Abstract
Tuberculosis (TB) is an important public health issue around the globe which is a chronic infectious disease and is still one of the major challenges for developing countries. The emergence of drug-resistant TB makes the condition worse and there is an urgent need of fast, highly sensitive diagnostic methods. This study was undertaken to evaluate the performance of GeneXpert® MTB/RIF assay and MTB culture for the detection of Mycobacterium tuberculosis (MTB) in sputum smear-negative pulmonary TB/drug-resistant tuberculosis (DR-TB) suspects. A total of 168 sputum smear-negative TB suspects were recruited for the study. Among the suspected TB cases, 52.98% were male and 47.02% were females with the mean age of 42 ± 17.6 years. All the sputum specimens collected from the study population were subjected to Ziehl–Neelsen (ZN) smear microscopy, GeneXpert MTB/RIF assay, and MTB culture. The results revealed that, out of 168 acid-fast bacilli (AFB)/ZN smear microscopy–negative sputum specimens, 48 (28.57%) and 58 (34.52%) were detected MTB positive by GeneXpert MTB/RIF assay and MTB culture, respectively, while 120 (71.43%) and 110 (65.48%) suspected TB cases were confirmed negative by GeneXpert MTB/RIF assay and MTB culture, respectively. The study concluded that GeneXpert assay was found to be a rapid and accurate tool for MTB detection in smear-negative sputum specimens. GeneXpert has advantage over ZN smear microscopy and MTB culture as it detects MTB and rifampicin resistance simultaneously within 2 h with minimal biohazards.
Keywords: AFB-negative smears, GeneXpert assay, MTB culture, pulmonary tuberculosis
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) bacteria is an important public health issue around the globe which is a chronic infectious disease. TB is transmitted primarily through sneezing and coughing and is considered among the oldest diseases recognized by the mankind. It affects mainly the lungs (pulmonary TB) but can also affect other organs or tissues of the body (extrapulmonary TB).1 According to World Health Organization (WHO) Global TB report published in 2016, Pakistan ranks fifth in high-TB burden countries and fourth for multi-drug-resistant tuberculosis (MDR-TB) among high-burden TB drug-resistant countries. In 2016, 27,000 drug-resistant TB cases were reported in Pakistan.2
Routine diagnostic methods for MTB include acid-fast bacilli (AFB) microscopy, MTB culture, conventional polymerase chain reaction (PCR), and GeneXpert® MTB/RIF assay. The early detection of the MTB and RIF/DR in TB suspects is crucial for disease management and to control the disease transmission from person to person and the emergence of drug-resistant tuberculosis (DR-TB). Sputum smear microscopy through Ziehl–Neelsen (ZN) staining is widely used in developing countries for the routine TB diagnosis due to cost effectiveness and high specificity, and does not require sophisticated equipments.3 Smear microscopy results can be obtained within 2 h; however, smear microscopy is less sensitive because it requires 5000–10,000 bacilli per mL of sputum for showing a positive result. Almost 13% of TB transmission occurs with smear-negative, culture-positive TB patients. Therefore, healthy individuals are at risk of MTB infection leading to active TB development when coming in close contact with sputum-negative TB suspects. Moreover, this test requires 3-day early morning sputum specimen collection protocol to enhance sensitivity. In addition to the lower sensitivity of sputum smear microscopy, it cannot differentiate MTB from MTB complex.4
A culture technique using Lowenstein–Jensen (LJ) medium for mycobacterial growth is being considered as the gold standard method for TB detection and it takes a longer time which is usually 3–4 weeks with a high sensitivity but requires biosafety level III laboratory. The efficiency of MTB culture using the LJ medium has been demonstrated to detect MTB when 10 viable bacilli per mL of sputum were present.5 GeneXpert MTB/RIF assay is one of the most advanced and rapid PCR-based methods recommended by WHO in 2010 for the detection of MTB DNA and rifampicin resistance. It is based on a hemi-nested real-time PCR assay utilizing five molecular beacon technology spanning the rpoB gene 81-bp rifampicin resistance determining region (RRDR).6 Hence, this study was planned to detect MTB in AFB smear-negative sputum specimens from pulmonary TB/DR-TB suspects through GeneXpert MTB/RIF assay and MTB culture.
Material and methods
This research work was carried out on sputum specimens from 168 AFB sputum smear microscopy–negative TB suspects. These patients belonged to various districts of Punjab including Sargodha, Khushab, Chiniot, Bhakkar, Mandi Bahauddin, and Mianwali. These patients were initially screened for TB through AFB sputum smear microscopy at Basic Monitoring Units for TB (BMUs) of the above districts. All the AFB smear-negative patients with persistent clinical symptoms of TB were referred to Programmatic Management Drug Resistant Tuberculosis Treatment (PMDT) site/MDR Unit, DHQ Teaching Hospital Sargodha for further confirmation of MTB/DR by GeneXpert MTB/RIF assay and MTB culture. The experimental work for AFB smear microscopy (to confirm sputum smear negativity) and GeneXpert MTB/RIF assay was carried out at Government TB Hospital Sargodha and PMDT site/MDR Unit, DHQ Teaching Hospital Sargodha, respectively. The MTB culture was also performed.
Before specimen collection, the patients were informed about the study and written consent was obtained. The patients were asked to collect the sputum specimens following the standard sputum collection technique using the recommended sterile plastic (50-mL Tarson) containers. One half of the collected sputum specimen was used for AFB sputum smear microscopy and MTB culture, while the remaining portion was used for GeneXpert MTB/RIF assay. The research study was conducted following the approved guidelines framed by the research scrutiny committee of the University of Sargodha.
Smear microscopy and culture
Direct smears were prepared for microscopic examination of AFB by applying one drop of sputum samples on a clean glass slide which was labeled with the ID number. The smear was air dried followed by heat fixation and staining using ZN staining methods. The stained smears were examined microscopically using 100× objective lens. Control slides (known positive and known negative) were included as procedural control with each run of ZN staining.7 For MTB culture, the sputum sample was added to an equal volume of 4% N-acetyl-L-cysteine (NALC)–NaOH solution in the centrifuge tube, vortex mixed, and digested for 15 min at 25°C–28°C. The mixture was then added with sterile phosphate-buffered saline (PBS) up to 50-mL mark followed by 15-min centrifugation at 3500 r/min. The upper layer of the mixture was decanted carefully, and the sediment was re-suspended using 0.3 mL of PBS. Deposit was inoculated into McCartney bottles which contain the LJ media and labeled with the patient ID number. The LJ media were kept at 37°C for 8 weeks and examined weekly. The whole procedure was performed inside biosafety cabinet class II. The LJ media were inoculated with H37RV, a known American Type Culture strain, as a positive control while as negative control the sterile PBS was also inoculated on random slants and incubated with each batch of test.8,9
GeneXpert MTB/RIF assay
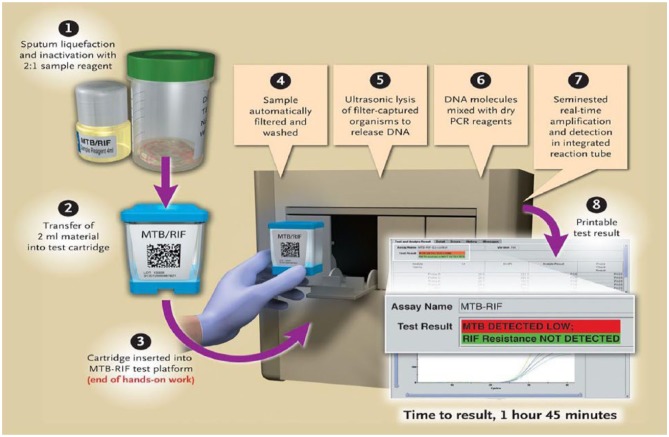
To 2 mL of sample reagent, 1 mL of sputum specimen was added and the sputum specimen was allowed to liquefy for 15 min. Using a sterile dropper provided with the kit, 2 mL of mixture was transferred into the GeneXpert cartridge. This cartridge contained the required reagents for nucleic acid amplification and RIF drug resistance detection. The inoculated cartridge was loaded into the GeneXpert machine, as shown in Figure 1. Results were displayed by the GeneXpert system automatically within 2 h. The operator can read and print the test results as “MTB detected; RIF resistance not detected.”
Figure 1.
Different procedural steps in the Xpert MTB/RIF assay process.6
Statistical analysis
The obtained data were analyzed for statistical significance using SPSS version 16.0 by applying Chi-square test.
Results
A total of 168 TB suspects were recruited in this study consisting of 79 (47.02%) females and 89 (52.98%) males with a male-to-female ratio of 1.13:1. Most of the subjects (25.60%) were under 31–40 years of age. The percent frequency of the study subjects belonging to different age groups is shown in Figure 2.
Figure 2.
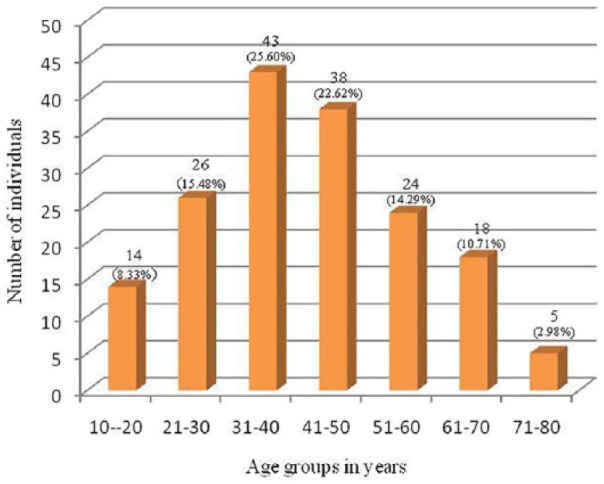
Diagrammatic representation of study subjects among different age groups.
Among the study population, the number of male participants was 89 (52.98%), while that of females was 79 (47.02%) out of the total 168 participants. Based on the locality of patients, 127 (75.60%) belonged to Sargodha district, 18 (10.71%) to Khushab, 15 (8.97%) to Bhakkar, 4 (2.38%) to Mandi Bahauddin, 2 (1.19%) to Chiniot, and 2 (1.19%) belonged to Mianwali district. The results stating the frequency distribution of the study subjects in terms of district-wise locality are shown in Figure 3. In this study, throughout the Central Punjab, a large number of cases belonged to Sargodha district, while the least numbers of cases were from the Chiniot and Mianwali districts.
Figure 3.
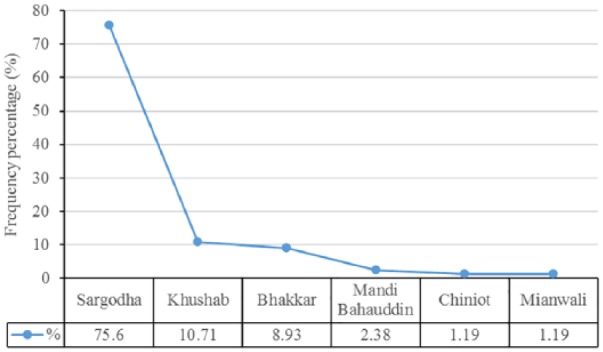
Frequency distribution graph showing the locality of participants in the study population.
All sputum samples were processed for smear microscopy to confirm smear negativity for AFB and found negative by ZN staining method. The sputum samples were further processed for GeneXpert MTB/RIF assay and MTB culture. A known AFB-positive specimen and AFB-negative specimens were also included to serve as controls for validation of the ZN staining protocol.
Among the 168 studied individuals, 48 (28.57%) were found MTB positive, while only 1 case was detected as MDR-TB for rifampicin resistance by GeneXpert MTB/RIF assay being a pulmonary TB suspect. The results revealed that GeneXpert MTB/RIF assay is a highly specific and sensitive method for MTB and MDR-TB detection as 28.57% of the cases were detected positive through this method which were smear negative for AFB by the ZN staining method.
Among the MTB culture specimens on the LJ medium, 58 (34.52%) were found culture and 110 (65.48%) were negative. On the LJ medium slants, MTB grows with characteristic features of its colonies. These are white to creamy white colored, in cauliflower shape, dry, rough, buff, and tough. AFB/ZN smear microscopy had 0, GeneXpert MTB/RIF assay gave 48 (28.57%), and MTB culture on the LJ medium resulted in 58 (34.52%) positive number of results for MTB, while 168 (100%), 120 (71.43%), and 110 (65.48%) were negative for all the three methods, namely, AFB sputum smear microscopy, GeneXpert MTB/RIF assay, and MTB culture, respectively, as given in Table 1. GeneXpert assay also detects one MDR-TB case in the studied population.
Table 1.
Comparison between ZN/AFB smear microscopy, GeneXpert MTB/RIF assay, and MTB culture of study subjects.
| Result/method | ZN/AFB smear microscopy | GeneXpert MTB/RIF assay | MTB culture on LJ medium |
|---|---|---|---|
| Positive | 0 | 48 (28.57%) | 58 (34.52%) |
| Negative | 168 (100%) | 120 (71.43%) | 110 (65.48%) |
| Total | 168 (100%) | 168 (100%) | 168 (100%) |
ZN/AFB: Ziehl–Neelsen/ acid-fast bacilli; LJ: Lowenstein–Jensen.
Hence, among the three used methods in this study for the detection of MTB and DR-TB, including ZN staining method, GeneXpert MTB/RIF assay, and MTB culture, we have found that MTB culture is a highly sensitive technique for the detection of MTB. MTB culture detected more MTB cases as compared to the rest of the two techniques, in ZN smear-negative specimens. Since ZN staining is the most widely used method for the initial diagnosis/screening of patients for TB infection, it has a lower sensitivity as compared to two other techniques because it requires 5000–10,000 bacilli per mL of sputum to give positive results. GeneXpert MTB/RIF assay gave a significantly high number of MTB-positive cases among ZN smear-negative specimens. It also detected rifampicin drug resistance (RIF/DR-TB). Thus, it is a more specific and sensitive method than ZN smear microscopy. It gives result in a very short time compared to MTB culture.
Discussion
Early TB diagnosis is essential for interrupting the transmission chain of TB disease. It is well known that AFB smear microscopy–positive TB patients are the major source of spreading TB to healthy individuals when left untreated. The studies also reported that approximately 17% of TB disease transmission is caused by AFB smear microscopy–negative TB suspects and therefore the risk of disease transmission by AFB-negative cases to healthy individuals could not be ignored.10 The continuously increasing frequency of TB in developing countries is a major threat to the life of human being and requires the use of highly sensitive and specific techniques for early detection of MTB.11
In this study, sputum specimens from presumptive TB cases from six districts of central Punjab were investigated to evaluate the efficiency of GeneXpert MTB/RIF assay against screened conventional ZN staining smear-negative results. MTB culture using the LJ medium was used as a gold standard method for MTB diagnosis. The ZN staining smear method was used to confirm the smear-negative results of sputum specimens. MTB culture verified the results of GeneXpert MTB/RIF assay for the detection of MTB. But the positivity rate for MTB was higher in the case of MTB culture by the LJ medium, since it is a more sensitive method. However, Xpert MTB/RIF assay demonstrated a high detection sensitivity for MTB complex in sputum specimens in comparison with conventional AFB smear microscopy.12
In combination with rapid MTB culture, Xpert MTB/RIF assay will definitely improve the detection rate of MTB bacteria. However, GeneXpert assay is advantageous over the culture technique as the results become readily available and in detecting both the MTB and MDR-TB simultaneously. Munir et al.5 in their study reported GeneXpert MTB/RIF assay as an effective tool for early diagnosis and treatment of TB.
Furthermore, ZN smear microscopy and culture are laborious and require more time to establish clinical diagnosis of TB. When compared to MTB culture by the LJ medium, GeneXpert MTB/RIF is less sensitive but a technique with more rapid and specific results readily available. In addition, it can detect DR-TB, that is, rifampicin resistance simultaneously with MTB DNA detection and its quantification. MTB culture by the LJ medium is a time-consuming technique, that is, up to 8 weeks, and requires additional 4–6 weeks for the diagnosis of DR-TB, that is, rifampicin resistance.13 Moreover, GeneXpert MTB/RIF assay is not prone to cross-contamination, requires minimal biosafety facilities, and has a high sensitivity in smear-negative pulmonary TB compared to MTB culture by the LJ method which required personal protective equipment (PPE) and special trainings for laboratory workers and biosafety level III laboratory requirement for its setting.
This study concluded that GeneXpert assay has shown a much higher sensitivity for the detection of MTB than ZN smear microscopy in pulmonary samples, while the culture technique is considered as the gold standard for MTB diagnosis but takes a longer time to develop MTB growth colonies and is unable to detect RIF/DR simultaneously. Nevertheless, the GeneXpert assay is considered a valuable diagnostic tool for the rapid detection of MTB in AFB smear-positive as well as smear-negative TB suspects with simultaneous detection of MDR-TB. This early detection facilitates in controlling the disease transmission and timely start of TB treatment therapy, and hence the introduction of GeneXpert assay results in a significant reduction of MDR-TB cases.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Riaz  https://orcid.org/0000-0002-5524-7735
https://orcid.org/0000-0002-5524-7735
References
- 1. Akram Y, Mahmood Z, Riaz M, et al. (2017) Biochemical profiling of tuberculosis patients co-infected with hepatitis C virus. European Journal of Inflammation 15(1): 42–45. [Google Scholar]
- 2. World Health Organization (2017) Global Tuberculosis Report 2017. Geneva: World Health Organization. [Google Scholar]
- 3. Riaz M, Mahmood Z, Javed MT, et al. (2016) Drug resistant strains of Mycobacterium tuberculosis identified through PCR-RFLP from patients of Central Punjab, Pakistan. International Journal of Immunopathology and Pharmacology 29(3): 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caulfield AJ, Wengenack NL. (2016) Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 4: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munir MK, Rehman S, Aasim M, et al. (2015) Comparison of Ziehl Neelsen microscopy with GeneXpert for detection of Mycobacterium tuberculosis. IOSR Journal of Dental and Medical Sciences 14(11): 56–60. [Google Scholar]
- 6. Boehme CC, Nabeta P, Hillemann D, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. The New England Journal of Medicine 363(11): 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheesbrough M. (2006) District Laboratory Practice in Tropical Countries. Cambridge: Cambridge University Press. [Google Scholar]
- 8. Abanda NN, Djieugoué JY, Lim E, et al. (2017) Diagnostic accuracy and usefulness of the Genotype MTBDRplus assay in diagnosing multidrug-resistant tuberculosis in Cameroon: A cross-sectional study. BMC Infectious Diseases 17(1): 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (2008) Global tuberculosis control: Surveillance, planning, financing. WHO report 2008, World Health Organization, Geneva. [Google Scholar]
- 10. Keflie TS, Ameni G. (2014) Microscopic examination and smear negative pulmonary tuberculosis in Ethiopia. The Pan African Medical Journal 19: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasool G, Riaz M, Mahmood Z, et al. (2018) Effects of household bleach on sputum smear microscopy to concentrate acid fast bacilli for the diagnosis of pulmonary tuberculosis. Journal of Biological Regulators and Homeostatic Agents 32(3): 607–611. [PubMed] [Google Scholar]
- 12. Geleta DA, Megerssa YC, Gudeta AN, et al. (2015) Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiology 15(1): 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shrestha P, Arjyal A, Caws M, et al. (2015) The application of GeneXpert MTB/RIF for smear-negative TB diagnosis as a fee-paying service at a South Asian General Hospital. Tuberculosis Research and Treatment 2015: 102430. [DOI] [PMC free article] [PubMed] [Google Scholar]