Abstract
Metabolic diseases are chronic disorders correlated to a greater risk of cardiovascular event and death. Recently, many data have sustained the biological link between microvascular dysfunction, oxidative stress, vascular inflammation, and metabolic diseases. The determination of new and specific blood biomarkers of vascular inflammation associated with obesity-related metabolic syndrome (MetS) and diabetes such as lipoprotein-associated phospholipase A2 (Lp-PLA2) could be useful to identify subject with high risk of cardiovascular events. Lp-PLA2 participates by a crucial role in microvascular dysfunction and oxidative stress showing positive association with metabolic disorders. In this review, we will argue the evolving role of Lp-PLA2 in predicting cardiovascular events in metabolic disease patients.
Keywords: biomarker, Lp-PLA2, vascular inflammation
Dear Editor,
Insulin resistance has a crucial function in the pathogenesis of type 2 diabetes mellitus (T2DM) and associated cardiovascular disease (CVD). Functional insulin signaling regulates glucose homeostasis by promoting glucose uptake in skeletal muscle and adipose tissue, inhibiting gluconeogenesis in the liver, and regulating nutrient delivery to target tissues by actions on microvasculature. Insulin-induced nitric oxide (NO) production from vascular endothelium acts to increase microvascular perfusion of skeletal muscle enhancing the delivery of hormones and nutrients to muscle cells.1 Dysregulation of insulin signaling results in insulin resistance, and the consequent hyperinsulinemia induces chronic low-grade inflammation.2 When insulin resistance develops, the activation of phosphatydilinositol (PI) 3-kinase/AKT pathway results markedly impaired and vascular dysfunction may occur because of the diminished glucose uptake in skeletal muscle, and the ability of insulin to stimulate NO production in the endothelium is impaired promoting in this way proatherogenic changes.3 In this context, several circulating vascular biomarkers such as lipoprotein-associated phospholipase A2 (Lp-PLA2) have been evaluated to predict CVD in patients with metabolic diseases. Lp-PLA2 is a calcium-independent serine lipase of 50-kDa which hydrolyzes the acetyl group at the sn-2 position of platelet-activating factor. About 80% of Lp-PLA2 circulates bound to low-density lipoprotein (LDL) particles, whereas the remaining 20% is bound to high-density lipoprotein (HDL). Lp-PLA2 participates in the oxidative modification of LDL in the vascular wall generating oxidized phospholipids such as lysophosphatidylcholine and oxidized non-esterified fatty acids. These molecules can promote vascular inflammation and atherosclerotic plaque development.4 Lp-PLA2 was considered as a biomarker of vascular dysfunction and as potential therapeutic target. Darapladib is a selective inhibitor of Lp-PLA2 that has been evaluated as a new therapeutic drug. However, in two large trials (STABILITY trial and SOLID-TIMI 52 study), Darapladib failed to reduce coronary events compared with placebo. Accordingly, it is unlikely that Lp-PLA2 could be a therapeutic target for the prevention of CVD.5
Lp-PLA2: laboratory methods
The methods developed to detect Lp-PLA2 in human blood can measure both its plasma concentration (mass) and enzymatic activity. However, Lp-PLA2 mass measurement has been gradually overcome because it resulted to be less accurate than enzymatic activity assessment for risk stratification and because it only detects a reduced percentage of total Lp-PLA2 as strongly inhibited by interaction with lipoprotein. Different assays for the measurement of Lp-PLA2 activity in human plasma and serum have been developed and all resulted more accurate than mass measurement in evaluating the total levels of circulating Lp-PLA2. The Food and Drug Administration (FDA) approved, in December 2014, the use of the PLAC® Test (diaDexus Inc, San Francisco, CA, USA) for the Lp-PLA2 enzymatic activity determination in clinical practice to predict CVD. The PLAC® Test method takes advantage of the hydrolysis operated by the Lp-PLA2 on sn-2 position of the substrate, 1-myristoyl-2-(4-nitrophenylsuccinyl) phosphatidylcholine, generating a colored reaction product 4-nitrophenol. The rate of 4-nitrophenol production at 405 nm is monitored spectrophotometrically and Lp-PLA2 activity is calculated by the rate of change of absorbance. The PLAC® Test Activity Kit has been validated on different automatic clinical chemistry analyzers, to encourage the method harmonization process for Lp-PLA2 activity assessment, method standardization and to increase the concordance between assays.6 Manufacturer declared a cut point at 225 nmol/min/mL which identifies high cardiovascular risk patients. Universal clinically appropriate cut-points for Lp-PLA2 activity are needed to improve the assessment of cardiovascular risk.6
Lp-PLA2 in metabolic diseases
CVD and T2DM correlate with the metabolic syndrome (MetS). As described, diabetic patients are more prone to CVD. The association between Lp-PLA2 and T2DM has been also reported in the Health Professionals Follow-Up Study (HPFS) and the Nurses’ Health Study (NHS), both showing a high relative risk of CV mortality and acute myocardial infarction of 1.75 (95% confidence interval (CI): 1.05–2.92).7 Moreover, Siddiqui et al.8 found the association between high Lp-PLA2 levels and risk of death and development of diabetic retinopathy independently of other traditional risk factors. HDL could be “dysfunctional” in type 2 diabetes. Some evidences suggested that the improving hypoglycemic therapy promotes atheroprotective changes by decreasing total Lp-PLA2 activity and triggering the reorganization of Lp-PLA2 activity to a higher fraction in HDL. The oxidative modification and glycation of HDL in patients with type 2 diabetes provoke the alteration of the HDL properties. Paraoxonase 1 (PON1) and Lp-PLA2 enzymatic activity alteration linked to HDL could stimulate pro-inflammatory effects reducing the ability of HDL to suppress inflammation in diabetes.9
In patients with metabolic syndrome, a chronic low-grade inflammation is observed, and MetS patients display higher Lp-PLA2 activity.10 In these patients, pro-inflammatory cytokines could stimulate and aggravate insulin resistance in adipose tissue and muscle. Human adipose tissue and adipocytes are active sources of Lp-PLA2 in patients with T2DM. The high expression of Lp-PLA2 protein in adipocytes in obesity and type 2 diabetes may induce the increase of circulating oxidized low-density lipoprotein (oxLDL), thus leading to inflammation and atherosclerosis. The novelty found in this study is that in T2DM, the Lp-PLA2 could be considered as a novel therapeutic target to reduce inflammation, atherosclerotic risk, and the development of cardiometabolic complications.11
Bariatric surgery in obese patients improves lipoprotein profile by increasing the antiatherogenic plasma HDL-2 subfraction and reducing oxLDL and Lp-PLA2 activity.12 In a study enrolling 67 lean and 66 obese age-matched children, higher levels of Lp-PLA2 were detected in the obese subjects compared with normal-weight ones, with a positive significant correlation between Lp-PLA2 and the body mass index. These findings suggest that Lp-PLA2 could represent a link between obesity and increased cardiovascular risk.
Discussion
Metabolic syndrome is the main risk factor for diabetes and coronary disease. Scientific evidence shows that abdominal obesity, elevated blood triglycerides, high non-HDL cholesterol and apolipoprotein B, insulin resistance, hyperglycemia, and high high-sensitivity C-reactive protein (hs-CRP) are risk factors for coronary artery disease (CAD). The discovery of new and specific blood biomarkers of plaque inflammation associated with obesity-related Mets and diabetes such as Lp-PLA2 could be useful in identifying low-risk subjects that have a high probability of developing CVD, as shown in Figure 1.
Figure 1.
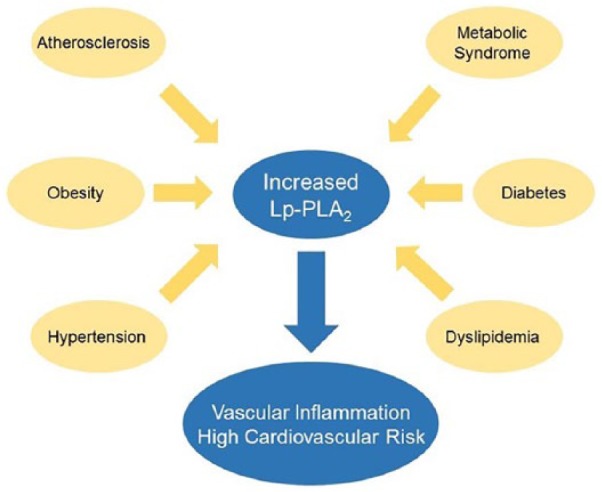
Lp-PLA2 in metabolic diseases.
Recent evidence suggests that Lp-PLA2 plays a pivotal role in the pathophysiology of atherosclerosis and as predictive biomarker for future cardiovascular events. The Lp-PLA2 assessment in the management of diabetes should be considered in addition to conventional cardiovascular risk factors in patients with type 2 diabetes undergoing drug therapy. Since Lp-PLA2 is a vascular-specific marker of inflammation, measuring Lp-PLA2 levels may be useful for a more complete evaluation of atherosclerosis in the metabolic syndrome, for an early classification of patients considered at high risk of CVD.10 The plasma concentration of C-reactive protein (CRP) reflects the low-grade inflammation and predicts the long-term risk of a coronary heart disease. CRP is accepted as a sensitive marker of chronic low-grade inflammation and predictor of atherosclerotic vascular disease evolution. Lp-PLA2 is a vascular-specific marker of inflammation, independent of other risk factors including CRP. The correlation of Lp-PLA2 with atherogenic risk is even greater considering that it is secreted by the macrophages of the atherosclerotic plaques and may well represent the transition to plaque instability. During inflammation of the arterial wall, Lp-PLA2 protein is localized within the plaque in high concentrations. The increase in Lp-PLA2 levels is associated with atherosclerotic plaque rupture and blood clots production that may cause cardiovascular events. Guidelines for dyslipidemia and CVD prevention recommend the importance of treating low-density lipoprotein cholesterol (LDL-C) and encourage the measurement of markers of vascular inflammation for risk stratification, especially in individuals with diabetes and familial hypercholesterolemia and in women and young subjects with dyslipidemia. The guidelines also suggest that individuals with T2DM, obesity or overweight, atherosclerosis, dyslipidemia, and high inflammatory biomarkers should be considered as atherosclerotic cardiovascular disease (ASCVD) patients. ASCVD risk is indicated by concomitant elevation of hs-CRP and Lp-PLA2 levels, which in combination correlate with a very high risk of CVD even in individuals with low or moderately elevated LDL-C levels.
Taken together, all these findings suggest that Lp-PLA2 could be considered as a new vascular-specific biomarker to predict CVD risk in patients with metabolic diseases.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Steven Nisticò  https://orcid.org/0000-0002-3828-0883
https://orcid.org/0000-0002-3828-0883
References
- 1. Grandl G, Wolfrum C. (2018) Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Seminars in Immunopathology 40: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tesauro M, Schinzari F, Rovella V, et al. (2008) Tumor necrosis factor-α antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care 31: 1439–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schinzari F, Tesauro M, Cardillo C. (2017) Endothelial and perivascular adipose tissue abnormalities in obesity-related vascular dysfunction: Novel targets for treatment. Journal of Cardiovascular Pharmacology 69: 360–368. [DOI] [PubMed] [Google Scholar]
- 4. Stafforini DM. (2015) Plasma PAF-AH (PLA2G7): Biochemical properties, association with LDLs and HDLs, and regulation of expression. The Enzymes 38: 71–93. [DOI] [PubMed] [Google Scholar]
- 5. Rymer JA, Newby LK. (2017) Targeting inflammation in acute coronary syndromes. JACC 4: 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Stefano A, Mannucci L, Massoud R, et al. (2017) Performance characteristics of lipoprotein-associated phospholipase A2 activity assay on the Dimension Vista analyser and preliminary study of a healthy Italian population. Biochemia Medica 27: 030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatoum IJ, Hu FB, Nelson JJ, et al. (2010) Lipoprotein-associated phospholipase A2 activity and incident coronary heart disease among men and women with type 2 diabetes. Diabetes 59: 1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddiqui MK, Kennedy G, Carr F, et al. (2018) Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: A longitudinal disease progression study. Diabetologia 61: 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, et al. (2017) The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids in Health and Disease 16: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acevedo M, Varleta P, Kramer V, et al. (2015) Comparison of lipoprotein-associated phospholipase A2 and high sensitive C-reactive protein as determinants of metabolic syndrome in subjects without coronary heart disease: In search of the best predictor. International Journal of Endocrinology 2015: 934681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackisch L, Kumsaiyai W, Moore JD, et al. (2018) Differential expression of Lp-PLA2 in obesity and type 2 diabetes and the influence of lipids. Diabetologia 61: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Julve J, Pardina E, Pérez-Cuéllar M, et al. (2014) Bariatric surgery in morbidly obese patients improves the atherogenic qualitative properties of the plasma lipoproteins. Atherosclerosis 234: 200–205. [DOI] [PubMed] [Google Scholar]


