Abstract
Introduction: Palliative surgeries were controversial for asymptomatic metastatic gastric cancer (mGC) patients. This study was aimed to evaluate survival benefit of palliative surgeries to gastric and/or metastatic tumors in mGC patients based on U.S population.
Materials and Methods: A total of 8345 gastric cancer patients diagnosed with synchronous distal metastasis between 2004 to 2013 from the Surveillance, Epidemiology, and End Results Program (SEER) database were divided into four groups according to surgery strategies: surgeries to both primary and metastatic tumors (SPM), gastrectomy only (GO), metastasectomy only (MO) and no surgery performed (NS). Their clinicopathological characteristics and overall survival (OS) were analyzed before and after propensity score matching (PSM) and coarsened exact matching (CEM).
Results: The median OS of SPM and GO patients was both significantly higher than NS patients (11 months vs. 8 months vs. 5 months; P<0.001, respectively) while that of MO was not (6 months vs. 5 months; P= 0.286). In comparisons between surgery strategies, survival benefit was similar between SPM and GO groups (P=0.389) and both showed significantly better survival than MO patients (P<0.001). All surgery strategies were proved to be favorable prognostic factors over non-surgical treatment (Hazard ratio (HR) for SPM: 0.60, P<0.001; HR for GO: 0.62, P<0.001; HR for MO: 0.91, P=0.046). Similar results were obtained after matching by PSM and CEM except that prognostic impact of MO deteriorated.
Conclusions: Gastrectomy plus metastasectomy or gastrectomy alone could be adopted as a choice of improving survival in the U.S population. Metastasectomy alone is not generally recommended.
Keywords: gastric cancer, gastrectomy, metastasectomy, survival, matching
1. Introduction
Gastric cancer (GC) ranks the fifth most common tumors and the fourth leading cause of cancer-related deaths in the United States, with approximately 22,220 new cases and 10,990 deaths in 2014. 1 Although an annual decline of the incidence rate of GC was observed since 1992 2, the 5-year overall survival (OS) remained unimproved. Especially for metastatic GC (mGC), the median survival was only 7.9 to 13.8 months as assessed in several randomized clinical trials, which however consisted of more than one third of initially diagnosed GC patients. 3 Developing best treatment strategies for mGC is undoubtedly of vital importance. Systemic treatment strategies with chemotherapy, target therapy and immunotherapy are universally adopted while performing surgery is controversial. Current guidelines approved of palliative gastrectomy in terms of obstruction or uncontrolled bleeding, but palliative surgeries for the purpose of tumor reduction to either primary or metastatic sites were not mentioned for recommendation. 4 There were some studies finding that surgical resection with therapeutic intent in mGC patients was associated with a relatively poor prognosis, and the REGATTA trial conducted in Eastern population negated gastrectomy followed by chemotherapy could improve survival in mGC patients compared to chemotherapy alone. 5, 6 Nevertheless, in recent years many retrospective studies challenged the negative results and have shown survival benefit in patients treated with gastrectomy and/or metastasectomy with or without chemotherapy. 7-14 Moreover, the GYMSSA trial proved regional treatment including maximal cytoreductive surgery and regional hyperthermic intraperitoneal chemotherapy combined with systemic chemotherapy could achieve prolonged survival in selected patients. 15 For now, the survival impacts of surgeries to mGC patients in Western population are still under investigation, and there also lacks evidence in comparing different surgery strategies.
In this study, we searched the Surveillance, Epidemiology, and End Results Program (SEER) database to evaluate survival benefit from surgeries to primary and/or metastatic tumors in comparison with non-surgeryin the U.S. population.
2. Material and methods
2.1 Patients and data collection
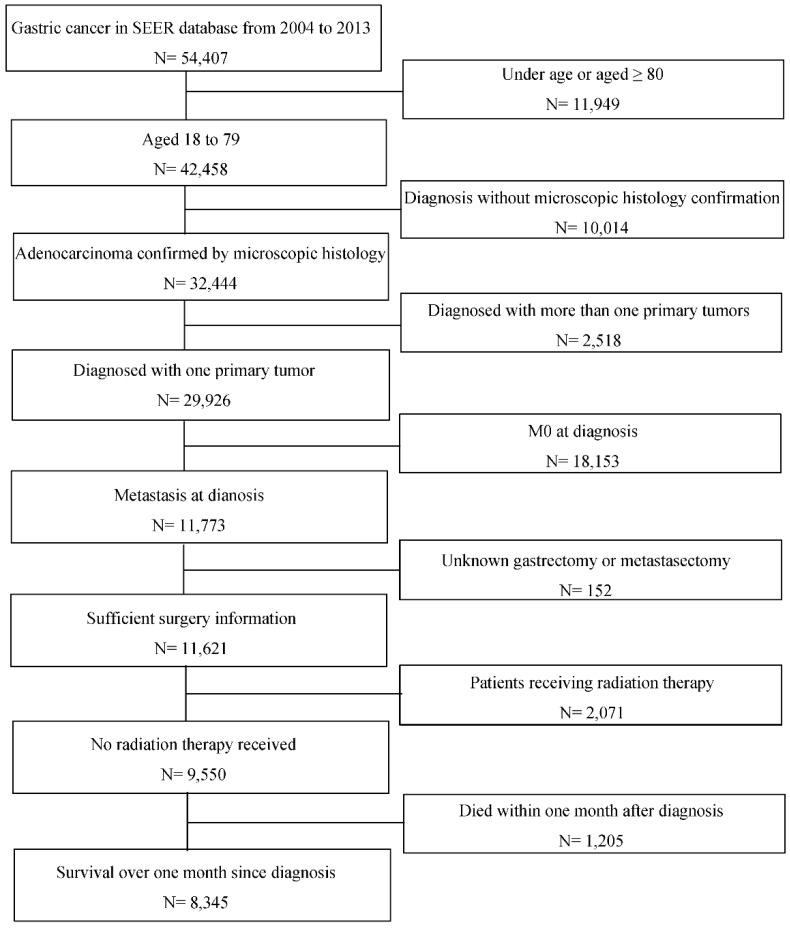
The study population and their clinicopathological data were searched and collected from Surveillance, Epidemiology, and End Results Program (SEER) database. Patients initially diagnosed as gastric adenocarcinoma by microscopically confirmation with synchronous distal metastasis between 2004 to 2013 at the age of 18 to 79 were included. GC was defined according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) with codes C160/C161/C162/C163/C164/C165/C166/C168/C169 for primary tumor location and codes 8140/8144/8145/8211/8260/8480/8490 for histological type. Patients aged 80 or more are excluded because they are not usually considered for surgery and thus not fit in this topic. Patients diagnosed with more than one primary tumor and patients undergoing radiation therapy were excluded to avoid distraction from study objectives. It was necessary to clarify each patient's surgery strategy that whether one underwent primary or metastatic tumor resection, and thus those without such information would also be excluded. Additionally, patients who died within one month after diagnosis were excluded for more sufficient analysis. Figure 1 presents the inclusion and exclusion process. A total of 8345 eligible patients were divided into four groups according to the surgery strategy, that is one group of patients who underwent surgeries to both primary and metastatic tumors (SPM), one group with gastrectomy only (GO), one group with metastasectomy only (MO) and one group of the other patients who was not surgically operated (NS).
Figure 1.
Flowchart of inclusion and exclusion process of the study population. Abbreviations: SEER, Surveillance, Epidemiology, and End Results; M0, without distant metastasis diagnosed.
No institutional approval or informed consent was required because SEER is a public-use database.
2.2 Statistical analysis
Patient characteristics were compared by the chi-square test for categorical variables. Spearman rank correlation analysis was used to evaluate time trends of surgery strategies. Overall survival (OS) was the primary endpoint outcome and was evaluated by Kaplan-Meier method. Log-rank test was used to compare survival between groups. To analyze prognostic factors, the univariable Cox regression analysis was performed and hazard ratio (HR) with 95% confidence interval (CI) were assessed, followed by a multivariable Cox analysis to evaluate prognostic impact of palliative surgeries with risk adjustment for the other factors whose P value <0.05 in the univariable Cox analysis. Furthermore, two matching methods were introduced to guarantee better balance in baseline characteristics between groups and to reduce confounding impacts on survival analysis. One matched subanalysis was based on the propensity score with 1-to-1-to-1-to-1 matching (PSM). 16 Coarsened exact matching (CEM) method, which is less fitting-model dependent than PSM and could improve balance for each covariate without influencing the others to achieve maximum of the balance, was used in the other subanalysis. 17-19 Population after matching was analyzed as it was before matching.
Information on chemotherapy in the SEER database was limited to patients with or without/unknown receiving chemotherapy, and was suggested to be used with cautions due to its incompleteness and potential biases according to the official data use agreement. However, systemic chemotherapy is a major treatment for mGC patients and have a strong impact on their survival, therefore we carried out sensitivity analyses to verify the survival benefit of surgery strategies in combination of chemotherapy after excluding patients receiving no/unknown chemotherapy.
All data analyses were performed using SPSS Statistics 22.0 (IBM Corp., Version 22.0, Armonk, NY) and the “MatchIt” and “cem” R packages (The R Foundation, version 3.4.2). A two-sided P value <0.05 was considered to be statistically significant.
3. Results
3.1 Patient characteristics
Table 1 shows the demographic and clinicopathological characteristics of the patients enrolled. The median age of the whole cohort was 61 years old and 62.3% was male. Non-Hispanic white people and west-region residents took the most proportion, respectively.
Table 1.
Baseline demographic and clinicopathological characteristics of the patients
| Variable | Number (%) | P value | ||||
|---|---|---|---|---|---|---|
| All cohort | SPM | GO | MO | NS | ||
| Total | 8345 (100.0%) | 359 (4.3%) | 1006 (12.1%) | 551 (6.6%) | 6429 (77.0%) | |
| Age, years | <0.001 | |||||
| < 50 | 1819 (21.8%) | 106 (29.5%) | 207 (20.6%) | 206 (37.4%) | 1300 (20.2%) | |
| 50-59 | 2028 (24.3%) | 87 (24.2%) | 254 (25.2%) | 138 (25.0%) | 1549 (24.1%) | |
| 60-69 | 2449 (29.3%) | 83 (23.1%) | 297 (29.5%) | 120 (21.8%) | 1949 (30.3%) | |
| ≥ 70 | 2049 (24.6%) | 83 (23.1%) | 248 (24.7%) | 87 (15.8%) | 1631 (25.4%) | |
| Sex | <0.001 | |||||
| Male | 5195 (62.3%) | 179 (49.9%) | 629 (62.9%) | 212 (38.5%) | 4175 (64.9%) | |
| Female | 3150 (37.7%) | 180 (50.1%) | 377 (37.5%) | 339 (61.5%) | 2254 (35.1%) | |
| Marriage status | 0.012 | |||||
| Married | 5005 (60.0%) | 227 (63.2%) | 631 (62.7%) | 327 (59.3%) | 3820 (59.4%) | |
| Widowed | 670 (8.0%) | 28 (7.8%) | 97 (9.6%) | 35 (6.4%) | 510 (7.9%) | |
| Other | 2670 (32.0%) | 104 (29.0%) | 278 (27.6%) | 189 (34.3%) | 2099 (32.6%) | |
| Race | <0.001 | |||||
| Non-Hispanic White | 3973 (47.6%) | 170 (47.4%) | 389 (38.7%) | 242 (43.9%) | 3172 (49.3%) | |
| Non-Hispanic Black | 1123 (13.5%) | 42 (11.7%) | 158 (15.7%) | 67 (12.2%) | 856 (13.3%) | |
| Hispanic | 2030 (24.3%) | 93 (25.9%) | 271 (26.9%) | 151 (27.4%) | 1515 (23.6%) | |
| Other | 1219 (14.6%) | 54 (15.0%) | 188 (18.7%) | 91 (16.5%) | 886 (13.8%) | |
| SEER region | <0.001 | |||||
| Mid-west | 917 (11.0%) | 43 (12.0%) | 69 (6.9%) | 71 (12.9%) | 734 (11.4%) | |
| Northeast | 1327 (15.9%) | 62 (17.3%) | 143 (14.2%) | 71 (12.9%) | 1051 (16.3%) | |
| South | 1339 (16.0%) | 67 (18.7%) | 162 (16.1%) | 85 (15.4%) | 1025 (15.9%) | |
| West | 4762 (57.1%) | 187 (52.1%) | 632 (62.8%) | 324 (58.8%) | 3619 (56.3%) | |
| Year of diagnosis | <0.001 | |||||
| 2004-2006 | 2387 (28.6%) | 130 (36.2%) | 389 (38.7%) | 179 (32.5%) | 1689 (26.3%) | |
| 2007-2009 | 2460 (29.5%) | 114 (31.8%) | 285 (28.3%) | 145 (26.3%) | 1916 (29.8%) | |
| 2010-2013 | 3498 (41.9%) | 115 (32.0%) | 332 (33.0%) | 227 (41.2%) | 2824 (43.9%) | |
| Primary tumor location | <0.001 | |||||
| Upper one third | 2537 (30.4%) | 52 (14.5%) | 138 (13.7%) | 105 (19.1%) | 2242 (34.9%) | |
| Middle one third | 841 (10.1%) | 35 (9.7%) | 93 (9.2%) | 64 (11.6%) | 649 (10.1%) | |
| Lower one third | 1568 (18.8%) | 105 (29.2%) | 333 (33.1%) | 99 (18.0%) | 1031 (16.0%) | |
| NOS | 3399 (40.7%) | 167 (46.5%) | 442 (43.9%) | 283 (51.4%) | 2507 (39.0%) | |
| Tumor grade | <0.001 | |||||
| G1/G2 | 1484 (17.8%) | 56 (15.6%) | 177 (17.6%) | 46 (8.3%) | 1205 (18.7%) | |
| G3/G4 | 5121 (61.4%) | 286 (79.7%) | 764 (75.9%) | 310 (56.3%) | 3761 (58.5%) | |
| Unknown | 1740 (20.9%) | 17 (4.7%) | 65 (6.5%) | 195 (35.4%) | 1463 (22.8%) | |
| Histology | <0.001 | |||||
| Adenocarcinoma NOS | 5774 (69.2%) | 219 (61.0%) | 628 (62.4%) | 270 (49.0%) | 4657 (72.4%) | |
| Mucinous adenocarcinoma | 135 (1.6%) | 9 (2.5%) | 31 (3.1%) | 10 (1.8%) | 85 (1.3%) | |
| Signet ring cell carcinoma | 2436 (29.2%) | 131 (36.5%) | 347 (34.5%) | 271 (49.2%) | 1687 (26.2%) | |
| Gastrectomy | ||||||
| Yes | 1365 (16.4%) | 359 (100.0%) | 1006 (100.0%) | 0 (0.0%) | 0 (0.0%) | |
| Total gastrectomy surgery | 401 (4.8%) | 115 (32.0%) | 286 (28.4%) | 0 (0.0%) | 0 (0.0%) | |
| Non-total gastrectomy surgery | 907 (10.9%) | 216 (60.2%) | 691 (68.7%) | 0 (0.0%) | 0 (0.0%) | |
| Not specified | 57 (0.7%) | 28 (7.8%) | 29 (2.9%) | 0 (0.0%) | 0 (0.0%) | |
| No | 6980 (83.6%) | 0 (0.0%) | 0 (0.0%) | 551 (100.0%) | 6429 (100.0%) | |
| Metastasectomy | ||||||
| Yes | 910 (10.9%) | 359 (100.0%) | 0 (0.0%) | 551 (100.0%) | 0 (0.0%) | |
| No | 7435 (89.1%) | 0 (0.0%) | 1006 (100.0%) | 0 (0.0%) | 6429 (100.0%) | |
Abbreviation: SPM, surgeries to both primary and metastatic tumors; GO, gastrectomy only; MO, metastasectomy only; NS, no surgery; SEER, Surveillance, Epidemiology, and End Results.
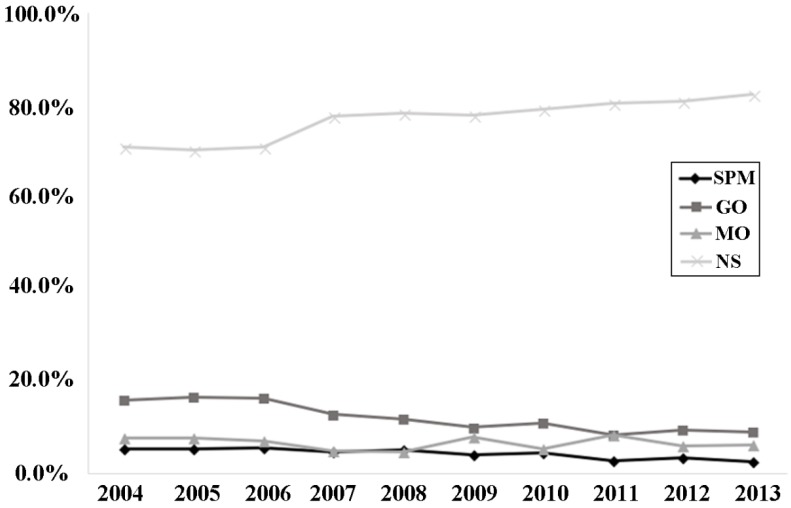
Seventy-seven percent of patients had no surgery, followed by 12.1% of patients with gastrectomy only and 6.6% with metastatic tumors resected only, and smallest proportion of patients underwent SPM. Imbalance between groups was found in all characteristics investigated. In SPM and MO groups, there were more younger patients (< 50 years old) and female than that in other groups. NS patients had more primary tumor located in the upper one third whereas SPM and GO patients had more in the lower one third stomach. Besides, more and more patients received non-surgical treatment and an over-ten-percent increase was observed from 2004 to 2013 while the rate of mGC patients undergoing gastrectomy dropped significantly as time went by. (P<0.001, Figure 2)
Figure 2.
Proportions of surgery strategies performed in each year from 2004 to 2013. Abbreviations: SPM, surgeries to both primary and metastatic tumors; GO, gastrectomy only; MO, metastasectomy only; NS, no surgery.
3.2 Survival analysis
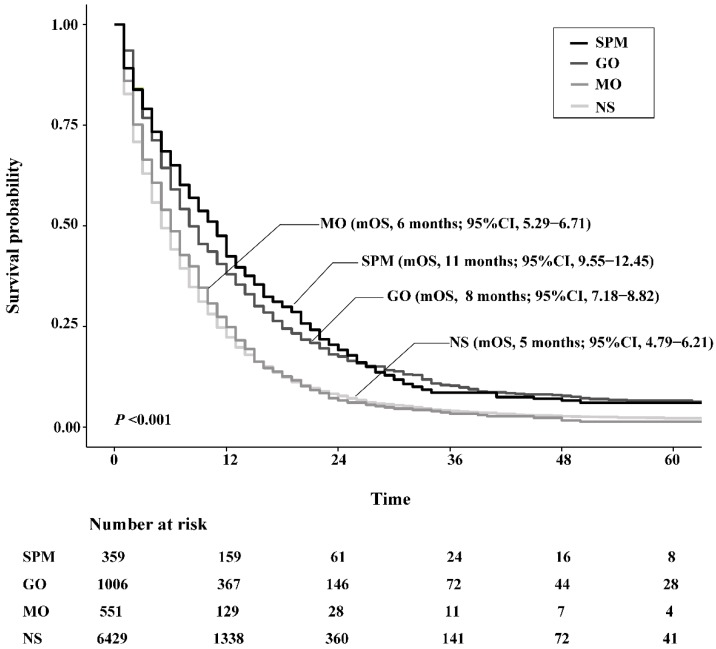
The OS of SPM and GO patients was both significantly higher than NS patients, with an improved median OS (mOS) of 6.0 months and 3.0 months, respectively. However, the mOS of MO only exceeded that of NS by 1.0 month, which was not a significant difference (P=0.286). In terms of comparisons between surgery strategies, survival benefit was similar between SPM and GO groups (P=0.389), while both showed significantly better survival than MO patients (P <0.001, respectively). Figure 3 depicts survival curves of patients stratified by surgical strategies.
Figure 3.
Kaplan-Meier survival curves of patients stratified according to their surgery strategies. Abbreviations: SPM, surgeries to both primary and metastatic tumors; GO, gastrectomy only; MO, metastasectomy only; NS, no surgery; mOS, median overall survival.
3.3 Univariable and multivariable Cox regression analysis
Age at diagnosis, marriage status, race, SEER region, location and histology of primary tumor, year of diagnosis and surgery strategies were proven to be independent prognostic factors (Table 2). After adjusting for these factors in multivariable Cox analysis, all surgery strategies were proved to have significantly better prognosis than NS ones (HR for SPM: 0.60, P<0.001; HR for GO: 0.62, P<0.001; HR for MO: 0.91, P=0.046), and gastrectomy with or without metastasectomy remained superior over MO.
Table 2.
Results of univariable and multivariable Cox regression analysis
| Variable | Univariable Cox analysis | Multivariable Cox analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | |||||
| < 50 | Reference | < 0.001 | Reference | < 0.001 | |
| 50-59 | 0.97 (0.91, 1.04) | 0.99 (0.93, 1.07) | |||
| 60-69 | 1.05 (0.98, 1.12) | 1.09 (1.02, 1.17) | |||
| ≥ 70 | 1.22 (1.14, 1.30) | 1.25 (1.16, 1.35) | |||
| Sex | |||||
| Male | Reference | 0.309 | - a | - a | |
| Female | 1.03 (0.98, 1.07) | - a | |||
| Marriage status | |||||
| Widowed | Reference | < 0.001 | Reference | < 0.001 | |
| Married | 0.79 (0.72, 0.86) | 0.85 (0.77, 0.92) | |||
| Other | 0.86 (0.78, 0.94) | 0.93 (0.85, 1.02) | |||
| Race | |||||
| Non-Hispanic White | Reference | 0.005 | Reference | 0.002 | |
| Non-Hispanic Black | 1.09 (1.02, 1.17) | 1.06 (0.98, 1.15) | |||
| Hispanic | 1.01 (0.96, 1.07) | 0.99 (0.93, 1.06) | |||
| Other | 0.93 (0.87, 0.99) | 0.90 (0.83, 0.96) | |||
| SEER region | |||||
| Mid-west | Reference | < 0.001 | Reference | < 0.001 | |
| Northeast | 0.83 (0.76, 0.90) | 0.83 (0.76, 0.91) | |||
| South | 1.02 (0.93, 1.11) | 1.03 (0.94, 1.13) | |||
| West | 0.99 (0.92, 1.07) | 1.02 (0.94, 1.11) | |||
| Year of diagnosis | |||||
| 2004-2006 | Reference | < 0.001 | Reference | < 0.001 | |
| 2007-2009 | 0.86 (0.81, 0.91) | 0.83 (0.78, 0.88) | |||
| 2010-2013 | 0.81 (0.76, 0.86) | 0.77 (0.73, 0.82) | |||
| Primary tumor location | |||||
| Upper one third | Reference | < 0.001 | Reference | < 0.001 | |
| Middle one third | 1.09 (1.01, 1.18) | 1.08 (0.99, 1.18) | |||
| Lower one third | 1.04 (0.98, 1.12) | 1.11 (1.03, 1.19) | |||
| NOS | 1.16 (1.10, 1.23) | 1.18 (1.12, 1.26) | |||
| Histology | |||||
| Adenocarcinoma NOS | Reference | 0.001 | Reference | < 0.001 | |
| Mucinous adenocarcinoma | 0.91 (0.76, 1.10) | 0.95 (0.79, 1.13) | |||
| Signet ring cell carcinoma | 1.09 (1.04, 1.15) | 1.11 (1.06, 1.18) | |||
| Surgery strategies | |||||
| NS | Reference | < 0.001 | Reference | < 0.001 | |
| GO | 0.67 (0.62, 0.72) | 0.62 (0.57, 0.67) | |||
| MO | 0.95 (0.87, 1.05) | 0.91 (0.83, 1.00) | |||
| SPM | 0.63 (0.57, 0.71) | 0.60 (0.54, 0.68) | |||
a The variable was not include in the multivariable Cox analysis because of its P value ≥ 0.05 in the univariable Cox analysis.
Abbreviations: HR, hazard ratio; SEER, the Surveillance, Epidemiology, and End Results Program; NS, no surgery; GO, gastrectomy only; MO, metastasectomy only; SPM, surgery to both primary and metastatic tumor; NOS, not otherwise specified.
3.4 Propensity score matching and coarsened exact matching analysis
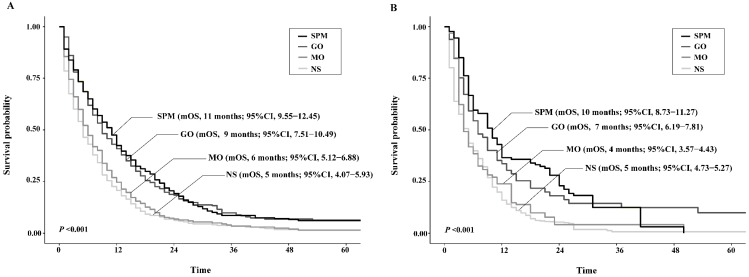
By PSM and CEM, the patient characteristics were well balanced between groups to diminish selection bias (Supplementary Table 1). The survival analyses based upon the matched population both showed consistent results with that upon the unmatched cohort, except in CEM-weighted analysis MO group presented poorer yet not significant survival than NS group (Figure 4).
Figure 4.
Kaplan-Meier survival curves of patients stratified according to their surgery strategies after propensity score matching (A) or coarsened exact matching (B). Abbreviations: SPM, surgeries to both primary and metastatic tumors; GO, gastrectomy only; MO, metastasectomy only; NS, no surgery; mOS, median overall survival.
In the univariable and multivariable Cox analysis, surgery strategies were demonstrated to be a prognostic factor (Table 3). Surgeries to the primary and/or metastatic tumors lowered the death risk from non-surgery, however MO did not function significantly in both matched subanalyses. Inbetween surgery strategies, SPM and GO held comparable survival impacts and both were more favorable than MO.
Table 3.
Multivariable Cox regression analysis with propensity score matching and coarsened exact matching method weighting
| Surgery strategies | Propensity score matching cohort | Coarsened exact matching method weighted cohort | ||
|---|---|---|---|---|
| HRa (95% CI) | P value | HRb (95% CI) | P value | |
| NS | Reference | Reference | ||
| GO | 0.59 (0.51, 0.69) | <0.001 | 0.57 (0.43, 0.77) | <0.001 |
| MO | 0.94 (0.80, 1.10) | 0.412 | 0.89 (0.65, 1.22) | 0.464 |
| SPM | 0.60 (0.51, 0.70) | <0.001 | 0.50 (0.35, 0.72) | <0.001 |
a After adjustment for age, year of diagnosis, primary tumor location and histology whose P value <0.05 in the univariable Cox regression analysis.
b After adjustment for age, sex, race, SEER region, year of diagnosis, primary tumor location and histology whose P value <0.05 in the univariable Cox regression analysis.
Abbreviations: HR, hazard ratio; CI, confidence interval; NS, no surgery; SPM, surgery to both primary and metastatic tumor; GO, gastrectomy only; MO, metastasectomy only.
3.5 Sensitivity analyses
A total of 5347 patients were enrolled in the sensitivity analyses, and the demographic and clinicopathological characteristics were still different between groups (Supplementary Table 2). Survival analyses found that mOS of SPM (13 months vs. 8 months; P<0.001) and GO (12 months vs. 8 months; P<0.001) patients were both significantly higher than that of NS patients, and MO was not (8 months vs. 8 months; P = 0.893). SPM and GO groups showed comparable survival (P = 0.748) and both were significantly better than MO patients (P<0.001). After matching by PSM and CEM, similar results were obtained. Table 4 illustrates death risks of surgery strategies over NS among patients receiving chemotherapy, that is SPM and GO rather than MO were proven to be favorable for survival over non-surgical treatment, even after matching by PSM or CEM. These consistent results implied that SPM and GO could also improve survival from MO or NS in patients who received chemotherapy.
Table 4.
Death risks of surgery strategies over NS among patients receiving chemotherapy for the sensitivity analyses
| Surgery strategies | All cohort | Propensity score matching cohort | Coarsened exact matching method weighted cohort | |||
|---|---|---|---|---|---|---|
| HR a (95% CI) | P value | HR b (95% CI) | P value | HR c (95% CI) | P value | |
| NS | Reference | Reference | Reference | |||
| GO | 0.61 (0.55, 0.67) | <0.001 | 0.56 (0.46, 0.69) | <0.001 | 0.64 (0.58, 0.70) | <0.001 |
| MO | 0.92 (0.82, 1.03) | 0.154 | 0.94 (0.77, 1.15) | 0.524 | 1.02 (0.95, 1.11) | 0.557 |
| SPM | 0.59 (0.51, 0.69) | <0.001 | 0.60 (0.49, 0.73) | <0.001 | 0.63 (0.57, 0.69) | <0.001 |
a After adjustment for age, race, SEER region, year of diagnosis, marriage status, primary tumor location and histology whose P value <0.05 in the univariable Cox regression analysis.
b After adjustment for year of diagnosis, primary tumor location and histology whose P value <0.05 in the univariable Cox regression analysis.
c After adjustment for age, sex, race, SEER region, year of diagnosis, marriage status, primary tumor location and histology whose P value <0.05 in the univariable Cox regression analysis.
Abbreviations: HR, hazard ratio; CI, confidence interval; NS, no surgery; GO, gastrectomy only; MO, metastasectomy only; SPM, surgery to both primary and metastatic tumor.
4. Discussion
In this study, we included 8345 patients from the SEER database and divided them into four groups according to the surgery strategies to evaluate survival benefit from surgeries to primary and/or metastatic tumors in comparison with non-surgery in the U.S. population.
Up to date, surgery has not been widely considered to treat mGC patients given that there was no solid evidence available to support its survival benefit. And high surgery-related mortality and morbidity rates and short life span of mGC patients have prevented aggressive treatment strategies from application. But with the progress of preoperative imaging diagnosis, anesthesia and surgical techniques, and nutritional support, the surgery-related mortality rate dropped markedly from more than 20% two decades ago to 4%. 20-23 Except the safety and feasibility of surgery, increasing awareness of clinical decision-making by the multidisciplinary team (MDT) 24, 25 also led to re-consideration of surgery as part of treatment to mGC.
Previous studies investigated whether gastrectomy and/or metastasectomy could be beneficial and the results were inconclusive. The most common surgery strategy investigated is gastrectomy. Although the negative result was drawn from the REGATTA trial, there are still doubts on its combination pattern of surgery followed by chemotherapy and also its validation in Western population, and therefore many researchers kept studying on it. Warschkow et al. carried out a study based on a large sample of 7026 mGC patients diagnosed within 2006-2012 from the National Cancer Database and suggested gastrectomy could improve survival from chemotherapy alone. 26 A systematic review and meta-analysis by Lasithiotakis, K. et al. implied a possible benefit of gastrectomy compared to non-resectional treatment for stage IV gastric cancer, and 40-70% of the patients received postoperative chemotherapy. 21 MO is also an option of surgery strategies. There were pooled studies showing long-term survival after metastasectomy, but the indication was quite restricted with solitary metastatic lesions in limited organs. 27-31 As a relatively aggressive and extensive surgery strategy, SPM was cautiously investigated and used, and obtained improved survival in several studies. He, M. M. et al. and Kim, K. H. et al. found gastrectomy or gastrectomy plus metastasectomy combined with systemic chemotherapy could improve survival for advanced GC or mGC patients, respectively, based on the Asian population. 7, 8 The GYMSSA trial indicated that maximal cytoreductive surgery combined with regional hyperthermic intraperitoneal chemotherapy and systemic chemotherapy could achieve prolonged survival, but the trial prematurely ended up with a small sample size and a deteriorated evidence level. 15 On the other hand, few studies compared different surgery strategies. Yang, S. W. et al.'s study which excluded chemotherapy-receivers implied better prognosis of patients with SPM than that with GO, but Chen, J. et al. found the advantage not significant based on SEER-based population. 9, 11
In the present study, the survival of the SPM and GO patients was demonstrated significantly better than MO and NS patients, respectively, and conducting metastasectomy did not improve survival significantly from gastrectomy or non-surgical treatment. Surgery strategies was proved as an independently favorable prognostic factor. Inbetween surgery strategies, SPM and GO held comparable survival impacts which were more favorable than MO. Chen, J. et al. obtained similar findings, but imbalanced baseline characteristics were not handled and consequent selection bias was aggravated by including factors like T and N stages, whose definition differed with or without resection, in the Cox regression analysis. 11 At an advantage, the present study removed post-surgery factors out of adjustment analysis and used PSM and CEM methods to avoid confounding effects, and also analyzed metastasectomy in addition. Furthermore, because chemotherapy is the mainstay of treatment for mGC patients, we carried out sensitivity analyses after excluding patients receiving no/unknown chemotherapy and obtained consistent results. To the best of our knowledge, this is the first study to demonstrate and compare survival benefits of palliative surgery strategies including gastrectomy and/or metastasectomy with PSM and CEM methods.
Results of the study cast light on the therapeutic role of palliative surgery strategies. Regional resection used to be applied for radical purpose, and it could be achieved in selected potentially resectable mGC patients after conversion chemotherapy with significant survival benefit. 13, 14, 32-36 However, since distal metastasis was a systemic manifestation, the complete resection of primary and metastatic tumors with proved survival benefit might actually be palliative as with a recurrence rate of more than 50%. 35, 37 Palliative gastrectomy with or without metastasectomy improved survival probably due to the debulking or cytoreductive effect at its source. Relieving symptoms caused by either the primary or metastatic tumors might as well positively influence mGC patients. However, metastasectomy seemed to obtain little additional survival improvement in the study, which might be attributed to heterogeneity of metastatic loci status of the study population. One research held the view that only metastasectomy could distinguish second primary tumors from metastasis. 29 Thus, MO is not recommended for general application and might be a choice among cautiously selected patients, and SPM could be adopted after adequate cost-and-effectiveness weighing for its numerically better OS compared with GO.
The study has several limitations. First, data on patient comorbidities and performance status, postoperative mortality and morbidity, quality of life, and detailed chemotherapy information are unavailable. Further studies are needed to explore the extra influence of these variables on survival and to select suitable surgery candidates. Second, the circumstances under which surgeries were conducted, like the surgeries are emergent or not and the patients are asymptomatic or not, were not specified in the SEER database. Such information could had better distinguished surgeries as a treatment alternative from emergency operations. Third, PSM and CEM analysis only deal with observable factors, thus other unobservable factors might be confounding and unmatched. Also, part of information is lost during the process of matching. Combination of the results of the two matching methods and multivariate Cox regression analysis in the present study is a dependable solution. Despite these limitations, given the large sample size and the population-based nature of the SEER database, we are able to perform adequately powerful survival analyses.
5. Conclusions
Poor prognosis of metastatic gastric cancer promotes oncologists to spare no effort finding and evaluating various treatment strategies for survival prolongation, and the present study demonstrated gastrectomy plus metastasectomy or gastrectomy alone could be adopted as a choice of improving survival in the U.S population. Metastasectomy alone is not recommended except for highly selected patients.
Supplementary Material
Supplementary tables.
Acknowledgments
We feel grateful that this study was funded by the Natural Science Foundation of Guangdong Province [grant number 2014A030312015]; Science and Technology Program of Guangdong [grant number 2015B020232008]; Science and Technology Program of Guangzhou [grant number 15570006, 201508020250, 201604020003]. We also hold gratitude towards staff members at the National Cancer Institute and their colleagues across the United States and at the Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology, and End Results (SEER) Program.
Abbreviations
- GC
gastric cancer
- mGC
metastatic GC
- SEER
Surveillance, Epidemiology, and End Results Program
- SPM
surgeries to both primary and metastatic tumors
- GO
gastrectomy only
- MO
metastasectomy only
- NS
not surgically operated
- PSM
propensity score matching
- CEM
coarsened exact matching
- MDT
multidisciplinary team.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ. et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shridhar R, Almhanna K, Hoffe SE, Fulp W, Weber J, Chuong MD. et al. Increased survival associated with surgery and radiation therapy in metastatic gastric cancer. Cancer. 2013;119:1636–42. doi: 10.1002/cncr.27927. [DOI] [PubMed] [Google Scholar]
- 4.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2014;40:584–91. doi: 10.1016/j.ejso.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Gold JS, Jaques DP, Bentrem DJ, Shah MA, Tang LH, Brennan MF. et al. Outcome of patients with known metastatic gastric cancer undergoing resection with therapeutic intent. Annals of surgical oncology. 2007;14:365–72. doi: 10.1245/s10434-006-9059-z. [DOI] [PubMed] [Google Scholar]
- 6.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU. et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. The Lancet Oncology. 2016;17:309–18. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 7.He MM, Zhang DS, Wang F, Wang ZQ, Luo HY, Jin Y. et al. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PloS one. 2013;8:e83921. doi: 10.1371/journal.pone.0083921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ. et al. Survival benefit of gastrectomy +/- metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2011;14:130–8. doi: 10.1007/s10120-011-0015-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang SW, Kim MG, Lee JH, Kwon SJ. Role of metastasectomy on overall survival of patients with metastatic gastric cancer. Journal of gastric cancer. 2013;13:226–31. doi: 10.5230/jgc.2013.13.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu JT, Liao JA, Chuang HC, Chen TD, Chen TH, Kuo CJ. et al. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC palliative care. 2017;16:19. doi: 10.1186/s12904-017-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Kong Y, Weng S, Dong C, Zhu L, Yang Z. et al. Outcomes of surgery for gastric cancer with distant metastases: a retrospective study from the SEER database. Oncotarget. 2017;8:4342–51. doi: 10.18632/oncotarget.14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y. et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2013;39:694–700. doi: 10.1016/j.ejso.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Gotz M, Scheuerlein H, Jandt K. et al. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2012;15:131–6. doi: 10.1007/s10120-011-0080-y. [DOI] [PubMed] [Google Scholar]
- 14.Iijima Y, Akiyama H, Atari M, Fukuhara M, Nakajima Y, Kinosita H. et al. Pulmonary Resection for Metastatic Gastric Cancer. Annals of thoracic and cardiovascular surgery: official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2016;22:230–6. doi: 10.5761/atcs.oa.16-00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T. et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. Journal of surgical oncology. 2014;110:275–84. doi: 10.1002/jso.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph JJ, Falbe K, Manuel AK & Balloun JL. A Step-by Step Guide to Propensity Score Matching in R. Practical Assessment, Research & Evaluation; 2014. p. 19. [Google Scholar]
- 17.Haider AH, David JS, Zafar SN, Gueugniaud PY, Efron DT, Floccard B. et al. Comparative effectiveness of inhospital trauma resuscitation at a French trauma center and matched patients treated in the United States. Annals of surgery. 2013;258:178–83. doi: 10.1097/SLA.0b013e31828226b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacus SM, King G, Porro G. Causal Inference without Balance Checking: Coarsened Exact Matching. Polit Anal. 2012;20:1–24. [Google Scholar]
- 19.Sutter R, De Marchis GM, Semmlack S, Fuhr P, Ruegg S, Marsch S. et al. Anesthetics and Outcome in Status Epilepticus: A Matched Two-Center Cohort Study. CNS drugs. 2017;31:65–74. doi: 10.1007/s40263-016-0389-5. [DOI] [PubMed] [Google Scholar]
- 20.Izuishi K, Mori H. Recent Strategies for Treating Stage IV Gastric Cancer: Roles of Palliative Gastrectomy, Chemotherapy, and Radiotherapy. Journal of gastrointestinal and liver diseases: JGLD. 2016;25:87–94. doi: 10.15403/jgld.2014.1121.251.rv2. [DOI] [PubMed] [Google Scholar]
- 21.Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer research. 2014;34:2079–85. [PubMed] [Google Scholar]
- 22.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M. et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy-Japan Clinical Oncology Group study 9501. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:2767–73. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SS, Yang HK. Lymphadenectomy for gastric adenocarcinoma: should west meet east? The oncologist. 2009;14:871–82. doi: 10.1634/theoncologist.2009-0070. [DOI] [PubMed] [Google Scholar]
- 24.Davies AR, Deans DA, Penman I, Plevris JN, Fletcher J, Wall L. et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2006;19:496–503. doi: 10.1111/j.1442-2050.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 25.Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P. et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer treatment reviews. 2016;42:56–72. doi: 10.1016/j.ctrv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Warschkow R, Baechtold M, Leung K, Schmied BM, Nussbaum DP, Gloor B. et al. Selective survival advantage associated with primary tumor resection for metastatic gastric cancer in a Western population. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2018;21:324–37. doi: 10.1007/s10120-017-0742-5. [DOI] [PubMed] [Google Scholar]
- 27.Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D. et al. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. Journal of surgical oncology. 2015;112:38–45. doi: 10.1002/jso.23945. [DOI] [PubMed] [Google Scholar]
- 28.Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M. et al. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Annals of surgery. 2016;263:1092–101. doi: 10.1097/SLA.0000000000001542. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Homma Y, Miyata N, Ushida S, Suzuki K, Otsuki Y. et al. Only surgical resection can identify the second primary lung cancer out of the metastasis after gastric cancer surgery. Japanese journal of clinical oncology. 2012;42:609–11. doi: 10.1093/jjco/hys067. [DOI] [PubMed] [Google Scholar]
- 30.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Borgonovo K, Lonati V. et al. Hepatic resection for gastric cancer liver metastases: A systematic review and meta-analysis. Journal of surgical oncology. 2015;111:1021–7. doi: 10.1002/jso.23920. [DOI] [PubMed] [Google Scholar]
- 31.Shiono S, Sato T, Horio H, Chida M, Matsuguma H, Ozeki Y. et al. Outcomes and prognostic factors of survival after pulmonary resection for metastatic gastric cancer. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2013;43:e13–6. doi: 10.1093/ejcts/ezs574. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Liang H, Zhang H, Wang X, Xue Q, Zhang R. Prognostic significance of radical surgical treatment for gastric cancer patients with synchronous liver metastases. Medical oncology (Northwood, London, England) 2014;31:258. doi: 10.1007/s12032-014-0258-3. [DOI] [PubMed] [Google Scholar]
- 33.Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K. et al. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer research. 2015;35:401–6. [PubMed] [Google Scholar]
- 34.Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J. et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA oncology. 2017;3:1237–44. doi: 10.1001/jamaoncol.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt T, Alldinger I, Blank S, Klose J, Springfeld C, Dreikhausen L. et al. Surgery in oesophago-gastric cancer with metastatic disease: Treatment, prognosis and preoperative patient selection. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41:1340–7. doi: 10.1016/j.ejso.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Polkowska-Pruszynska B, Rawicz-Pruszynski K, Cisel B, Sitarz R, Polkowska G, Krupski W. et al. Liver metastases from gastric carcinoma: A Case report and review of the literature. Current problems in cancer. 2017;41:222–30. doi: 10.1016/j.currproblcancer.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Tatsubayashi T, Tanizawa Y, Miki Y, Tokunaga M, Bando E, Kawamura T. et al. Treatment outcomes of hepatectomy for liver metastases of gastric cancer diagnosed using contrast-enhanced magnetic resonance imaging. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20:387–93. doi: 10.1007/s10120-016-0611-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.