawn during the study period for routine care and sufficient serum remaining were tested for HIV, hepatitis B virus, and hepatitis C virus infection; HIV viral load (VL); and presence of antiretroviral (ARV) drugs. We also estimated cross-sectional incidence using the Limiting-Antigen Avidity assay and HIV VL. The study was conducted between September and November 2016 at the Frere Hospital Emergency Department in East London, South Africa. The overall HIV prevalence in our study population was 26.9% [95% confidence interval (CI): 25.0–28.8; n = 2,100]. The highest prevalence was observed among females in the 30–39 years age group [60.3% (95% CI: 53.2–67.1)]. HIV prevalence was significantly higher among females compared with males in both the 20–29 years age group and 30–39 years age group (p < .05), but nearly identical to older age groups. ARV drugs were detected in 53.5% (95% CI: 48.1–58.9) of HIV-infected subjects. The frequency of HIV viral suppression (< 1,000 copies/mL) was 48.5% (95% CI: 44.3–52.7), and was not statistically different between males and females (age-adjusted prevalence ratio = 1.15, 95% CI: 0.95–1.39). The HIV incidence rate was estimated to be 2.6% (95% CI: 1.2–3.9). The Frere Hospital ED has an extremely high burden of HIV infection. The detection of ARV drugs and prevalence of viral suppression fall short of the World Health Organization 90-90-90 goals in this population. Furthermore, there were a large number of patients with recent infection in the ED. The ED is a critical venue for testing and linkage to care of high-yield population who are likely missed by current testing and linkage-to-care programs.
Keywords: HIV epidemiology, HIV incidence, South Africa, emergency medicine
Introduction
South Africa has the greatest burden of HIV disease in the world with >7 million infected individuals.1 The 2016 South Africa country fact sheet reports an HIV prevalence of 18.9% with an annual incidence of ∼1 per 100 adults between the ages of 15 and 49 years.2,3 These modeling estimates were derived using the SPECTRUM© platform based on HIV prevalence from surveillance of pregnant women attending antenatal care clinics, and from nationally representative population-based surveys.4 Expanding HIV surveillance to a variety of health care venues will better allow policymakers to better identify other testing venues with high HIV incidence to better target testing resources.
For decades, emergency department (ED)-based serosurveys have been used in the United States as a robust approach for obtaining population-level estimates of HIV prevalence, incidence, and more recently metrics along the HIV care cascade.5,6 ED-based surveillance has allowed policymakers and implementers to advocate for routine ED-based testing services in the United States.7,8 The ED provides a valuable window into the community delivering care to high numbers of patients with a wide range of clinical conditions.9 Furthermore, patients with substance abuse, homelessness, mental health problems, and victims of violent crime are more likely to seek care in the ED compared with other primary care settings.10 Finally, in the United States, ED populations have been shown to have a higher prevalence of HIV infection compared with antenatal clinics and other primary care settings.5,11–14 This study sought to estimate HIV and hepatitis prevalence, HIV incidence, and the HIV care cascade, in a South African ED setting, using a blinded serosurvey approach.
Materials and Methods
A cross-sectional, identity-unlinked serosurvey was conducted at Frere Hospital in the Eastern Cape region of South Africa from August 29 to November 30, 2016. Frere Hospital is a government-funded tertiary care hospital that provides 24-h emergency services. Patients attending the ED come from an ∼100-km radius catchment area, and are either self-referred or referred from district hospitals and clinics in the region. The blinded serosurvey methodology has been previously described in depth elsewhere.5,15,16 During the 3-month study period, excess blood samples (i.e., samples that had completed clinical testing and undergone mandatory storage for 7 days) were retained from all patients aged ≥18 years who presented in the ED. Samples were stripped of protected health information (PHI) except for age and sex, and were then centrifuged and screened in the hospital laboratory for HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV). Extended serum analysis for HIV incidence, the presence of antiretrovirals (ARVs) and viral loads (VLs), was conducted at the NIAID International HIV/STD Laboratory located in Baltimore, Maryland.
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, the University of Cape Town, and the Walter Sisulu University Human Research Ethics Committees. Informed consent was deemed unnecessary given the use of remnant samples due to be discarded and collected during routine clinical care. All patients presenting to the ED for care were informed of the blinded serosurvey through educational posters and by hospital staff during triage. Given that patient identifiers were stripped of PHI before testing, it was not possible to share testing results with the patient. To overcome this quandary, a parallel study was conducted in the ED that offered voluntary counseling and testing to all ED patients during the study period using 24 h a day 7 days a week lay counselors.17
Laboratory testing
All tests were conducted based on sample availability; tests undertaken in the Frere Hospital laboratory required a minimum of 800 μL, and all testing at the Johns Hopkins University (JHU) laboratory required 400 μL of excess serum. In the Frere Hospital laboratory, samples were tested for HIV using the fourth-generation ARCHITECT HIV Ag/Ab Combo ELISA, HCV antibodies using the ARCHITECT anti-HCV assay, hepatitis B surface antigen using the ARCHITECT HBsAg Qualitative II assay, and HBV core IgM antibody using the ARCHITECT Anti-HBc IgM Assay (Abbott Laboratories, Abbott Park, IL). At the JHU laboratory, HIV-positive samples were further analyzed for HIV VL using the Abbott m2000 RealTime System (Abbott Laboratories) (limit of detection, 320 copies/mL). HIV-positive samples were also tested using the LAg-Avidity assay (Maxim HIV-1 Limiting Antigen Avidity EIA; Maxim Biomedical, Inc., Rockville, MD). After all tests were performed, a subset of HIV-positive samples with available specimen (≥250 μL) were assessed for the presence of ARV drugs using high-performance liquid chromatography (HPLC)–high-resolution accurate mass (HRAM) spectrometry, which detects all commonly used ARVs reliably.18
Statistical analysis
Comparisons of prevalence estimates were made using Pearson's chi-squared tests, and 95% confidence interval (CI) was estimated from a binomial distribution. Adjusted prevalence ratios (aPRs) of ARV detection and HIV viral suppression were calculated among HIV-positive patients using multivariable log-binomial regression models that included an adjustment for age group and sex. ARV detection was defined as the presence of any ARVs. For purposes of HIV incidence estimation and cascade description, HIV viral suppression was defined as a VL <1,000 copies/mL.3,19,20 For HIV incidence and 95% CI estimates patients with a LAg Avidity assay <1.5 OD-n and HIV VL >1,000 copies/mL were considered recently infected; this Centers for Disease Control and Prevention incidence testing algorithm has a window period of 144 days and a false-recent rate of 0%.21–25 The analysis was conducted using STATA version 14.2 (StataCorp LP, College Station, TX).
Results
Sample population
During the study period, the ED had 9,583 patient visits, of which 2,289 patients had blood drawn for routine clinical care in the ED and were included in the study. One hundred eighty-nine patients did not have sufficient excess serum (400 μL) for any testing, and thus were excluded from the analysis. The remaining 2,100 samples were all tested for HIV infection, of which 1,351 were also tested for HBV infection (HBsAg+ and/or HBcAb+), and 1,353 samples were also tested for HCV IgG antibody. Seven hundred forty-seven samples did not have sufficient excess serum (800 μL) for hepatitis testing. Of the 565 samples identified to be HIV positive, 542 samples were assessed for HIV VL and cross-sectional incidence biomarkers, the remaining 23 had insufficient serum. Only 326 of the HIV-positive samples had sufficient volume (250 μL) to complete testing for detectable ARVs.
HIV prevalence and incidence
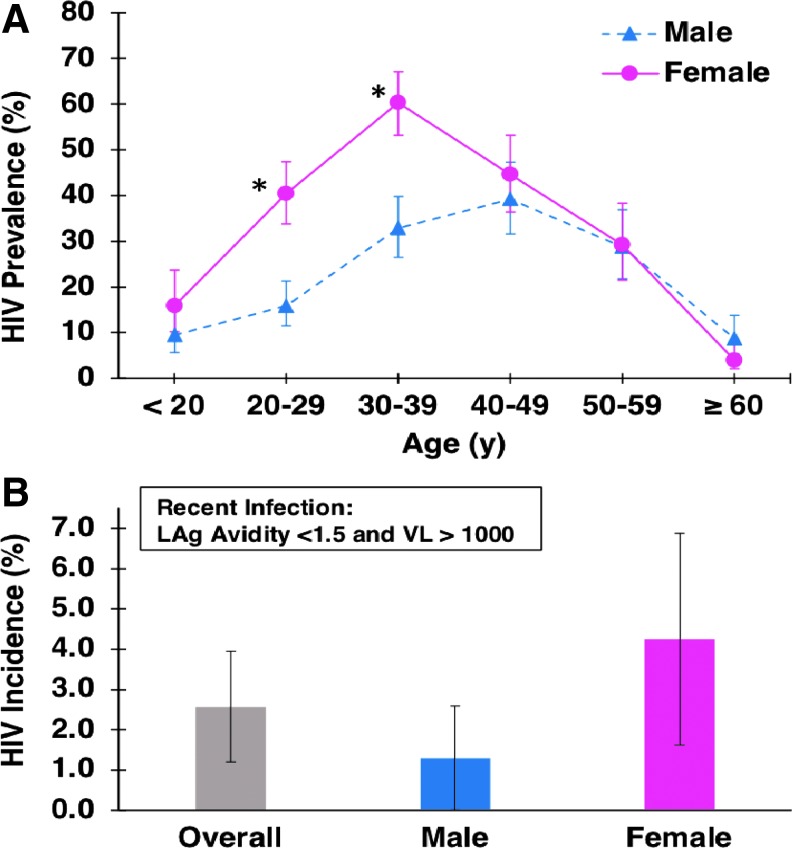
Of 2,100 patients, 565 were HIV positive yielding an overall HIV prevalence of 26.9% (95% CI: 25.0–28.8). The HIV prevalence among males was 21.9% (95% CI: 19.5–24.5; n = 1,049) compared with 32.2% (95% CI: 29.3–35.1; n = 998) among females (p < .001). In females, HIV prevalence was highest among patients aged 20–29 years [40.4% (95% CI: 33.8–47.4); n = 198] and 30–39 years [60.3% (95% CI: 53.2–67.1); n = 189] (Fig. 1A). In males, HIV prevalence was highest among patients aged 40–49 years [39.2% (95% CI: 31.6–47.3); n = 58] (Fig. 1A). HIV prevalence was significantly higher among females compared with males in the 20–29 and 30–39 years age groups (p < .05), but nearly identical among older age groups (Fig. 1A).
FIG. 1.
HIV prevalence (A) and incidence (B) by age and sex. *p < .05. LAg, limiting antigen; VL, viral load.
The incidence testing algorithm identified 4 males (of 220 HIV+ tested) and 11 females (of 311 HIV+ tested) as recently infected (i.e., infected within 144 days), yielding an overall annual HIV incidence estimate of 2.6% (95% CI = 1.2–3.9). HIV incidence was estimated to be 4.3% in females (95% CI: 1.6–6.9) and 1.3% in males (95% CI: 0.0–2.6; Fig. 1B). Thirty-four patients did not have sufficient serum for the incidence testing algorithm, and thus were excluded for this analysis.
HIV care continuum
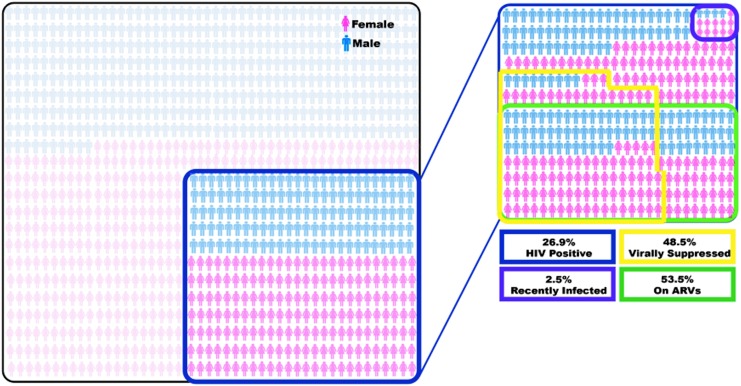
ARVs were detected in 53.5% (95% CI: 48.1–58.9; n = 326) of HIV-infected subjects with sufficient sample volumes available. Of the 174 patients with detectable ARVs, 67.2% (95% CI: 59.9–73.9) were virally suppressed (HIV VL <1,000 copies/mL). Approximately half of all HIV-infected patients [48.5% (95% CI: 44.3–52.7); n = 542] were virally suppressed (HIV VL <1,000 copies/mL). An infographic quantifying the burden of HIV, percentage on ARVs and percentage viral suppressed is provided in Figure 2.
FIG. 2.
Infographic demonstrating proportion of emergency department patients who are HIV positive, virally suppressed, on antiretrovirals, and recently infected during the study period.
The prevalence of HIV viral suppression (<1,000 copies/mL) was significantly higher among the 40–49 year olds when compared with 20–29 year olds (aPR = 1.37, 95% CI: 1.04–1.82), independent of sex (Table 1).
Table 1.
Antiretroviral Detection and Viral Suppression in HIV-Positive Patients by Sex and Age Category
| ARV detection | Viral suppression | |||||
|---|---|---|---|---|---|---|
| N tested | No. on ARV (%) | Adjusted PR (95% CI) | N tested | No. virally suppressed (%) | Adjusted PR (95% CI) | |
| Sex | ||||||
| Male | 131 | 69 (52.7) | Reference | 218 | 97 (44.5) | Reference |
| Female | 185 | 102 (55.1) | 1.11 (0.91–1.37) | 310 | 159 (51.3) | 1.15 (.95–1.39) |
| Unknown | 10 | 4 (40.0) | — | 14 | 7 (50.0) | — |
| Age group, years | ||||||
| 0–19 | 11 | 3 (27.2) | 0.58 (0.22–1.54) | 33 | 19 (57.6) | 1.38 (0.95–2.01) |
| 20–29 | 73 | 37 (50.7) | Reference | 113 | 45 (39.8) | Reference |
| 30–39 | 101 | 59 (58.4) | 1.17 (0.88–1.55) | 167 | 82 (49.1) | 1.20 (0.91–1.58) |
| 40–49 | 72 | 47 (65.3) | 1.31 (0.98–1.76) | 112 | 62 (55.4) | 1.37 (1.04–1.82)* |
| ≥50 | 55 | 25 (45.5) | 0.92 (0.63–1.33) | 95 | 42 (44.2) | 1.11 (0.81–1.53) |
| Unknown | 14 | 4 (28.6) | — | 22 | 13 (59.1) | — |
| Total | 326 | 175 (53.5) | 542 | 263 (48.5) | ||
Multivariable log-binomial model for each outcome was adjusted for sex and age group. Individuals with missing data on sex and age group were excluded from the model.
p < .05.
ARV, antiretroviral; CI, confidence interval; PR, prevalence ratio.
Coinfections
The prevalence of active HBV infection (HBsAg+) was 5.2% (95% CI: 4.1–6.5; n = 1,346). Of the patients with active HBV infection, 7.1% (5/70) were acutely infected (HBc IgM+). HIV-positive patients were significantly more likely to have active HBV infection than HIV-negative patients [8.5% (95% CI: 6.0–11.9) vs. 4.0% (95% CI: 2.9–5.4); p = .001]. Prevalence of HCV IgG antibody was 1.0% (95% CI: 0.6–1.7; n = 1,349). HIV-positive patients were significantly more likely to have been infected with HCV in comparison with HIV-negative patients [2.2% (95% CI: 1.1–4.3) vs. 0.6% (95% CI: 0.3–1.4); p = .012].
Discussion
This anonymous serosurvey revealed a much higher HIV prevalence (26.9%) than that nationally reported for the local district (13.6%). The observed prevalence of >60% in females between 30 and 39 years is almost double the national estimates of 36% in the same group.26 The high HIV prevalence in this venue is in keeping with other African ED HIV prevalence studies of 23%–43%.26–30
The HIV incidence estimate observed in this ED (2.9%) is also higher than the national estimate (1.7%).26 The high incidence and prevalence likely reflect ED population biases, which attract patients who have higher risk of acquiring HIV, such as those who are homeless, victims of crime and substance-related comorbidities.17,31 Furthermore, it is possible that current sampling strategies (i.e., antenatal clinical and population-based surveys) that are used for national incidence estimates may underestimate the national incidence.3
ARVs were detected in only 53% of persons/people living with HIV (PLWH) falling short of the World Health Organization (WHO) 90-90-90 goals (81% of PLWH). Unfortunately, while this demonstrates some access to ARVs, the region is far short of reaching the WHO 2020 goals.32 Part of the shortfall in ARV treatment observed is accounted for by the fact that a proportion of HIV-positive individuals in this study may have been unaware of their serostatus. Notably, as well, only 67.2% of those taking ARVs were virally suppressed, again falling short of the third 90% target. This is concerning given the increased risk of ongoing transmission of HIV, and potential for emergence of drug resistance associated with poor adherence.
The increased burden of HBV and HCV among the HIV-infected and non-HIV-infected patients is a universal finding, most likely due to shared transmission routes. The 8.5% HBV coinfection of HIV patients is higher than that reported in urban and rural South African HIV cohorts of 4.8% and 7.1%, respectively.33,34 The HCV seroprevalence (2.2%) among HIV-infected patients is similar to other South African studies (2.9%).35 The higher prevalence of HBV in our study population may be attributable to risky sexual behavior, given the young median age of the ED population. However, given the overall low numbers of the HBV- and HCV-positive individuals and the lack of additional demographic data further interpretation is limited.
A limitation of this study is that the blood sampling was not universal or random across the ED patients, but according to clinical indication and availability of excess blood samples. This may overestimate the actual HIV prevalence, as sicker patients would be more likely to have their blood drawn. Furthermore, the quantity of excess serum available was not equal among participants, and thus a small number of patients were excluded from extended serum analyses such as hepatitis screening, ARV presence, and the incidence algorithm. Another limitation is that due to the identity-unlinked nature of this study, we are unable to ascertain if patients were aware of their HIV status. Finally, the generalizability of these results to the surrounding community is unknown, given the unique nature of the ED population and the referral pathways within the health care system.
Conclusion
This anonymous serosurvey of a South African ED demonstrates that the ED population has almost double the HIV prevalence and incidence rates compared with national estimates. The ED population likely represents a potential transmission hot spot, given the high burden of both undiagnosed and new HIV infections. Furthermore, the proportion of HIV-infected patients in the ED taking ARVs and achieving viral suppression falls short of WHO targets. Thus, the ED is a critical venue for targeted interventions focused on HIV prevention, testing, treatment initiation, and linkage to care.
Acknowledgments
Research reported in this publication was funded by the South African Medical Research Council and in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (TCQ). The authors would also like to acknowledge the staff in the Frere Hospital Emergency Department for making this research possible, the HIV Counseling and Testing team for their dedication and hard work during the study, Nadia Yimer and Pankati Thaker for their role in data validation, and the Walter Sisulu Infectious Diseases Screening in Emergency Departments (WISE) study team.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. AVERT: HIV and AIDS in South Africa. Available at www.avert.org/professionals/hiv-around-world/sub-saharan-africa/south-africa (2017), accessed August1, 2018
- 2. HSRC: Research Methodology and Data Centre, SA. Available at www.hsrc.ac.za/en/departments/rmdc (2018), accessed August1, 2018
- 3. Rehle T, Johnson L, Hallett T, et al. : A comparison of South African national HIV incidence estimates: A critical appraisal of different methods. PLoS One 2015;10:e0133255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. UNAIDS: UNAIDS HIV Data and Estimates. Available at www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf (2017)
- 5. Hsieh YH, Kelen GD, Laeyendecker O, Kraus CK, Quinn TC, Rothman RE: HIV care continuum for HIV-infected emergency department patients in an inner-city academic emergency department. Ann Emerg Med 2015;66:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelen GD, Hsieh Y-H, Rothman RE, et al. : Improvements in the continuum of HIV care in an inner-city emergency department. AIDS 2016;30:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothman RE: Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: Critical role of and a call to action for emergency physicians. Ann Emerg Med 2004;44:31–42 [DOI] [PubMed] [Google Scholar]
- 8. Brown J, Shesser R, Simon G, et al. : Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines: Results from a high-prevalence area. J Acquir Immune Defic Syndr 2007;46:395–401 [DOI] [PubMed] [Google Scholar]
- 9. Coffey R, Houchens R, Chu B, et al. : Emergency Department Use for Mental and Substance Use Disorders. Agency for Health care Research and Quality (AHRQ), Rockville, MD, 2010, p. 131 [Google Scholar]
- 10. La Fleur F: Determinants of physician utilization, emergency room use, and hospitalizations among populations with multiple health vulnerabilities. Health 2010;15:491–516 [DOI] [PubMed] [Google Scholar]
- 11. Copeland B, Shah B, Wheatley M, Heilpern K, del Rio C, Houry D: Diagnosing HIV in men who have sex with men: An emergency department's experience. AIDS Patient Care STDs 2012;26:202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng R, Kendall CE, Burchell AN, et al. : Emergency department use by people with HIV in Ontario: A population-based cohort study. CMAJ Open 2016;4:E240–E248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohareb AM, Rothman RE, Hsieh YH: Emergency department (ED) utilization by HIV-infected ED patients in the United States in 2009 and 2010—A national estimation. HIV Med 2013;14:605–613 [DOI] [PubMed] [Google Scholar]
- 14. Hsieh Y-H, Kelen GD, Beck KJ, et al. : Evaluation of hidden HIV infections in an urban ED with a rapid HIV screening program. Am J Emerg Med 2016;34:180–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelen GD, DiGiovanna T, Bisson L, Kalainov D, Sivertson KT, Quinn TC: Human immunodeficiency virus infection in emergency department patients. Epidemiology, clinical presentations, and risk to health care workers: The Johns Hopkins experience. JAMA 1989;262:516–522 [DOI] [PubMed] [Google Scholar]
- 16. Kelen GD, Johnson G, DiGiovanna TA, Loring K, Sivertson KT: Profile of patients with human immunodeficiency virus infection presenting to an inner-city emergency department: Preliminary report. Ann Emerg Med 1990;19:963–969 [DOI] [PubMed] [Google Scholar]
- 17. Hansoti B, Stead D, Parrish A, et al. : HIV testing in a South African emergency department: A missed opportunity. PLoS One 2018;13:e0193858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marzinke MA, Breaud A, Parsons TL, et al. : The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta 2014;433:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuma K, Shisana O, Rehle TM, et al. : New insights into HIV epidemic in South Africa: Key findings from the National HIV Prevalence, Incidence and Behaviour Survey, 2012. Afr J AIDS Res 2016;15:67–75 [DOI] [PubMed] [Google Scholar]
- 20. Huerga H, Van Cutsem G, Ben Farhat JB, et al. : Progress towards the UNAIDS 90-90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: A household-based community cross-sectional survey. BMC Public Health 2018;18:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kassanjee R, McWalter TA, Bärnighausen T, Welte A: A new general biomarker-based incidence estimator. Epidemiology 2012;23:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brookmeyer R, Quinn TC: Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. Am J Epidemiol 1995;141:166–172 [DOI] [PubMed] [Google Scholar]
- 23. CDC: Estimates of new HIV infections in the United States. Available at www.cdc.gov/nchhstp/newsroom/docs/fact-sheet-on-hiv-estimates.pdf (2018), accessed August1, 2018
- 24. Janssen RS, Satten GA, Stramer SL, et al. : New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 1998;280:42–48 [DOI] [PubMed] [Google Scholar]
- 25. Brookmeyer R, Konikoff J, Laeyendecker O, Eshleman SH: Estimation of HIV incidence using multiple biomarkers. Am J Epidemiol 2013;177:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simbayi L, Shisana O, Rehle T, et al. : South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Human Sciences Research Council, Pretoria, 2014 [Google Scholar]
- 27. Waxman MJ, Kimaiyo S, Ongaro N, Wools-Kaloustian KK, Flanigan TP, Carter EJ: Initial outcomes of an emergency department rapid HIV testing program in western Kenya. AIDS Patient Care STDs 2007;21:981–986 [DOI] [PubMed] [Google Scholar]
- 28. Waxman MJ, Muganda P, Carter EJ, Ongaro N: The role of emergency department HIV care in resource-poor settings: Lessons learned in western Kenya. Int J Emerg Med 2008;1:317–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakanjako D, Kyabayinze DJ, Mayanja-Kizza H, Katabira E, Kamya MR: Eligibility for HIV/AIDS treatment among adults in a medical emergency setting at an urban hospital in Uganda. Afr Health Sci 2007;7;124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haac BE, Charles AG, Matoga M, LaCourse SM, Nonsa D, Hosseinipour M: HIV testing and epidemiology in a hospital-based surgical cohort in Malawi. World J Surg 2013;37:2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peden M, Van der Spuy J, Smith P, Bautz P. Substance abuse and trauma in Cape Town. S Afr Med J 2000;90:251–255 [PubMed] [Google Scholar]
- 32. Iwuji CC, Orne-Gliemann J, Larmarange J, et al. : Uptake of home-based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu-Natal, South Africa: Descriptive results from the first phase of the ANRS 12249 TasP cluster-randomised trial. PLoS Med 2016;13:e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Firnhaber C, Reyneke A, Schulze D, et al. : The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J 2008;98:541–544 [PMC free article] [PubMed] [Google Scholar]
- 34. Boyles TH, Cohen K: The prevalence of hepatitis B infection in a rural South African HIV clinic. S Afr Med J 2011;101:470–471 [PubMed] [Google Scholar]
- 35. Azevedo TCL, Zwahlen M, Rauch A, Egger M, Wandeler G: Hepatitis C in HIV-infected individuals: A systematic review and meta-analysis of estimated prevalence in Africa. J Int AIDS Soc 2016;19:20711. [DOI] [PMC free article] [PubMed] [Google Scholar]