Abstract
Papillary thyroid carcinoma (PTC) is the most commonly diagnosed endocrine cancer, and those with BRAFV600E mutation have high recurrence rate and less favorable clinical behavior. Genistein having anti-carcinoma effects in various types of carcinomas as an estrogen analog, but the mechanism of Genistein in the progression of PTC remains unknown. Genistein significantly inhibits the proliferation and the invasion (P < 0.01), and the apoptosis (P < 0.001) of all tumor cell lines, which was probably due to the inducing of the arrest in G2/M phase of the cell cycle (P < 0.001). The anti-proliferation and apoptosis inducing effects are more obvious in BCPAP, IHH4 cell lines harboring BRAFV600E mutation. Genistein significantly decreased the invasion of PTC cell lines and partially reverses epithelial mesenchymal transition in PTC cell lines. Functional study indicated that small interfering RNA (siRNA) knockdown of β-catenin significantly reverses the effect of genistein on EMT at protein levels. In conclusion, for the first time, our study suggested that genistein has anticarcinoma effect for PTC patients in the range of 2.5 and 80 μg/ml in thyroid carcinoma cells, which was probably through cytoplasmic translocation of β-catenin. Further study will be needed to determine whether genistein could be used in clinical trial of high-risk PTC.
Keywords: Genistein, Papillary thyroid carcinoma, Proliferation, Epithelial mesenchymal transition, BRAFV600E mutation
Introduction
Thyroid carcinoma is the most common malignant tumor of the endocrine system and accounts for approximately 1% of all newly diagnosed cancer cases 1, 2. Papillary thyroid carcinoma (PTC) represents the most frequent malignancy comprising 80% of thyroid malignancies with the continuously increasing incidence 3, 4. It has been shown that some environmental and lifestyle factors increase the risk to development thyroid cancer, e.g., female gender, radiation exposure, familiar genetic tendency, and aging 5. The genetic alterations occur to some PTC patients and the most prevalent changes are BRAF mutations (29%-70%) and RET/PTC translocation (13%-43%). These mutations not only correlate with the tumor differentiation and metastasis, but also affects the therapy efficacy, e.g., surgery tolerance, thyrotropin suppression, and radioactive iodine treatment 6, 7.Thus, it is urgently needed to uncover the novel targets to supplement clinical therapy of PTC.
Bioactive, natural compounds from plants, called Phytochemicals, or their derived compounds, are being increasingly accepted as potentially potent complementary treatments for cancer due to their apparent safety, efficacy and multi-specific actions 5. Flavonoid is a great group of phytochemical compounds widely spread in the vegetables. Its basic structure is composed by two aromatic rings linked by a three-carbon chain. Flavonoids are not synthesized by human beings but we can obtain them through the diet 8. A variety of biological activities has been attributed to flavonoids, such as protection against ultraviolet and visible rays, prevention of insects, viruses and bacteria invasion, and antioxidant effect and hormonal modulation 9. Isoflavones belongs to the group of flavonoids, which are found in large quantities in soybeans and have a number of biological properties, including anti-proliferative, vasculo-protective effects and cancer prevention 10, 11. It was reported that isoflavones had the potential to inhibit the synthesis of thyroid hormones by acting as alternative substrate for thyroperoxidase (TPO), the key enzyme in the synthesis of thyroid hormones 12. These findings indicate that flavonoids might exert regulatory functions on thyroid in physiological and pathological milieu.
Genistein (4',5,7-trihydroxyisoflayine) is the most extensively studied soy isoflavone, which is mainly absorbed from the intestine and is readily bioavailable. This makes it a promising candidate for disease prevention 13. Owing to its structural similarity to endogenous estrogen 17 estradiol, Genistein is also called phytoestrogens and could bind with estrogen receptor and activate its downstream signaling pathway 14, 15. Lu et al. found when the mice were administrated with genistein at 100 mg/kg, the serum concentrations of genistein was at an average of approximately 60 ng/ml, and stachyose could enhance the absorption of genistein in mice 16. Genistein was shown to be a potent inhibitor of the tyrosine-specific protein kinase RET, and has been used for clinical treatment of prostate carcinoma and non-small cell lung cancer 17-22. However, there is no report about the effect of Genistein on the growth and metastasis of PTC. In the present study, we demonstrated that Genistein inhibited the proliferation of PTC cell lines and induced G2/M phase arrest and apoptotic cell death. It significantly suppressed the motility potential and the EMT tendency of PTC cell lines, and β-catenin reduction was involved in this action. Our findings shed new light on the clinical application of Genistein and might become a potential candidate for clinical therapy of PTC.
Materials and methods
Cell lines and Reagents
Normal human follicular epithelial cell line Nthy-ori 3-1 was obtained from JENNIO biological company (Guangzhou, China), human PTC-derived BHP10-3 cells (with RET/PTC 1 rearrangement) were purchased from Chinese Academy of Sciences (Shanghai, China). Human PTC cell lines BCPAP and IHH4 (with BRAFV600E mutation) were kindly provided by Endocrine Laboratory and Department of Pathology, Wakayama Medical University. Genistein was purchased from BOC Sciences (New York, USA).
Cell proliferation assays
The cell proliferation assay was conducted by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay according to the manufacturer's instructions. Cells were treated with different concentrations of Genistein (2.5, 5, 10, 20, 40 and 80μg/ml) and were incubated for another 24h, 48h and 72h, and cells treated with 1% DMSO were set as negative control. All cell treatments were carried out in triplicate. Results are expressed as inhibition rate of viability of treated cells compared to non-treated cells calculated according to the following equation: the inhibition rate (%) = [1 - (average absorbance of experimental group/average absorbance of cells treated with control group)] × 100. At the concentration of 80 μg/ml, almost 90% cell died. Thus, 5, 10, 20 and 40 μg/ml of Genistein was used in the following assays.
Wound healing and invasion assay
Wound healing and invasion assay was performed as previously described 23. For quantification of invading cells, cells were counted in five randomly selected microscopic fields (×200). Migration was measured on photographs. The average width of the remaining wounded gaps was measured from two different locations per wounded gap per time point. Invasion assay was performed in 24-well Boyden chambers precoated with Matrigel Matrix (8mm pore size, BD Biosciences). Cells that invaded through the pores of the inserts were fixed with 4% paraformaldehyde for 10 min and stained with crystal violet staining fluid (Beyotime). Three independent experiments were performed.
Cell cycle analysis
Cell cycle analysis was done using propidium iodide staining (BestBio). Nthy-ori 3-1, BCPAP, IHH4 and BHP10-3 were synchronized in G0 by serum starvation for 24h in serum-free medium and treated with different concentrations of Genistein for 72h. One million of cells were washed twice with ice-cold PBS and resuspended in 1ml ice cold 70% ethanol. After centrifugation, the cells were washed with ice-cold PBS and then resuspended with 200μl PBS and add 20 μl DNase-free Rnase A for 30 minutes in the 37℃. Then, they were stained with 400 μl of a propidium iodide working solution. At least 10,000 events were collected by flow cytometry. Results are presented as cell percentage distributed in each phase of cell cycle. The percentage of cells in each cell cycle stage (G0/G1, S or G2/M) was calculated by using WinCycle32 software. Three independent assays were performed.
Cell apoptosis assay
Apoptosis was determined by flow cytometry analysis using Annexin-V kit (BestBio). Cells were treated with gradient concentrations of Genistein for 24h. Then, the floating and adherent cells were pooled, washed three times with ice-cold PBS, and stained with 400μl of 1X Annexin binding buffer and 5μl Annexin V-FITC. After incubation for 15 min in the dark at 4℃, 10μl of propidium iodide were added to the cell suspension and incubated for 5 minutes at 4℃. Flow cytometry analysis was immediately performed. Three independent experiments were performed and data represent the average percentage of apoptotic cells.
Quantitative Real-Time PCR
After treatment with different concentration of Genistein (5, 10, 20, 40μg/ml), total RNA was isolated with TRIzol (invitrogen, CA) and then reversed into cDNA using First Strand cDNA Synthesis Kit (TOYOBO, Japan). Quantification of target and reference (GAPDH) genes were performed with Fast Start Universal SYBR Green Master (Roche, USA) in triplicate using C1000TN Real-Time PCR system (BioRad, USA), and the primers of cyclinD1, cyclinA2, and cyclinB1 for RT-qPCR were provided in Table 1.
Table 1.
List of primary primer sequence used for RT-qPCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| CyclinA2 | 5'-GGATGGTAGTTTTGAGTCACCAC-3' | 5'-CACGAGGATAGCTCTCATACTGT-3' |
| CyclinB1 | 5'-TTGGGGACATTGGTAACAAAGTC-3' | 5'-ATAGGCTCAGGCGAAAGTTTTT-3' |
| CyclinD1 | 5'-GCTGCGAAGTGGAAACCATC-3' | 5'-CCTCCTTCTGCACACATTTGAAA-3' |
| β-actin | 5'- AAGAGAGGCATCCTCACCCT-3' | 5'- TACTATGGCTGGGGTGTTGAA-3' |
Western blot assay
Total protein extracted by RIPA lysis or nuclear and cytoplasmic protein by extraction kit (BestBio) was separated by 10% SDS PAGE and transferred to nitrocellulose membranes after electrophoresis. The membranes were incubated overnight with antibodies against cyclinD1 (1:5000; Abcam), cyclinA2 (1:1000; Abcam), cyclinB1 (1:5000; Abcam) and EMT related antibodies against E-cadherin (1:5000; CST), N-cadherin (1:5000; CST), Vimentin (1:5000; CST), and Snail (1:5000; CST). The secondary antibody was a goat anti-rabbit antibody (ZSGB-BIO, China) at dilution of 1:5000 and the membranes were probed using an enhanced chemiluminescence ECL kit (Millipore Corporation, USA). Three independent experiments were performed. Intensity of ECL bands was digitally analyzed using ImageJ software.
RNA interference and cell transfection
Short interfering RNAs (siRNA) for β-catenin and control RNAs were purchased from Thermo Fisher Scientific (Massachusetts). The siRNAs were transfected into BHP10-3 and BCPAP cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Specific siRNA sequences targeting the human β-catenin were designed and synthesized respectively (GenePharma Pharmaceutical Co., Shanghai, China), and the sequence were provided in Table 1. The NC group was defined as negative control. The efficacy of the siRNA knockdown of β-catenin was verified by in vitro Trans well assay.
Immunofluorescence and Confocal Image
BHP10-3 and BCPAP were grown and treated on micro cover lips, then fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.3% Triton X-100 for 10min after Genistein treatment. Goat serum was used for blockage for 1h at room temperature. Cells were then incubated with primary antibodies against E-cadherin (1:100, CST, USA), Vimentin (1:100, CST), N-cadherin (1:100 CST), snail (1:100 CST) at 4°C overnight. Cells were incubated with Alexa Fluor 488-conjugatedsecondary antibodies (1:100, Beyotime) for 1hr at room temperature. Samples were examined with Confocal Laser Scanning Microscopy (LSM780, Zeiss) to analyze the expression of E-cadherin and Vimentin, N-cadherin and Snail, respectively.
Statistical analysis
Statistical differences between groups were analyzed using Student's t-tests. Data were expressed as mean ± SD of 3 individual experiments performed in triplicate and analyzed using 2 way ANOVA analysis. Differences with P < 0.05 were considered to be statistically significant.
Results
The role of Genistein in PTC cell proliferation and cell death
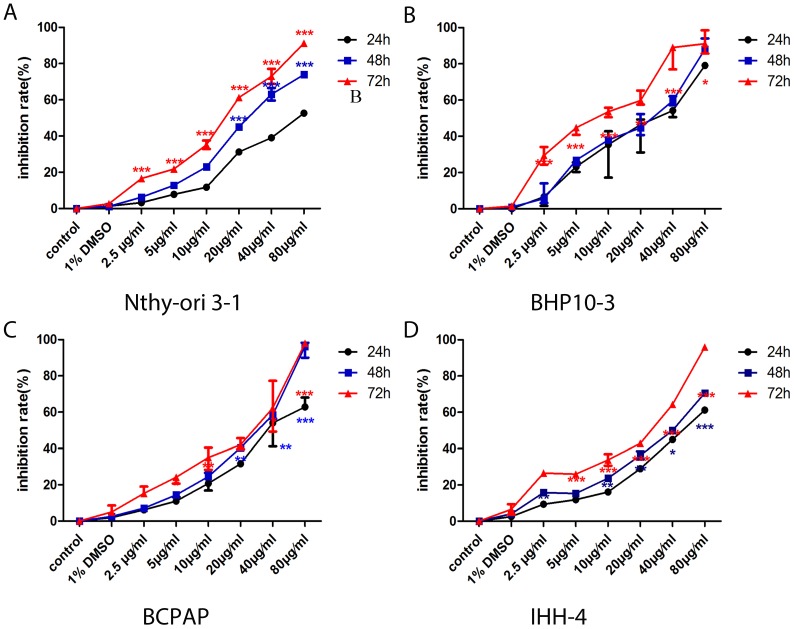
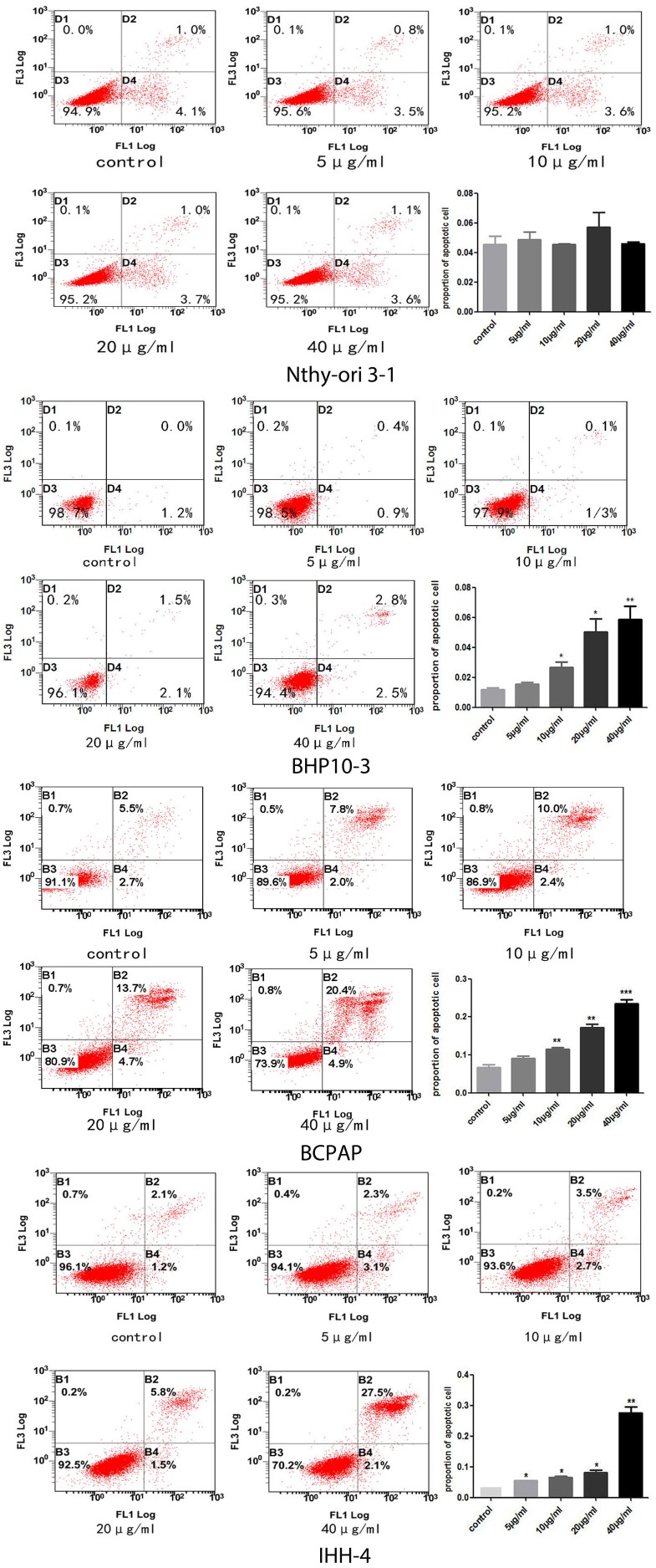
In consistent with the previous findings in cancer cells of the prostate and kidney 22, 24, the proliferation of PTC cells with different genetic background and the normal thyroid follicular cell were significantly inhibited in a concentrate and time-dependent manner (Figure 1). Almost all cells dying at the concentration of 80μg/ml, therefore, we chose the four concentrations of Genistein (5μg/ml, 10μg/ml, 20μg/ml, and 40μg/ml) for the following experiments. The cell death inducing effect was different in PTC cell lines and normal thyroid follicular cells (Figure 2). Significant effect was determined in all the PCT cell lines (Figure 2B-D) with different genetic background comparing with the normal thyroid follicular cells (Figure 2A). These results suggest that Genistein could inhibit PTC cell growth and inducing death comparing with the normal human thyroid follicular cell line.
Figure 1.
A-D, The proliferation of normal human thyroid follicular cell and PTC cells with both BRAFV600E mutation and RET/PTC1 was significantly inhibited in concentrate and time-dependent manner. However, the proliferation inhibition rate is very low comparing with those carcinoma cell lines.
Figure 2.
A-C, No significant cell death inducing effect by Genistein was demonstrated in the normal human thyroid follicular cells (A). However, significant cell death inducing effect was found in all the PTC cell lines with deferent genetic background (B-D) in a concentrate-dependent manner, especially when the concentration is more than 10 ug/ml.
Genistein leads to PTC cell cycle arrest in G2/M phase of PTC cells
To validate the effect of Genistein on PTC cell growth, we analyzed Cell cycle progression. Flow cytometry analysis demonstrated that Genistein-treated tumor cells had the decreased G1 phase and S phase ratio, but increased G2/M phase ratios (Figure 3B-C). While, the Nthy-ori 3-1 had only decreased G1 phase ratio, and increased G2/M phase and S-phase ratio (Figure 3A). RT-PCR and Western Blot analysis further confirmed these effects according to the decreased expression of Cyclin A2 and Cyclin B1 in a concentrate-dependent manner in both PTC carcinoma cells and normal thyroid follicular cell (Figure 4B-D and F-H). Although increased expression of Cyclin D1 was found in PTC cells (Figure 4B-D and F-H), decreased expression was examined in Nthy-ori 3-1 normal thyroid follicular cell (Figure 4A and E). These results indicated that Genistein leads to cell cycle arrest, which is consistent with its growth inhibition on PTC cells.
Figure 3.
Decreased G1 phase and increased G2 phase ratio of the Genistein-treated normal human thyroid follicular cell and PTC cell lines were demonstrated by Flow cytometry analysis. However, decreased S phase ratio (A) was examined, which are reverse to all the PTC cell lines by (B-D).
Figure 4.
A-F, After treated with Genistein, the expression of Cyclin B1 and Cyclin A2 was significantly down-regulated in a concentrate-dependent manner in normal human thyroid follicular cell (A and E) and PTC carcinoma cells (B-D and F-H) as demonstrated by RT-PCR and Western Blot analysis, while Cyclin D1 expression increased in the carcinoma cells (B-D and F-H) and decreased in the normal human thyroid follicular cell (A and E) in a concentrate-dependent manner. *: P<0.05, **: P<0.01, ***: P<0.001.
The role of Genistein in PTC cell motility
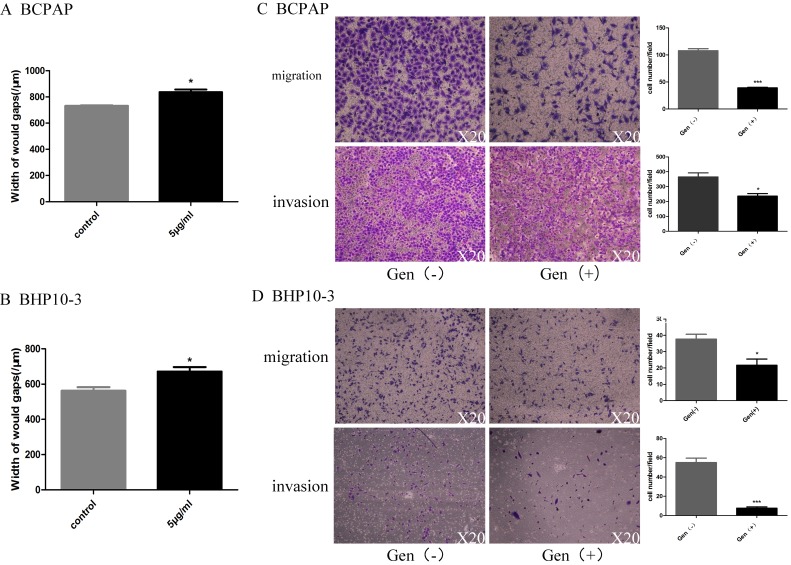
As PTC with BRAFV600E mutation was reported to be high-risk PTC and accounts for 60~80 of the PTCs, the two cell lines BCPAP and BHP10-3 were included in the consequent study. Considering its regulatory functions of Genistein in cell motility, the migration and invasion ability of PTC cells by was detected by wound-healing assay and in vitro Trans well assay with 5μg/ml Genistein. Genistein significantly decreased the motility (Figure 5A and B) and reduced the migration and invasion ability (Figure 5C, D) of PTC cell lines.
Figure 5.
In vitro data clearly showed that Genistein represses PTC cell motility. Figure 5A and 5B shows that Genistein treatment decreased the motility of BCPAP and BHP10-3 cells as demonstrated by the wound healing assay. This effect was further confirmed by Trans well assay in Figure 5C and 5D. *: P<0.05, **: P<0.01, ***: P<0.001.
Genistein reverses epithelial mesenchymal transition tendency of PTC cells
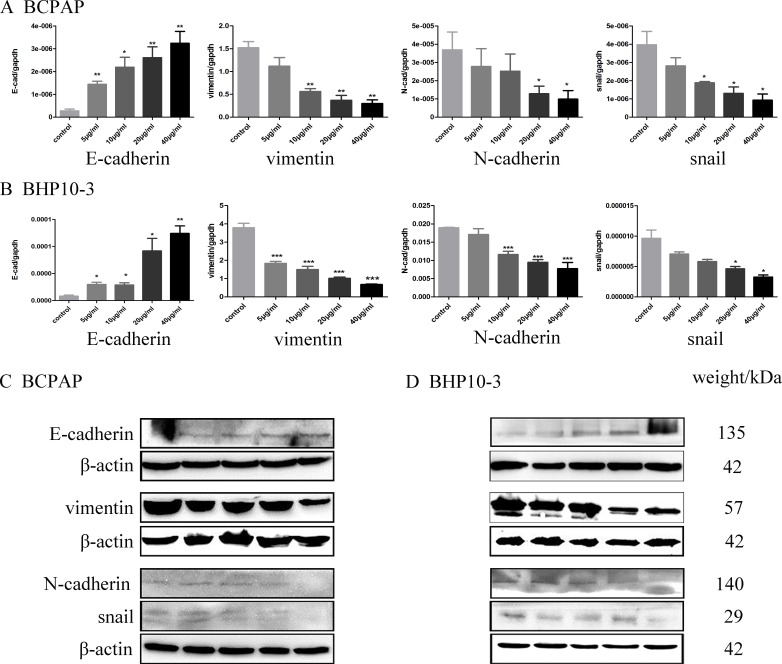
An EMT is a differentiation process accompanied by various biochemical alterations that drives apicobasal polarized epithelial cells to switch into front-rear polarity and acquire mesenchymal features, which gives the newly transformed mesenchymal cells the ability to migrate and invade25. Therefore, the effect of Genistein on the epithelial marker (E-cadherin) and mesenchymal marker (N-cadherin, Vimentin), and the critical transcription factors for EMT (Snail) were further detected by RT-qPCR, Western blot and immunofluorescence staining assay. Real-time PCR analysis showed that Genistein significantly down-regulated the expression of N-cadherin, Vimentin and Snail, while up-regulated E-cadherin in both mRNA and protein levels in a concentrate-dependent manner (Figure 6 and 7). These results indicated that Genistein reverses the EMT tendency of the PTC cells, which is coincident with its inhibition on cell migration and invasion.
Figure 6.
Genistein down-regulated the mRNA expression of N-cadherin, vimentin and snail, while up-regulated E-cadherin mRNA level in a concentrate-dependent manner as demonstrated by Real-time PCR analysis in Figure 6A-B. In parallel, Western blot and immunofluorescence staining assay demonstrated increased E-cadherin protein level and decreased N-cadherin, vimentin and snail level in Genistein-treated BCPAP and BHP10-3 cells in Figure 6C-D. *: P<0.05, **: P<0.01, ***: P<0.001.
Figure 7.
Immunofluorescence staining assay demonstrated increased E-cadherin protein level and decreased N-cadherin, Vimentin and Snail level in Genistein-treated BCPAP and BHP10-3 cells in Figure 7A-B (×200).
The translocation of β-catenin by Genistein
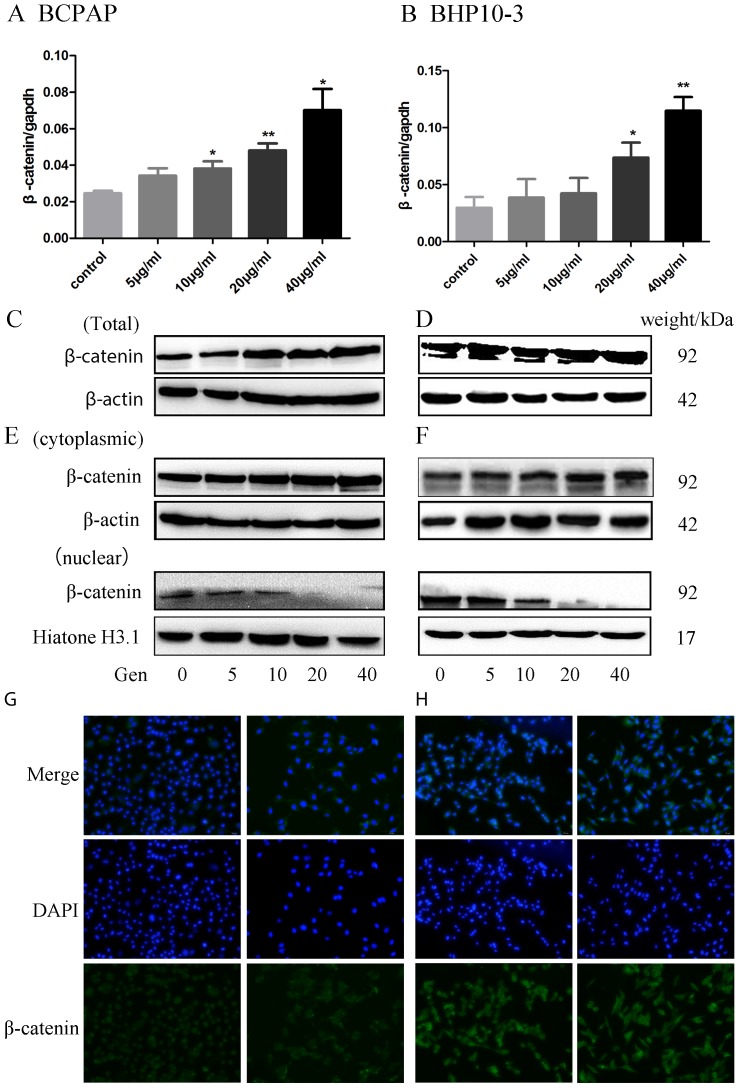
β-catenin is the key protein in the WNT signaling and its disassembly from the destruction complex in the cytoplasm facilitates the nuclear translocation and subsequent transcriptional activation of several genes in EMT. In order to further study the potential mechanism of Genistein in regulating EMT, we further detected the alterations of β-catenin expression and location induced by Genistein treatment. β-catenin mRNA and protein were up-regulated in PTC cell lines in both mRNA level (Figure 8A-B) and protein level (Figure 8C-D) after treated with different concentration of Genistein in a time dependent manner. Subcellular fraction analysis showed that the cytoplasmic β-catenin increased with Genistein treatment, nuclear β-catenin decreased after treated with Genistein in a concentrate-dependent manner (Figure 8E-F). This finding was further confirmed by immunofluorescence staining assay (Figure 9A-B).
Figure 8.
The whole expression of β-catenin mRNA and protein were proved to be concentrate-dependently up-regulated in BCPAP and BHP10-3 cells after treatment with Genistein showing by RT-qPCR (A-B) and Western Blot (Figure C-D). E-F, Nuclear β-catenin significantly decreased after exposure to Genistein in a concentrate-dependent manner as confirmed by further subcellular fraction analysis. G-H, Immunofluorescence staining assay further confirmed that Genistein induced the translocation of β-catenin from the nucleus into the cytoplasm (×200). *: P<0.05, **: P<0.01.
Figure 9.
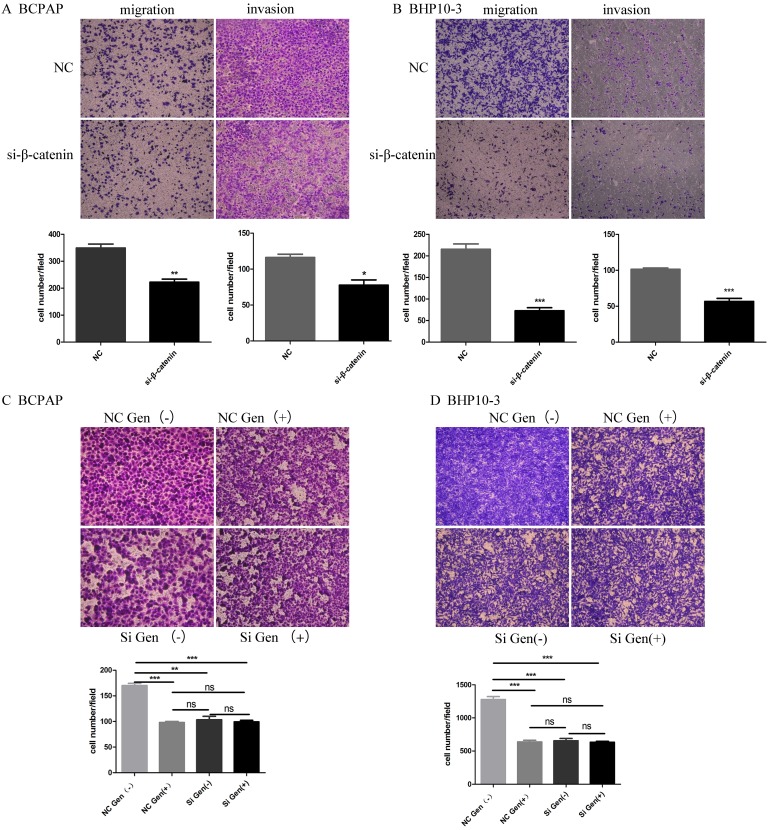
A-B, Knockdown the β-catenin using siRNA significantly inhibited the migration and invasion of BCPAP and BHP10-3 cells as proved by in vitro Trans well assay. C-D, Genistein significantly inhibited the migration and invasion of BCPAP and BHP10-3 cells, β-catenin knock down almost abrogated these effects according to the effects of Genistein on cell motility using cells with β-catenin knockdown (×200). *: P<0.05, **: P<0.01, ***: P<0.001.
β-catenin silencing reduces migration and invasion BCPAP and PHP10-3 cells
Knockdown the β-catenin using siRNA markedly inhibited the migration and invasion of PTC carcinoma cells as proved by in vitro Trans well assay (Figure 9A-B). Although Genistein significantly inhibited the migration and invasion of BCPAP and BHP10-3 cells, β-catenin knock down almost abrogated these effects according to the effects of Genistein on cell motility using cells with or without β-catenin knockdown (Figure 9 C-D).
Overall, our data suggested that β-catenin plays an important role in the inhibition of the invasion and metastasis as a target molecule of Wnt/β-catenin signal way by genistein in PTC.
Discussion
PTC has been reported to be the most common thyroid carcinoma with a favorable clinical behavior 26. However, our previous study suggested that PTC with more than 20% of tumor cells with loss of cellular polarity/cohesiveness (LOP/C) is high-risk thyroid carcinoma with higher recurrence rate and less favorable clinical behavior 27. We further confirmed those tumor cells with LOP/C to have aberrant epithelial marker and mesenchymal marker expression, and these morphological features were proposed to be a symbol of EMT in PTC 28. Hobnail variant PTC with predominant LOP/C was classified as a new aggressive subvariant of PTC in the current new WHO classification, and BRAFV600E mutation was reported to correlated with this kind of morphology29, 30. The clinical treatment of PTC is mainly surgery and radio-iodine refractory, and traditional biochemical therapy does not work well 31. Therefore, it is necessary to develop potential therapeutic method for high risk PTC with BRAFV600E mutation.
As a phytoestrogen bioactive compound, Genistein has displayed its potential anti-cancer bioactivity in several types of tumors, such as breast cancer, prostate cancer, and renal cancer 24, 32. However, the effects of Genistein on PTC have not been investigated. Mechanistically, Genistein was demonstrated to interfere with the activation of protein tyrosine kinase (PTK), modulate apoptotic cell death, induce cell differentiation, and scavenge oxygen free radicals in cancer cells 33. We firstly demonstrated that Genistein significantly inhibits the proliferation and lead to cell death of several PTC cell lines in a time and concentrate dependent manner. In current study, downregulation of CyclinB1 and Cyclin A2 was confirmed in both PTC cell lines and normal human thyroid follicular cells. However, decreased expression of Cyclin D1 was found in the normal thyroid follicular cells, comparing with the upregulation of Cyclin D1 in PTC carcinoma cells. These result suggest that the inhibition of the proliferation of the tumor cells was mainly through inducing G2/M phase arrest caused by manipulating the expression of cell cycle regulators, which are consistent with the previous study in hepatocellular, breast and nasopharyngeal carcinoma cells 34-36. Genistein induces G2/M phase arrest caused by downregulation of CyclinB1 and Cyclin A2 and upregulation of Cyclin D1 34-36.
Simultaneously, Genistein leads to increased cell death in all PTC cell lines. The anti-proliferation effect was examined to be obvious in both PTC cells with BRAFV600E mutation and cells with RET/PTC1 rearrangement. Slight inhibitory effect was also demonstrated in the normal thyroid follicular cells. These results suggested that Genistein plays a protective effect in both neoplastic and non-neoplastic thyroid tissue. A recent clinic trail suggested that Genistein improves thyroid function in Hashimoto's thyroiditis patients through regulating Th1 cytokines 37. As Hashimoto's thyroiditis is an autoimmune thyroiditis with variable regeneration and degeneration of the thyroid follicular cells, anti-proliferation of Genistein should be a possible mechanism in addition to anti-inflammatory effect in autoimmune thyroiditis.
EMT is a major pathologic mechanism in tumor progression, which promotes polarized epithelial cells to lose cellular adhesion and polarity and to acquire the phenotype of mesenchymal cells. Numerous studies have demonstrated that EMT facilitates the progression of thyroid cancer from PTC to dedifferentiated, anaplastic thyroid carcinomas (ATCs), leading to high metastatic property and poor prognosis 38, 39. We found that Genistein significantly inhibited the ability of migration and invasion in PTC cell lines. In addition to the inhibition of cell migration, increased expression of epithelial marker E-cadherin and β-catenin, and decreased expression of mesenchymal markers N-cadherin, Vimentin and EMT transcription factor Snail were further confirmed. These results suggested that Genistein could reverse the EMT progression, and thereby inhibit the invasive and metastatic ability of the PTC carcinoma cells.
WNT/β-catenin signaling largely contributes to loss of E-cadherin and leads to the initiation and development of various types of human cancers, including thyroid cancer 13. The nucleus translocation and activation of β-catenin was reported to be the central event for canonical WNT signaling pathway 40. Using immunofluorescence assay, we further confirmed that Genistein impeded the translocation of β-catenin into nucleus in the PTC cells. When knock down β-catenin using siRNA, the inhibitory effects of Genistein on PTC migration and invasion was reversed, which further confirmed that the Genistein regulate the EMT of PTC tumor cells mainly through the translocation of β-catenin from the nucleus to the cytoplasm.
In conclusion, our studies firstly demonstrate that Genistein inhibits PTC cell proliferation, induces cell death and cell cycle arrest, and reverses EMT tendency by preventing nucleus translocation of β-catenin. Our findings here provide novel evidences for the potential therapeutic application of Genistein in high-risk PTC with BRAFV600E mutation.
Acknowledgments
This research was supported by Science Foundation of Shandong Province China (Grant No. ZR2011HQ040), Fundamental Research Fund of Shandong University-Clinical Research Fund of Qilu Hospital [Grant No. 2014QLKY20], Research Fund of Affiliated Qilu Hospital of Shandong University [Grant No. 2015QLMS30].
References
- 1.Liu D, Liu Z, Jiang D, Dackiw AP, Xing M. Inhibitory effects of the mitogen-activated protein kinase kinase inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab. 2007;92:4686–95. doi: 10.1210/jc.2007-0097. [DOI] [PubMed] [Google Scholar]
- 2.Amacher AM, Goyal B, Lewis JS Jr, El-Mofty SK, Chernock RD. Prevalence of a hobnail pattern in papillary, poorly differentiated, and anaplastic thyroid carcinoma: a possible manifestation of high-grade transformation. Am J Surg Pathol. 2015;39:260–5. doi: 10.1097/PAS.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 3.Lubitz CC, Economopoulos KP, Pawlak AC, Lynch K, Dias-Santagata D, Faquin WC. et al. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014;24:958–65. doi: 10.1089/thy.2013.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D. et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves CFL, de Freitas ML, Ferreira ACF. Flavonoids, Thyroid Iodide Uptake and Thyroid Cancer-A Review. Int J Mol Sci; 2017. p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho NL, Lin CI, Whang EE, Carothers AM, Moore FD Jr, Ruan DT. Sulindac reverses aberrant expression and localization of beta-catenin in papillary thyroid cancer cells with the BRAFV600E mutation. Thyroid. 2010;20:615–22. doi: 10.1089/thy.2009.0415. [DOI] [PubMed] [Google Scholar]
- 7.Salvatore G, De Falco V, Salerno P, Nappi TC, Pepe S, Troncone G. et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006;12:1623–9. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 8.Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 9.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 10.Qian K, Gao AJ, Zhu MY, Shao HX, Jin WJ, Ye JQ. et al. Genistein inhibits the replication of avian leucosis virus subgroup J in DF-1 cells. Virus Res. 2014;192C:114–20. doi: 10.1016/j.virusres.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Spoerlein C, Mahal K, Schmidt H, Schobert R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J Inorg Biochem. 2013;127:107–15. doi: 10.1016/j.jinorgbio.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Divi RL, Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9:16–23. doi: 10.1021/tx950076m. [DOI] [PubMed] [Google Scholar]
- 13.Rahman Mazumder MA, Hongsprabhas P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed Pharmacother. 2016;82:379–92. doi: 10.1016/j.biopha.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 2013;9:1089–98. doi: 10.7150/ijbs.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavese JM, Krishna SN, Bergan RC. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am J Clin Nutr. 2014;100:431S–6S. doi: 10.3945/ajcn.113.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Lin D, Li W, Yang X. Non-digestible stachyose promotes bioavailability of genistein through inhibiting intestinal degradation and first-pass metabolism of genistein in mice. Food Nutr Res. 2017;61:1369343. doi: 10.1080/16546628.2017.1369343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinets A, Hulchiy M, Sofiadis A, Ghaderi M, Hoog A, Larsson C. et al. Clinical, genetic, and immunohistochemical characterization of 70 Ukrainian adult cases with post-Chornobyl papillary thyroid carcinoma. Eur J Endocrinol. 2012;166:1049–60. doi: 10.1530/EJE-12-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M, Evdokimova V, Nikiforov YE. Mechanisms of chromosomal rearrangements in solid tumors: the model of papillary thyroid carcinoma. Mol Cell Endocrinol. 2010;321:36–43. doi: 10.1016/j.mce.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielecka ZF, Czarnecka AM, Solarek W, Kornakiewicz A, Szczylik C. Mechanisms of Acquired Resistance to Tyrosine Kinase Inhibitors in Clear - Cell Renal Cell Carcinoma (ccRCC) Curr Signal Transduct Ther. 2014;8:218–28. doi: 10.2174/1574362409666140206223014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rusin A, Krawczyk Z, Grynkiewicz G, Gogler A, Zawisza-Puchalka J, Szeja W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochim Pol. 2010;57:23–34. [PubMed] [Google Scholar]
- 21.Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs. 2009;18:957–71. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavese JM, Krishna SN, Bergan RC. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am J Clin Nutr. 2014;100(Suppl 1):431S–6S. doi: 10.3945/ajcn.113.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Wang L, Su B, Lu N, Song J, Yang X. et al. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate. 2014;74:689–701. doi: 10.1002/pros.22787. [DOI] [PubMed] [Google Scholar]
- 24.Hirata H, Ueno K, Nakajima K, Tabatabai ZL, Hinoda Y, Ishii N. et al. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br J Cancer. 2013;108:2070–8. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–49. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZY, Zhou GY, Kakudo K, Lam AK. [Update on 2017 Who classification of tumors of thyroid gland] Zhonghua Bing Li Xue Za Zhi. 2018;47:302–6. doi: 10.3760/cma.j.issn.0529-5807.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y. et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008;99:1908–15. doi: 10.1111/j.1349-7006.2008.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T, Miyauchi A. et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol. 2011;64:325–9. doi: 10.1136/jcp.2010.083956. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd R, Osamura R, Klöppel G, Rosai J. WHO classification of tumours:Pathology and genetics of tumours of endocrine organs. 4th ed: Lyon: IARC; 2017. [Google Scholar]
- 30.Teng L, Deng W, Lu J, Zhang J, Ren X, Duan H. et al. Hobnail variant of papillary thyroid carcinoma: molecular profiling and comparison to classical papillary thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. Oncotarget. 2017;8:22023–33. doi: 10.18632/oncotarget.15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM. et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid. 2017;27:481–3. doi: 10.1089/thy.2016.0628. [DOI] [PubMed] [Google Scholar]
- 32.Wu TC, Lin YC, Chen HL, Huang PR, Liu SY, Yeh SL. The enhancing effect of genistein on apoptosis induced by trichostatin A in lung cancer cells with wild type p53 genes is associated with upregulation of histone acetyltransferase. Toxicol Appl Pharmacol. 2016;292:94–102. doi: 10.1016/j.taap.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Barnes S, Peterson TG. Biochemical targets of the isoflavone genistein in tumor cell lines. Proc Soc Exp Biol Med. 1995;208:103–8. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Xunsun, Liu JY, Yang D, Yang LL, Kong DX. et al. The effect of cell cycle and expression of cyclin B1 and cyclin C protein in hepatocellular carcinoma cell line HepG2 and SMMC-7721 after of silencing beta-catenin gene. Hepatogastroenterology. 2012;59:515–8. doi: 10.5754/hge11534. [DOI] [PubMed] [Google Scholar]
- 35.Han H, Zhong C, Zhang X, Liu R, Pan M, Tan L. et al. Genistein induces growth inhibition and G2/M arrest in nasopharyngeal carcinoma cells. Nutr Cancer. 2010;62:641–7. doi: 10.1080/01635581003605490. [DOI] [PubMed] [Google Scholar]
- 36.Li JP, Yang YX, Liu QL, Zhou ZW, Pan ST, He ZX. et al. The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells. Drug Des Devel Ther. 2015;9:1027–62. doi: 10.2147/DDDT.S74412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, Wang Y, Ma W, Hu Z, Zhao P. Genistein improves thyroid function in Hashimoto's thyroiditis patients through regulating Th1 cytokines. Immunobiology. 2017;222:183–7. doi: 10.1016/j.imbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Zhang W, Zhang L, Chen X, Liu F, Zhang J. et al. miR-146a-5p mediates epithelial-mesenchymal transition of oesophageal squamous cell carcinoma via targeting Notch2. Br J Cancer. 2016;115:1548–54. doi: 10.1038/bjc.2016.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Ma B, Hottiger MO. Crosstalk between Wnt/beta-Catenin and NF-kappaB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]