Abstract
The authors describe a 12-year-old girl with an atypical presentation of Bartonella encephalitis. She presented with fever and altered mental status and developed flaccid paralysis of her left upper extremity a day later. An electroencephalogram showed slowing over her right hemisphere. She had mild leukocytosis and bandemia, but her imaging and cerebrospinal studies were unrevealing. After five days, her symptoms resolved and she was discharged home on doxycycline due to suspicion for Bartonella encephalitis. The patient admitted to playing with a kitten two months prior, but she lacked the classic regional lymphadenopathy. Bartonella titers were sent during her hospitalization and returned positive after her discharge. Cat scratch disease neurologic manifestations are uncommon, with hemiplegia being exceedingly rare. This case illustrates that focal neurologic signs may develop during cat scratch disease infection and suggests that cat scratch disease encephalitis should be considered during evaluation of a pediatric patient with acute flaccid paralysis.
Keywords: EEG, electroencephalogram, pediatric, adolescents, encephalitis, Todd paralysis, infectious disease, cat scratch disease, epidemiology
In the United States, there are an estimated 22000 cases per year of cat scratch disease (CSD), with over 2000 cases requiring hospital admission.1 Cat scratch disease is caused by Bartonella henselae and most often presents with fever and regional lymphadenopathy.1 Over 90% of patients recall contact with a cat prior to illness.1 It is primarily a pediatric disease: One study reported 84% of cases occurred in patients younger than 18 years.2 The higher incidence in the younger population may be attributed to children having an immature immune system or children playing with cats more frequently than adults do. Neurological manifestations occur in up to 7% of patients and on average appear within 2 weeks after fever and lymphadenopathy onset.1,2 The authors present a case of cat scratch disease encephalitis with acute flaccid paralysis of the left arm alongside a electroencephalogram (EEG) correlate of right hemispheric slowing.
Case Presentation
A 12-year-old girl presented to the emergency department in the early morning after her parent found her hiding under and hitting herself against her bed while jerking and crying. This pediatric patient with acute onset of altered mental status and possible seizure had a recorded temperature of 103.1°F and a Glasgow Coma Scale of 12. She opened her eyes to voice, she had confused speech, and she localized to pain in all extremities. She had no known prior episodes and her family history was negative for seizures, although she had a personal history of anxiety treated with sertraline. A rapid antigen detection test for group A streptococcus was positive, but she had no sore throat or tonsillar erythema on examination, indicating carrier status. A complete blood count demonstrated mild leukocytosis (white blood cells, 14.6/µL) and bandemia (18%). Urine drug screen, complete metabolic panel, cerebrospinal fluid (CSF) cell counts, urinalysis, and head computed tomography scan were all within normal limits. The patient’s initial presentation of altered mental status with a high fever and abnormal peripheral white blood cell count prompted treatment with vancomycin, ceftriaxone, and acyclovir due to a concern for infectious encephalitis. Despite the normal CSF, infectious encephalitis was still in the differential diagnosis. The patient’s presentation raised clinical suspicion for an encephalitis and some studies demonstrate normocellular CSF can occur in encephalitis, although more rarely.3 She was started on levetiracetam for seizure prophylaxis due to concern that her initial presentation may have represented a postictal state after an unwitnessed seizure, particularly since herpes simplex virus encephalitis was in the differential diagnosis.
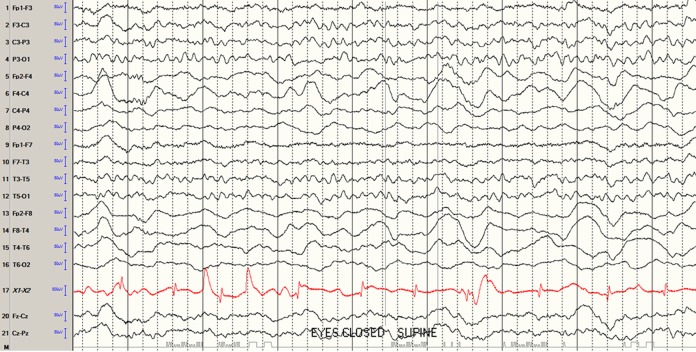
The morning after admission, she was found to have left upper extremity flaccid paralysis that improved after two days. She also experienced urinary incontinence overnight. Magnetic resonance imaging (MRI) with and without contrast of the brain and magnetic resonance angiography of the head showed no abnormalities except for mild paranasal sinus inflammation. A routine EEG showed slowing over her right hemisphere consistent with a widespread functional disturbance in that hemisphere (Figure 1). With these EEG and head imaging findings, the patient’s paralysis was attributed to a Todd paralysis phenomenon. Her urinary incontinence was attributed to subclinical seizures versus encephalopathy.
Figure 1.
Electroencephalogram showing slowing over right hemisphere consistent with a widespread functional disturbance in that hemisphere.
Blood, urine, and CSF cultures were sent and returned no growth. Herpes simplex virus polymerase chain reaction (PCR) and varicella virus PCR were both negative in the CSF. The treatment team discontinued her vancomycin, ceftriaxone, and acyclovir on day three of hospitalization. West Nile virus immunoglobulin M and Epstein-Barr virus PCR were negative. Serum Mycoplasma pneumoniae antibodies demonstrated elevated immunoglobulin M at 1861 U/mL, but PCR was negative. Serum anti-streptolysin (ASO) titers were elevated at 675 IU/mL, and anti-DNAse B antibodies were high at 583 U/mL. Serum arbovirus panel was sent and resulted negative. Serum Bartonella titers were sent as well.
The patient improved clinically with return of her left arm function on the fifth day of hospitalization. Infectious disease team was consulted, and they interviewed the family with targeted questions to look for unusual exposures to more uncommon infectious agents. The team learned through their questions that the patient had played with a friend’s kitten two months prior to admission. Empiric treatment with doxycycline was initiated due to suspected Bartonella encephalitis. The patient was discharged on doxycycline 100 mg twice daily and completed a total 14-day course. Bartonella titers came back after time of discharge at 1:2560. At her follow-up appointment one week later, the patient had made a complete recovery. She stayed on levetiracetam for six months and then was weaned off the medication, as she was seizure-free at the six-month follow-up.
Discussion
Bartonella henselae bacteria can be transmitted from a cat scratch or bite, from cat saliva that contacts broken skin or mucosal surfaces, or from a cat or dog with fleas.4 Although our patient recalled playing with a kitten, her presentation did not include any enlarged and erythematous lymph nodes as is typical for this disease.4
The patient’s elevated anti-DNAse B antibodies and elevated ASO titers are indicative of past infection with group A streptococcus.5 In addition, all treatment teams felt that pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections was an unlikely diagnosis, as the patient did not exhibit features of tic disorder or obsessive compulsive disorder associated with the syndrome.6
The patient’s positive Mycoplasma pneumoniae immunoglobulin M must be considered in combination with the patient’s negative Mycoplasma pneumoniae PCR: this combination of results is not necessarily diagnostic of acute infection.7–10 The Infectious Disease Society of America recommends PCR as the diagnostic test of choice due to high specificity.11 One small study suggests that serologic tests do not distinguish Mycoplasma pneumoniae disease status from carrier status.12 A larger study looking at neurologic manifestations of Mycoplasma pneumoniae noted 2 distinct disease patterns: one characterized by high immunoglobulin M and frequent respiratory symptoms and the other with neither trait.13 Our patient did not fit either clinical picture in that larger study.13 Nevertheless, a coinfection picture cannot be completely ruled out in our patient.
Diagnosis of Bartonella henselae infection is also challenging due to several factors. The length of time from obtaining serum for testing to receiving the test result is over one week in our hospital’s laboratory. Specificity and sensitivity of IgG titers have been studied with varying results.14–17 For high titers like our patient’s, one study suggests that there is a 100% sensitivity and a 98.5% specificity for cat scratch disease.17 Generally, a titer result of greater than 1:256 is suggestive of acute infection.14–17 High titers alongside encephalopathy support the diagnosis of Bartonella encephalitis.
The infectious disease team chose doxycycline monotherapy rather than adding rifampin due to concerns about the family’s compliance with taking antibiotics as well as levetiracetam when discharged from the hospital. Doxycycline with rifampin is the preferred regimen for cat scratch disease–associated neurologic disease.18
Focal neurologic signs are common when considering all etiologies of pediatric infectious encephalitis.19 In one large study from Toronto, 60% of pediatric encephalitis cases presented with focal seizures and 50% had other focal neurologic signs.19 Of the patients with cat scratch disease who have neurological manifestations, encephalopathy, status epilepticus, convulsions, retinitis, cerebral vasculitis, and transverse myelitis are all documented presentations.20–24 Encephalopathy is the most common neurologic manifestation of cat scratch disease, and it can present several weeks after initial exposure to a cat.2,25
One case report from Brazil describes a child who had cat scratch disease–associated left hemiplegia and a right frontoparietal lesion on head imaging.26 Another case report from Peru describes a child who had temporary right hemiparesis after cat scratch disease–associated status epilepticus, suggestive of Todd paralysis.27 There have also been at least two case reports of cat scratch disease–associated vasculitis as etiology for stroke and resultant hemiparesis.2 One retrospective review found 20 cases of cat scratch disease–related vertebral osteomyelitis, and within that case series, there is one patient who had severe transient paresis.28 These case reports suggest that the flaccid paralysis from cat scratch disease encephalitis could be due to a direct infection of the right hemisphere, a postictal Todd paralysis, or spinal cord involvement. However, the mechanism by which Bartonella causes CNS disease is currently not well understood. One unfortunate case describes a patient who died from cat scratch disease–associated encephalitis and microglial nodules were found on his brain biopsy at autopsy.29 Further study on this mechanism through animal models may assist with developing treatment approaches that shorten duration of symptoms.
In our patient, focal arm paralysis with confusion and incontinence was found the morning after the patient had been admitted, on morning rounds. Since she went the night without these changes being noted on examination, our working hypothesis was that she had an unwitnessed seizure during the night. Whether she had a focal motor seizure (resulting in Todd paralysis) followed by secondary generalization (resulting in confusion and incontinence postictally) is difficult to ascertain. She may also have had two separate seizures during the night, 1 focal motor, and 1 with secondary generalization.
As illustrated in the worldwide cases discussed above, an important differential the team considered was direct infection of the contralateral hemisphere. The MRI obtained did not have evidence of direct infection on postcontrast sequences. Another differential considered was spinal cord involvement due to the urinary incontinence. Magnetic resonance imaging of the spine was not obtained during her hospitalization. On repeated physical examinations during the hospital stay, she did not develop increased tone or hyperreflexia in her left upper extremity, as would be expected for spinal cord lesions (upper motor neuron findings after early lower motor neuron findings), and her incontinence also resolved. The patient also had no personal or family history of classic migraine or hemiplegic migraine, the latter of which is a diagnostic possibility in children with acute flaccid paralysis.
Conclusion
To our knowledge, our patient’s case is the second reported case of cat scratch disease associated with focal weakness. The report of her disease adds to the literature, demonstrating that focal neurologic signs on examination, including acute flaccid paralysis, may indicate cat scratch disease encephalopathy. Given the right clinical history, this disease should be considered, even without the classic regional lymphadenopathy. Our case also illustrates that in cases of fever and focal paralysis, if initial imaging is negative, EEG can provide valuable diagnostic assistance and help to guide medical management.
Acknowledgment
Grateful acknowledgement is made to Dr Gloria Heresi for her assistance in taking care of this patient and Dr Jeremy Lankford for his academic guidance in publishing this article.
Footnotes
Author contributions: LR, KR, CM and AB contributed to conception and design. All authors drafted the manuscript and gave final approval. All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The patient’s information has been anonymized according to ICMJE guidelines.
References
- 1. Opavsky MA. Cat scratch disease: the story continues. Can J Infect Dis. 1997;8(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis DW, Tucker SH. Central nervous system involvement in cat scratch disease. Pediatrics. 1986;77(5):714–721. [PubMed] [Google Scholar]
- 3. Saraya AW, Wacharapluesadee S, Petcharat S, et al. Normocellular CSF in herpes simplex encephalitis. BMC Research Notes, 2016;9 doi:10.1186/s13104-016-1922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartonella Infection (Cat Scratch Disease, Trench Fever, and Carrion’s Disease). Centers for disease control and prevention. https://www.cdc.gov/bartonella/index.html.
- 5. Bryant AE, Stevens DL. Streptococcus pyogenes In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed Philadelphia, PA: Elsevier Saunders; 2015. [Google Scholar]
- 6. Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev. 2018;86:51–65. [DOI] [PubMed] [Google Scholar]
- 7. Thurman KA, Walter ND, Schwartz SB, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis. 2009;48(9):1244. [DOI] [PubMed] [Google Scholar]
- 8. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Touati A, Benard A, Hassen AB, Bébéar CM, Pereyre S. Evaluation of five commercial real-time PCR assays for detection of mycoplasma pneumoniae in respiratory tract specimens. J Clin Microbiol. 2009;47(7):2269–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loens K, Goossens H, Ieven M. Acute respiratory infection due to mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010;29(9):1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57(4):e22–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med. 2013;10(5):e1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Zaidy SA, MacGregor D, Mahant S, Richardson SE, Bitnun A. Neurological Complications of PCR-proven M. pneumoniae infections in children: prodromal illness duration may reflect pathogenetic mechanism. Clin Infect Dis. 2015;61(7):1092–1098. [DOI] [PubMed] [Google Scholar]
- 14. Bergmans AM, Peeters MF, Schellekens JF, et al. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol. 1997;35(8):1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dupon M, Savin De Larclause AM, Brouqui P., et al. Evaluation of serological response to Bartonella henselae, Bartonella quintana and Afipia felis antigens in 64 patients with suspected cat-scratch disease. Scand J Infect Dis. 1996;28(4):361–366. [DOI] [PubMed] [Google Scholar]
- 16. Sander A, Posselt M, Oberle K, Bredt W, et al. Seroprevelance of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: evaluation and comparison of two commercial serological tests. Clin Diagn Lab Immunol. 1998;5(4):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klotz SA, Ianas V, Elliott SP. Cat-scratch disease. Am Fam Physician. 2011;83(2):152–155. [PubMed] [Google Scholar]
- 18. Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48(6):1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolski H, Ford-Jones EL, Richardson S, et al. Etiology of acute childhood encephalitis at the hospital for sick children, Toronto, 1994-1995. Clin Infect Dis. 1998;26(2):398–409. [DOI] [PubMed] [Google Scholar]
- 20. Reed JB, Scales DK, Wong MT, Lattuada CP, Jr, Dolan MJ, Schwab IR. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology. 1998;105(3):459–466. [DOI] [PubMed] [Google Scholar]
- 21. Marra CM. Neurologic complications of Bartonella henselae infection. Curr Opin Neurol. 1995;8(3):164–169. [DOI] [PubMed] [Google Scholar]
- 22. Selby G, Walker GL. Cerebral arteritis in cat-scratch disease. Neurology. 1979;29(10):1413–1418. [DOI] [PubMed] [Google Scholar]
- 23. Baylor P, Garoufi A, Karpathios T, Lutz J, Mogelof J, Moseley D. Transverse myelitis in 2 patients with Bartonella henselae infection (cat scratch disease). Clin Infect Dis. 2007;45(4):e42–e45. [DOI] [PubMed] [Google Scholar]
- 24. Farooque P, Khurana DS, Melvin JJ. Persistent focal seizures after cat scratch encephalopathy. Pediatr Neurol. 2010;42(3):215–218. [DOI] [PubMed] [Google Scholar]
- 25. Cherinet Y, Tomlinson R. Cat scratch disease presenting as acute encephalopathy. EMJ. 2008;25(10):703–704. [DOI] [PubMed] [Google Scholar]
- 26. Rocha JL, Pellegrino LN, Riella LV, Martins LT. Acute hemiplegia associated with cat-scratch disease. Braz J Infect Dis. 2004;8(3):263–266. [DOI] [PubMed] [Google Scholar]
- 27. Cerpa PR, Orellana G, Silva Caso W, et al. Encephalitis with convulsive status in an immunocompetent pediatric patient caused by Bartonella henselae. Asian Pac J Trop Dis. 2016;9(6):610–613. [DOI] [PubMed] [Google Scholar]
- 28. Vermeulen MJ, Rutten GJ, Verhagen I, Peeters MF, van Dijken PJ. Transient paresis associated with cat-scratch disease: case report and literature review of vertebral osteomyelitis caused by Bartonella henselae. Pediatr Infect Dis J. 2006;25(12):1177–1181. [DOI] [PubMed] [Google Scholar]
- 29. Fouch B, Coventry S. A case of fatal disseminated Bartonella henselae infection (cat-scratch disease) with encephalitis. Arch Pathol Lab Med. 2007;131(10):1591–1594. [DOI] [PubMed] [Google Scholar]