Abstract
Introduction
Human induced pluripotent stem cells (hiPSCs) harboring cardiac myosin heavy chain 6 promoter can differentiate into functional cardiomyocytes called “iCell cardiomyocytes” under blasticidin treatment condition. While iCell cardiomyocytes are expected to be used for predicting cardiotoxicity of drugs, their responses to antiarrhythmic agents remain to be elucidated. We first examined electrophysiological properties of iCell cardiomyocytes and mRNA levels of ion channels and Ca handling proteins, and then evaluated effects of class I antiarrhythmic agents on their Na+ and Ca2+ currents.
Methods
iCell cardiomyocytes were cultured for 8–14 days (38–44 days after inducing their differentiation), according to the manufacturer's protocol. We determined their action potentials (APs) and sarcolemmal ionic currents using whole-cell patch clamp techniques, and also mRNA levels of ion channels and Ca handling proteins by RT-PCR. Effects of three class I antiarrhythmic agents, pirmenol, pilsicainide and mexiletine, on Na+ channel current (INa) and L-type Ca2+ channel current (ICaL) were evaluated by the whole-cell patch clamp.
Results
iCell cardiomyocytes revealed sinoatrial node-type (18%), atrial-type (18%) and ventricular-type (64%) spontaneous APs. The maximum peak amplitudes of INa, ICaL, and rapidly-activating delayed-rectifier K+ channel current were −62.7 ± 13.7, −8.1 ± 0.7, and 3.0 ± 1.0 pA/pF, respectively. The hyperpolarization-activated cation channel and inward-rectifier K+ channel currents were observed, whereas the T-type Ca2+ channel or slowly-activating delayed-rectifier K+ channel current was not detectable. mRNAs of Nav1.5, Cav1.2, Kir2.1, HCN4, KvLQT1, hERG and SERCA2 were detected, while that of HCN1, minK or MiRP was not. The class Ia antiarrhythmic agent pirmenol and class Ic agent pilsicainide blocked INa in a concentration-dependent manner with IC50 of 0.87 ± 0.37 and 0.88 ± 0.16 μM, respectively; the class Ib agent mexiletine revealed weak INa block with a higher IC50 of 30.0 ± 3.0 μM. Pirmenol, pilsicainide and mexiletine blocked ICaL with IC50 of 2.00 ± 0.39, 7.7 ± 2.5 and 5.0 ± 0.1 μM, respectively.
Conclusions
In iCell cardiomyocytes, INa was blocked by the class Ia and Ic antiarrhythmic agents and ICaL was blocked by all the class I agents within the ranges of clinical concentrations, suggesting their cardiotoxicity.
Keywords: iCell cardiomyocytes, class I antiarrhythmic agents, Na+ channel current, L-type Ca2+ channel current
Abbreviations: AP, action potential; APA, AP amplitude; APD, AP duration; ET-1, Endothelin 1; HP, holding potential; hiPSC, human induced pluripotent stem cell; IGF-1, insulin-like growth factor-1; MDP, maximum diastolic potential; minK, minimal potassium channel subunit; MiRP, minK related protein; MYH, myosin heavy chain; OS, overshoot potential; SAN, sinoatrial node
Highlights
-
•
iCell cardiomyocytes primarily showed ventricular-type spontaneous APs.
-
•
INa, ICaL, IKr, If and IK1, but not ICaT or IKs, were detected in the myocytes.
-
•
mRNAs of Nav1.5, Cav1.2, Kir2.1, HCN4, KvLQT1, hERG and SERCA2 were detected.
-
•
INa was blocked by the class Ia and Ic agents at clinical concentrations.
-
•
ICaL was blocked by all the class I agents at clinical concentrations.
1. Introduction
Cardiomyocytes derived from the human induced pluripotent stem cell (hiPSC) as well as the human embryonic stem cell provide new in vitro platforms for drug discovery and evaluation of cardiotoxicities of compounds. iCell cardiomyocytes are well known to be hiPSCs-derived cardiomyocytes harboring blasticidin resistance gene driven by the cardiac myosin heavy chain 6 gene (MYH6) promoter; in the presence of blasticidin, therefore, iCell cardiomyocytes expressing MYH6 are selectively purified as human ventricular myocytes [1]. Thus, the iCell cardiomyocyte is an appropriate model for evaluating adverse effects of antiarrhythmic agents on human ventricular myocytes. However, electrophysiological properties of iCell cardiomyocytes, their expressions of ion channel and Ca handling protein mRNAs, and effects of therapeutic drugs, especially class I antiarrhythmic agents, on their electrophysiological properties remain to be elucidated.
Class I antiarrhythmic agents, blocking Na+ channels, are world-widely used for patients with supraventricular and ventricular arrhythmias [2]. However, several clinical trials such as Cardiac Arrhythmia Suppression Trial (CAST-I) demonstrated that class Ic antiarrhythmic agents significantly increased the mortality of patients with myocardial infarction [3], [4]. Although mechanisms responsible for adverse effects of class Ic antiarrhythmic agents remain unknown, their negative inotropic actions may trigger proarrhythmic events by negatively affecting hemodynamic conditions of the patients [5], [6]. To predict cardiotoxicity of class I antiarrhythmic agents, heterologous cell models expressing ion channels of the human heart or animal models are frequently used in the preclinical stage; however, the differences in cell types or species hinder the accurate prediction of their cardiotoxicity in human cases.
In the present study, we confirmed the electrophysiological properties of iCell cardiomyocytes as well as their expressions of mRNAs, and examined blocking actions of class I antiarrhythmic agents on the Na+ channel current (INa) and L-type Ca2+ channel current (ICaL) in the iCell cardiomyocyte.
2. Materials and methods
2.1. Cell culture
iCell cardiomyocytes [Cellular Dynamics International (CDI), Madison, WI, USA; Lot#: CMC021544, 1290129] were cryopreserved and stored in liquid nitrogen; they were grown for 30 days by the manufacturer CDI before being shipped. For this study, single vials containing 1.5 × 106 cardiomyocytes were thawed by immersing the frozen cryovial in a 37 °C water bath; thawed cardiomyocytes were transferred into a 15-ml tube, and diluted with 10 ml of ice-cold plating medium [iCell Cardiomyocytes Plating Medium (iCPM); CDI]. iCell cardiomyocytes can be maintained in culture for 14 days using the Maintenance Medium (CDI) without appreciable loss of purity, according to the manufacturer's manual. iCell cardiomyocytes were cultured for another 8–14 days, i.e., cultured for 38–44 days after inducing differentiation, until they were subjected to patch-clamp experiments and RT-PCR analysis.
2.2. Electrophysiology
iCell cardiomyocytes (4 × 104 to 8 × 104 cells/well) were cultured on cover slips coated with fibronectin and were subjected to patch clamp experiments. The action potential (AP) and sarcolemmal ion channel currents were measured by the whole-cell patch-clamp technique with an Axopatch-200 B amplifier (Molecular Devices, San Jose, CA, USA) as described elsewhere [7]. Generation of command voltage pulses, data acquisition and data analyses were performed with the pCLAMP 9 software (Molecular Devices). Cells were perfused with normal Tyrode's solution of the following composition (mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaHPO4, 5 HEPES, and 5 glucose (pH 7.4 with NaOH). The internal pipette solution contained (in mM) 130 K-glutamate, 1 MgCl2, 15 KCl, 5 NaOH, 5 HEPES, and 5 Mg-ATP (pH 7.3 with KOH). Recording pipettes had tip resistances of <5 MΩ when filled with the internal solution. Cell membrane capacitances were 41.0 ± 4.8 pF (mean ± SEM). INa was elicited at 0.2 Hz by 300-ms depolarization pulses of −80 to +40 mV (in 10 mV increments) from a HP of −90 mV; ICaL and outward K+ currents were eliminated by 5 μM Cd2+ and 5 μM E4031 which almost completely block ICaL and the rapid component of delayed-rectifier K+ channel currents (IKr), respectively. ICaL was elicited at 0.2 Hz by 300-ms depolarization pulses of −40 to +40 mV (in 10 mV increments) from a HP of −50 mV. IKr and the slow (IKs) components of the delayed-rectifier K+ channel current were elicited every 5 s by 2-s depolarization pulses of −40 to +40 mV (in 10 mV increments) from a HP of −50 mV. The hyperpolarization-activated cation channel current (If) was elicited every 10 s by 3-s hyperpolarizing pulses of −60 to −150 mV (in 10 mV decrements) from a HP of −50 mV. In addition, the inward-rectifier K+ channel current (IK1) was recorded using a ramp pulse protocol with a function generator (FG-122, NF Corporation, Yokohama, Japan); the ramp pulses from −120 to 0 mV with a HP of −40 mV were applied at 1.5 V/s every 3 s. Most of experiments were carried out at 37 °C, while INa was measured at 27 °C.
To determine the blocking effects of class I antiarrhythmic agents on Na+ and L-type Ca2+ channels of iCell cardiomyocytes, INa and ICaL were measured during a train of 20-ms step depolarizations to −20 and 0 mV, respectively, at 0.2 Hz in the presence of the agents; the amplitudes of peak INa and ICaL reached steady-state levels at the 10th pulse, their steady-state values determined at the 20th pulse. Concentration dependence of the effects of each drug on peak INa and ICaL was fitted using the following equation:
| I% = (100−R%)·IC50h / (IC50h + [D]h) + R% | (1) |
where I% represents the percentages of peak INa and ICaL determined at −20 mV and 0 mV, respectively, in the presence of a drug at a given concentration ([D]), with those in the absence of the drug being set as 100%. R% denotes the residual (non-blocked) current (%) at higher concentrations. IC50 and h are the half-maximal inhibitory concentration and Hill coefficient, respectively. All data are presented as the mean ± SEM.
2.3. RT-PCR assay
Total RNAs of iCell cardiomyocytes were isolated using an RNeasy Micro Kit (QIAGEN, Tokyo, Japan). cDNAs were synthesized using PrimeScript® RT reagent Kit with gDNA Eraser Perfect Real Time (TaKaRa BIO, Otsu, Japan). RT-PCR was performed using EmeraldAmp® MAXPCR Master Mix (TaKaRa). Primer sequences are listed in Table 1.
Table 1.
Primer sets for RT-PCR.
| Forward (5′→3′) | Reverse (5′→3′) | |
|---|---|---|
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
| HCN1 | GGTGACAGAAAGCAGGGGTA | CTTCCACACCAGTTGGGATT |
| HCN2 | GTCCGATGGCTCCTACTTCG | TGGTTGTTGAATACGCCCGA |
| HCN4 | ACTGTGCTGGCTCAGGAGTT | CGCATTTACCGGTTTCTGTT |
| Nav1.5 | TTTGAGTCCAGTGTGGGACA | GTGACAGCCAGGCTAAAAGC |
| Cav1.2 | ATCAGCCTACCTGGTGGGAT | GGGAAGAGGGAGCTTTGCTT |
| hERG | CGCTACCACACACAGATGCT | ATGAAGTACAGGGCGGTGAG |
| KvLQT1 | AGGAAATGCTGACCCATGGG | CTTCTGTGTGTTTGGCTGGC |
| Kir2.1 | GGCACTGTTGTCATTTCCAA | AGCGGAAACCCAAAATTACC |
| mink | ATGCCTGGCAAGAGAAGGAC | CACCCCTCACCCCTTACAAC |
| MiRP | CATGAGAACATTGGTGCGGC | TGACATTCACTTGCCCGGAA |
| RYR2 | GGGGAGGGTAAGAAAAGCAG | CTGATCACAGGTGGCTGAAA |
| ITPR2 | GTCCCCAGGACTGAGAATGA | TGGTGTGCACCTCACAATTT |
| SERCA2a | TGAGACGCTCAAGTTTGTGG | CCTCTTGCAGCAAAGAAACC |
| MLC2a | ACATCATCACCCACGGAGAAGAGA | ATTGGAACATGGCCTCTGGATGGA |
| MLC2v | CCTTGGGCGAGTGAACGT | GGGTCCGCTCCCTTAAGTTT |
3. Results
3.1. Electrical properties of APs in iCell cardiomyocytes
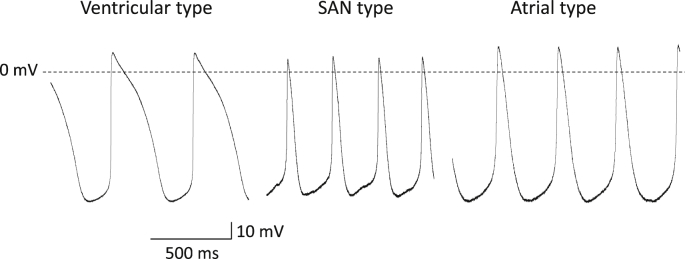
iCell cardiomyocytes revealed automaticity of sinoatrial node (SAN)-type, atrial-type and ventricular-type APs, which are classified according to the criteria of Ma et al. [1] (Fig. 1). SAN-type iCell cardiomyocytes showed clearer phase 4 depolarization than atrial-type cells. Ventricular-type APs had longer duration and the prominent plateau phase. Out of 22 cells, SAN-, atrial- and ventricular-type APs were observed in 4 (18%), 4 (18%) and 14 (64%) cells, respectively. The SAN-type APs were characterized by the maximum diastolic potential (MDP) of −52.3 ± 2.7 mV, over shoot potential (OS) of 29.8 ± 5.2 mV, AP amplitude (APA) of 72.0 ± 5.4 mV, AP duration (APD) at 50% repolarization (APD50) of 110 ± 10 ms, APD at 90% repolarization (APD90) of 260 ± 20 ms, and AP interval of 470 ± 50 ms. The atrial-type APs were characterized by the MDP of −57.2 ± 3.3 mV, OS of 26.6 ± 1.0 mV, APA of 73.7 ± 4.1 mV, APD50 of 130 ± 10 ms, APD90 of 290 ± 30 ms, and AP interval of 490 ± 60 ms. The ventricular-type APs were characterized by the MDP of −61.1 ± 3.0 mV, OS of 37.7 ± 5.3 mV, APA of 88.8 ± 7.0 mV, APD50 of 200 ± 40 ms, APD90 of 340 ± 50 ms, and AP interval of 640 ± 50 ms.
Fig. 1.
Characterization of spontaneous action potentials (APs) in iCell cardiomyocytes. Representative waveforms of ventricular-, SAN- and atrial-type APs recorded at 37 °C are shown.
3.2. Characteristics of sarcolemmal ionic currents in iCell cardiomyocytes
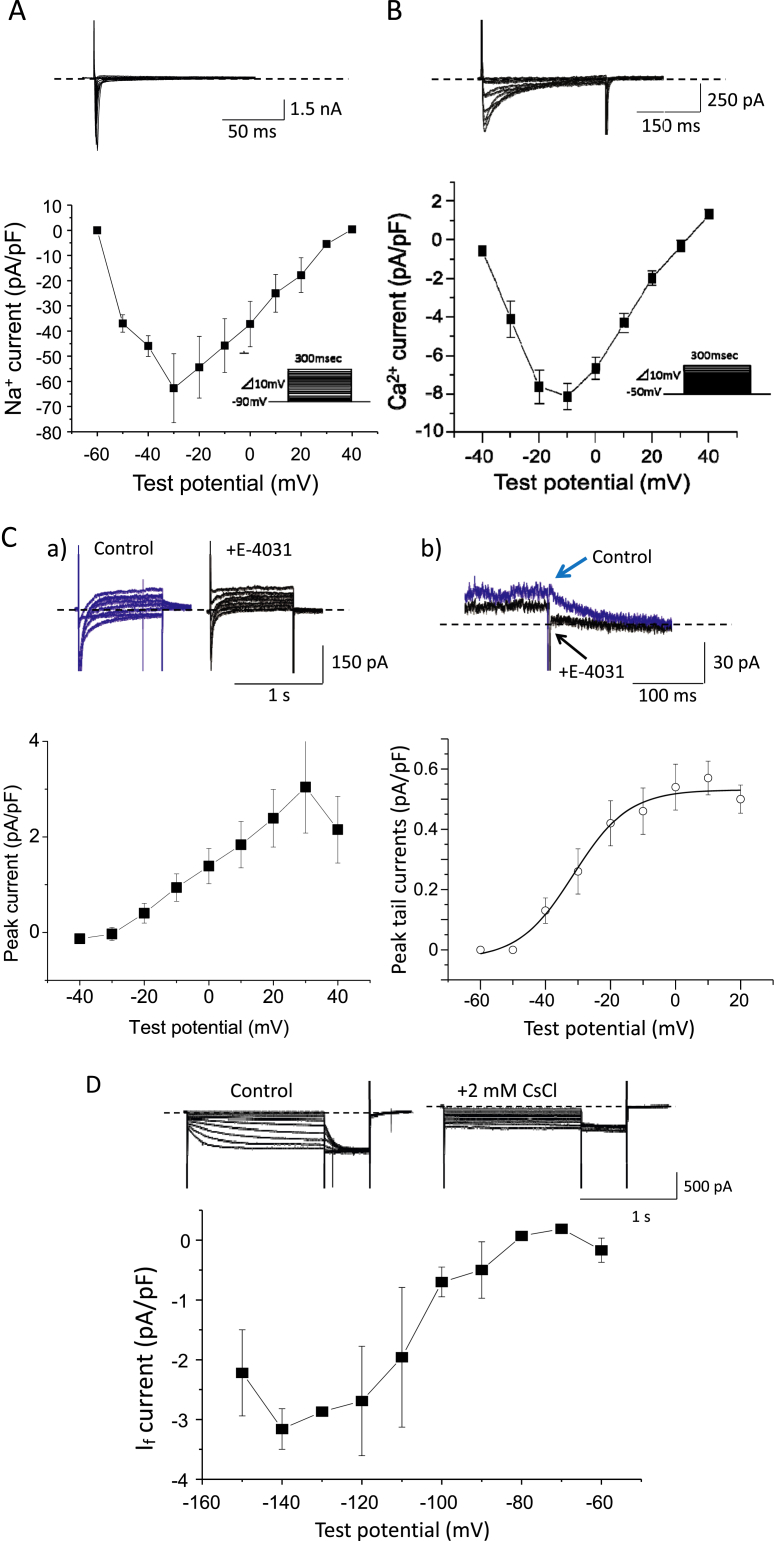
We next tried recording sarcolemmal ionic currents, INa, ICaL, T-type Ca2+ channel current (ICaT), IKr, IKs, If and IK1, in iCell cardiomyocytes. Fig. 2A shows the original traces of INa elicited by 300-ms depolarizing pulses from a HP of −90 mV. Peak current-voltage relationship of INa revealed the threshold potential of −60 mV and the maximum peak current of −62.7 ± 13.7 pA/pF at −30 mV (n = 10). Fig. 2B shows the original traces of ICaL elicited by 300-ms depolarization pulses from a HP of −50 mV. Peak current-voltage relationship revealed the threshold potential of −40 mV and the maximum peak current of −8.1 ± 0.7 pA/pF at −10 mV (n = 10). We also tried to detect ICaT using more negative HP, but could not demonstrate its presence. IKr was determined as the E4031-sensitive current, i.e., the difference between currents recorded in the absence and presence of 1 μM E4031, during 2-s depolarizing pulses from a HP of −50 mV and repolarization pulses to the HP (Fig. 2C); E4031 at 1 μM abolished tail currents during the step repolarization, isolating the E4031-sensitive IKr. Current-voltage relationship of IKr determined at the end of depolarizing pulses revealed the threshold potential of −30 mV and the maximum current amplitude of 3.0 ± 1.0 pA/pF at +30 mV. The voltage-dependence of IKr peak tail currents showed that IKr activated at the potentials positive to −40 mV to reach maximum at +10 mV, with the maximum peak tail currents of 0.57 ± 0.06 pA/pF. On the other hand, the chromanol 293 B-sensitive IKs was not observed in iCell cardiomyocytes (Supplemental Fig. S1A); chromanol 293 B did not prolong their APD (Supplemental Fig. S1B). Fig. 2D shows original traces of membrane currents elicited by 3-s hyperpolarizing pulses from a HP of −50 mV in the absence and presence of 2 mM Cs+ by which If is almost completely blocked. A threshold potential of the Cs+-sensitive If was −80 mV and the maximum currents of 3.2 ± 1.8 pA/pF were evoked at −140 mV. Membrane current recording by ramp pulses with a HP of −40 mV demonstrated the presence of IK1 with the slope conductance of 102 ± 10 pS/pF (n = 10), which was almost completely blocked by Ba2+ at 3 mM (Supplemental Fig. S2).
Fig. 2.
Characterization of sarcolemmal ionic currents in iCell cardiomyocytes. A: Representative original traces of the Na+ channel current (INa) elicited by 300-ms depolarization pulses from the HP of −90 mV (top) and current-voltage relationship for peak INa obtained from 10 cells (bottom). B: Representative original traces of the L-type Ca2+ channel current (ICaL) elicited by 300-ms depolarization pulses from the HP of −50 mV (top) and current-voltage relationship for peak ICaL obtained from 10 cells (bottom). C: Representative original traces of membrane currents elicited by 1-s depolarization pulses from the HP of −50 mV (a), as well as an expanded scale view of the tail currents during step repolarizations to the HP from 30 mV (b), in the absence (blue) and presence (black) of 1 μM E4031 are shown at the top. Note that 1 μM E4031 completely blocked the tail current. Current-voltage relationships for the E4031-sensitive current IKr determined at the end of depolarizing pulses (left) and peak tail currents during step repolarizations (right) are shown at the bottom (n = 10). D: Representative original traces of membrane currents evoked by 3-s hyperpolarizing pulses in the absence and presence of 2 mM CsCl (top) and current-voltage relationship for the Cs-sensitive If obtained from 10 cells (bottom).
3.3. mRNA expression profile of iCell cardiomyocytes
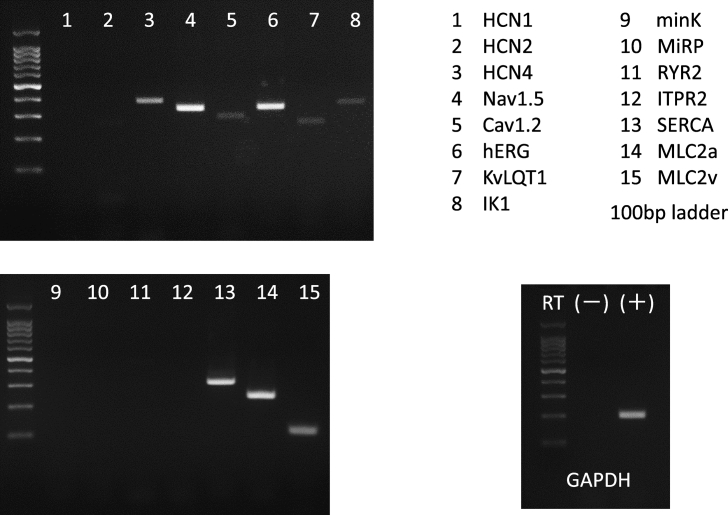
Fig. 3 shows mRNA levels of ion channels and Ca handling proteins in iCell cardiomyocytes on 44 days after differentiation. mRNAs of the ion channels Nav1.5, Cav1.2, Kir2.1, HCN4, KvLQT1 and hERG as well as the myocin light chain 2v (Mlc2v) and 2a (Mlc2a) were detected, while that of HCN1, minK or MiRP was not. With respect to Ca handling proteins, mRNAs of RyR2 and SERCA2, but not that of the inositol 1,4,5-triphosphate receptor type 2 (ITPR2), were detected.
Fig. 3.
mRNA levels of ion channels and Ca handling proteins in iCell cardiomyocytes.Target genes for RT-PCR are numbered serially (1–15), with their names listed on the top-right. PCR of GAPDH with RNA sample without reverse transcriptase (RT) showed no contamination of cDNA by genomic DNA.
3.4. Concentration-dependent inhibitory effects of class I antiarrhythmic agents on INa and ICaL in iCell cardiomyocytes
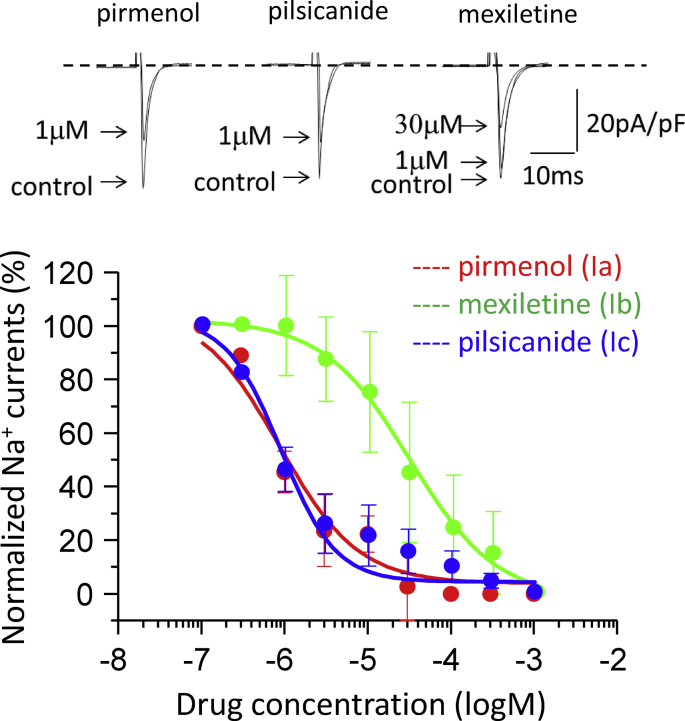
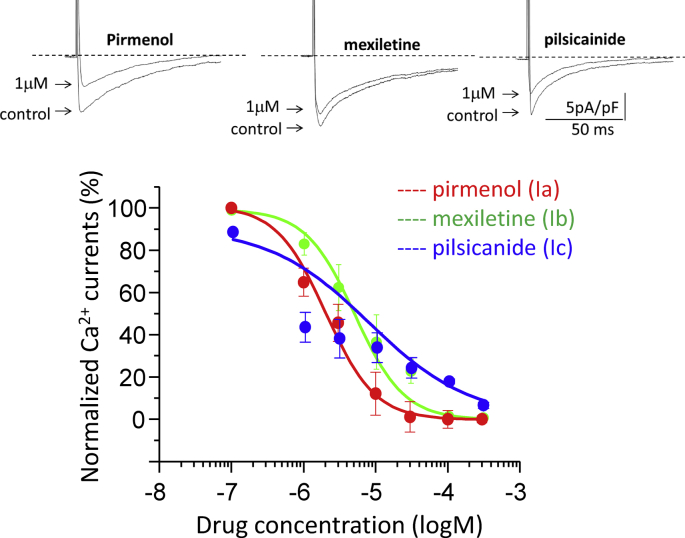
Next, we studied the concentration-dependent effects of class I antiarrhythmic agents, pirmenol (class Ia), mexiletine (class Ib) and pilsicainide (class Ic), on INa and ICaL. Fig. 4 shows the original traces of INa recorded at −20 mV in the absence and presence of these agents. Pirmenol and pilsicainide at 1 μM each remarkably blocked INa, while mexiletine at the same concentration of 1 μM did not. Concentration dependence curves obtained from multiple experiments (n = 7–11) indicated that pirmenol, mexiletine and pilsicainide blocked INa with IC50 values of 0.87 ± 0.37, 30.0 ± 3.0, and 0.88 ± 0.16 μM, respectively, and the Hill coefficients of 0.91 ± 0.32, 0.84 ± 0.56 and 1.30 ± 0.24, respectively. Fig. 5 shows the original traces of ICaL recorded at 0 mV in the absence and presence of these agents. Pirmenol and pilsicainide at 1 μM each significantly blocked ICaL, while mexiletine at the same concentration only slightly blocked ICaL. Concentration dependence curves obtained from multiple experiments (n = 7–11) showed that pirmenol, mexiletine and pilsicainide blocked ICaL with IC50 of 2.0 ± 0.4, 5.0 ± 0.1, and 7.7 ± 2.5 μM, respectively, and the Hill coefficient of 1.27 ± 0.25, 1.28 ± 0.10 and 0.62 ± 0.07, respectively.
Fig. 4.
Blocking effects of class Ia, Ib and Ic antiarrhythmic agents on INa. Top: Representative original traces of INa recorded in the absence and presence of one of the three class I antiarrhythmic agents at indicated concentrations. INa was elicited by a train of 20-ms test pulses to −20 mV from a HP of −90 mV at 0.2 Hz; steady-state currents determined at the 20th pulses are shown. Bottom: Concentration-dependent inhibitory effects of the three class I antiarrhythmic agents on INa (n = 7–10). The curves are fits by Eq. (1) with the mean IC50 and Hill coefficient values given in the text.
Fig. 5.
Blocking effects of class Ia, Ib and Ic antiarrhythmic agents on ICaL. Top: Representative original traces of ICaL recorded in the absence and presence of the class I antiarrhythmic agents at indicated concentrations. ICaL was elicited by a train of 300-ms test pulses to 0 mV from the HP of −50 mV at 0.2 Hz, with steady-state currents determined at the 20th pulses. Bottom: Concentration-dependent inhibitory effects of the three class I antiarrhythmic agents on ICaL (n = 7–10). The data were fitted by Eq. (1) with the mean IC50 and Hill coefficient values given in the text.
4. Discussion
In the present study, we confirmed the electrophysiological properties and mRNA expressions of iCell cardiomyocytes, and then examined the effects of three class I antiarrhythmic agents on INa and ICaL.
4.1. Electrophysiological properties and mRNA expression of iCell cardiomyocytes
We classified spontaneous APs of iCell cardiomyocytes into three different types of ventricular-, SAN- and atrial-type APs, based on the following criteria of Ma et al. [1]: Ventricular-type APs have larger amplitude with hyperpolarized MDP and longer APD with distinct plateau phase, while SAN- and atrial-type APs have smaller amplitude with minimal plateau phase. SAN-type APs show more depolarized MDP with more distinct phase 4 depolarization than atrial-type APs. In this study, the percentages of the ventricular-, SAN-, and atrial-type APs were 64%, 18%, and 18%, respectively, which are very close to those in the previous report of Ma et al. [1] (58%, 22% and 24%, respectively). Taken together, iCell cardiomyocytes showed predominantly ventricular-type APs, but revealed the heterogeneous characteristics including various types of cardiac phenotypes even when cells were purified using MYH6 reporter assays, as reported previously [1].
The largest and the second-largest currents in iCell cardiomyocytes recorded in the present study were INa and ICaL, respectively; the maximum peak current densities of INa and ICaL averaged −62.7 and −8.1 pA/pF, respectively, which were smaller than those in the previous reports for adult human atrial and ventricular myocytes [8], [9] as well as hiPSC-derived cardiomyocytes [1]. The APD values of ventricular-type APs were smaller in this study than in the previous reports, probably reflecting the smaller ICaL. However, the threshold and peak potentials of both currents were comparable to those in the previous studies. In the present study, we used iCell cardiomyocytes on 38–44 days after differentiation. According to a previous report [10], mRNA levels of Nav1.5 and Cav1.2 in hiPSC-derived cardiomyocytes on 45 days after differentiation were comparable to those in human adult heart tissues, suggesting that the functions of Na+ and Ca2+ channels (densities of INa and ICaL) in the hiPSC-derived cardiomyocyte may be close to those in human adult heart tissues. Thus, the iCell cardiomyocyte could be useful for evaluating the toxicity of Na+ and Ca2+ channel blockers to human hearts, e.g., their effects on contractility and conductivity of the human ventricle.
Delayed-rectifier K+ channel currents (IKr and IKs) in human cardiac myocytes were reported to be small in amplitude and in some cases not detectable [[11], [12], [13], [14], [15]]. The maximum density of peak IKr during depolarizing pulses, averaging 3.0 pA/pF at +30 mV (Fig. 2C), was larger than that in the previous report [1]; however, IKr peak tail currents were smaller than those in the previous report [1]. The smaller IKr tail current in our cardiomyocytes may be attributable to more negative repolarizing pulse of −50 mV (smaller driving force for K+) and/or different voltage-dependent kinetics of hERG channels. Viraq et al. [16] and Ma et al. [1] reported that IKs was detected in less than 50% of human ventricular myocytes and hiPSC-derived cardiomyocytes. In the present study, the IKs inhibitor chromanol 293 B little affected the APD of iCell cardiomyocytes (Supplemental Fig. S1B), which is consistent with the lack of IKs in the voltage clamp experiment (Supplemental Fig. S1A). Further experiment is necessary to determine the average density of IKs in iCell cardiomyocytes. The reasons why the amplitude of IKr tail currents was smaller than that in the previous report and why IKs was undetectable remain unknown. It has been shown that accessory proteins, minimal potassium channel subunit (minK) and minK related protein (MiRP), bind to the α subunits of KvLQT1 and hERG, and facilitate their transport to the sarcolemmal membrane [17], [18]. Therefore, impaired expressions of minK and MiRP may reduce both IKs and IKr in iCell cardiomyocytes. Further study is necessary to determine why mRNA levels of minK and MiRP were decreased in iCell cardiomyocytes.
The present study also demonstrated the presence of IK1 in ventricular-type iCell cardiomyocytes, as previously reported in human ES cell-derived cardiac myocytes [7] and iCell cardiomyocytes [1]. Larger IK1 is likely to contribute to more negative MDP values in ventricular-type iCell cardiomyocytes. The slope conductance of IK1 was 102 ± 10 pS/pF in the present study, while it was reported to be more than 500 pS/pF in human ventricular myocytes [19]; the MDP of ventricular–type spontaneous APs in iCell cardiomyocytes was much more positive than the resting potential of human ventricular myocytes, which is attributable to the smaller IK1 conductance. Since IK1 is well-known to contribute to the resting potential and phase-3 repolarization of ventricular myocytes, the effects of IK1 inhibitors on APs may be less prominent in iCell cardiomyocytes than in native human ventricular myocytes. The mRNA level of Kir2.1 in iCell cardiomyocytes on 45 days after differentiation was much less than that in human adult heart tissues [10]. Thus, in iCell cardiomyocytes on 38–44 days after differentiation, the expression of Kir2.1 would be less than that in human adult heart tissues, which is consistent with the present electrophysiological data of the reduced expression of Kir2.1 mRNA.
It is of interest that iCell cardiomyocytes expressed If channel, which is known to be encoded mainly by the HCN gene family, the marker for SAN cells [20]. Previous studies using hiPSC-derived cardiac myocytes indicated that they expressed If channels encoded by HCN1, 2 and 4. It was reported that in human ES cell-derived cardiomyocytes on 57 days after differentiation, HCN1 mRNA decreased without changes in HCN4 and the activation of If slowed, suggesting that both the reduction of HCN1 and slowed activation of If may reflect maturation of hiPSC-derived cardiac myocytes as well [21]. In the present study, iCell cardiomyocytes on 38–44 days after differentiation did not express HCN1 mRNA but expressed HCN2 and 4, suggesting that iCell cardiomyocytes reach a steady state of maturation on 38 days or later. We could not tell maturation from the aspect of If activation kinetics, since we could not precisely measure the rate of If activation at the early stage of iCell cardiomyocytes.
Taken together, the electrophysiological properties of iCell cardiomyocytes determined in the present study were quantitatively different in AP waveforms and channel current densities from those of human ventricular myocytes in the previous reports, but were qualitatively the same as in the previous reports. These results suggest that iCell cardiomyocytes could be useful for evaluating the cardiotoxicity of antiarrhythmic drugs, although their AP responses to the agents may vary depending on lots (clones).
The maturity of iCell cardiomyocytes has been evaluated by measurements of gene expression, ion channel functions, intracellular Ca2+ cycling, responsiveness to cardioactive pharmacological stimuli and metabolism [10]: on 45 days after differentiation, mRNA levels of Nav1.5 and Cav1.2 in iCell cardiomyocytes reached as high as those in the adult heart tissue, associated with increases of INa and ICaL densities as well as increased intracellular Ca2+ concentrations and mRNA level of SERCA2. Responsiveness to endothelin 1 (ET-1) and insulin-like growth factor-1 (IGF-1) can be used as a benchmark for measuring mature degrees of signaling pathways in hiPSC-derived cardiomyocytes relative to those in isolated mature human cardiomyocytes. iCell cardiomyocytes on 45 days after differentiation responded to both ET-1 and IGF-1, indicating that the myocytes acquire a functionally mature phenotype by 45 days after differentiation [10]. It has also been reported that in cardiac myocytes derived from the human ES cell (H1), mRNAs of HCN1 and HCN4 were less expressed at the late stage (day 57–110) than at the early stage (day 16–25), while mRNAs of Kir2.1, Kv4.3 and Kv1.4 increased at the late stage [21]. iCell cardiomyocytes did not express HCN1 but expressed Nav1.5, Cav1.2, Kir2.1, SERCA2 and Mlc2v, suggesting that their maturation level is comparable to that at the late stage of hiPSC-derived cardiomyocytes.
4.2. Effects of class I antiarrhythmic agents on INa of iCell cardiomyocytes
Class I antiarrhythmic agents block INa to suppress supraventricular and ventricular arrhythmias. They are categorized into 3 types of class Ia, Ib and Ic based on the Vaughan Williams classification, i.e., from the aspect of their actions on APDs of ventricular myocytes [22]. As shown in Table 2, the class Ia antiarrhythmic agent pirmenol [23] and class Ic agent pilsicainide [24] are used to treat supraventricular and ventricular arrhythmias at clinical blood concentrations of 5.23 ± 0.59 and 2.08 ± 0.53 μM, respectively. The class Ib antiarrhythmic agent mexiletine [25] is used for the treatment of ventricular arrhythmias at a clinical concentration of 6.08 ± 1.12 μM. Qu et al. [26] reported that human ES cell-derived cardiomyocytes were less sensitive to these Na+ channel blockers. Gibson et al. [27] showed that a class Ic antiarrhythmic agent, flecainide, slowed AP upstroke in iCell cardiomyocytes, but did not examine the direct action of the agent on INa. This is the first report to show the inhibitory actions of class I antiarrhythmic agents on INa in iCell cardiomyocytes. In the present study, the IC50 values of INa block by pirmenol and pilsicainide were lower than their clinical blood concentrations in patients who have been prescribed with pirmenol or pilsicainide for ventricular arrhythmias, suggesting that the class Ia and Ic antiarrhythmic agents sufficiently block INa of human ventricular myocytes in the clinical setting. On the other hand, the IC50 value of mexiletine-induced INa block (30 μM on average) was much larger than its clinical concentrations. In the present study, the steady-state amplitude of peak INa in the presence of the agents was measured at the 20th pulse during a train of the 20-ms step depolarizations at 0.2 Hz. Different potencies of the agents for INa block could be due to the differences in kinetics of the binding to and unbinding from Na+ channels [28]: Since pirmenol and pilsicainide belong to the slow kinetic drug [29], blocked Na+ channels are accumulated even when depolarization pulses are applied at the low frequency of 0.2 Hz as in the present study, suggesting that they sufficiently block INa at the clinical concentrations. In contrast, mexiletine belongs to the fast kinetic drug [29] so that blocked Na+ channels are not accumulated during the low frequency depolarizing pulses. Mexiletine would not significantly block INa of the human ventricular myocyte at a clinical concentration, although it could block INa under tachycardia or in a state of membrane depolarization.
Table 2.
IC50 values of pirmenol, mexiletine and pilsicainide block of INa and ICaL in iCell cardiomyocytes, with those of Nav 1.5-mediated currents in transfected cells and clinical concentrations of the agents.
| iCell cardiomyocytes (present study) |
Expression systems |
Clinical concentrations (Cmax) |
MW |
|||
|---|---|---|---|---|---|---|
| INa block (μM) | ICaL block (μM) | Nav 1.5 block (μM) | (ng/mL) | (μM) | (g/mol) | |
| pirmenol | 0.87 ± 0.37 | 2.00 ± 0.39 | ND | 1770 ± 200 | 5.23 ± 0.59 | 338.4864 |
| mexiletine | 30 ± 3.0 | 5.0 ± 0.1 | 159a | 1090 ± 200 | 6.08 ± 1.12 | 179.2588 |
| pilsicainide | 0.88 ± 0.16 | 7.7 ± 2.5 | 307 ± 19b | 566.8 ± 145.2 | 2.08 ± 0.53 | 272.3853 |
The inhibitory actions of class I antiarrhythmic agents on INa have been evaluated using cultured cells transfected with cDNA of human Nav1.5 α subunit. As shown in Table 2, IC50 values of INa block by mexiletine [30] and pilsicainide [31] obtained using transfected cells were much higher than their clinical concentrations, suggesting that co-expression of α and β subunits is necessary for the agents to block Na+ channels on the plasma membrane potently. Thus, iCell cardiomyocytes could be useful for evaluation of actions of class I antiarrhythmic agents on human ventricular Na+ channels, while not suitable for evaluating the action of IKs inhibitors because of the lack of minK expression and resulting very small IKs.
Na+ channel blockers are well known to decrease the intracellular Na+ activity in ventricular myocytes and thereby cause the Na+/Ca2+ exchanger-mediated reduction of the intracellular Ca2+ concentration which leads to negative inotropic effects [32]. Thus, the inhibition of INa by pirmenol and pilsicainide at clinical concentrations, stronger than that by mexiletine, may impair the cardiac contractile function through decreases in the intracellular Na+ activity.
4.3. Effects of class I antiarrhythmic agents on ICaL of iCell cardiomyocytes
It is also well known that the amount of Ca2+ releases from the sarcoplasmic reticulum depends on the sarcolemmal Ca2+ influx through L-type Ca2+ channels [33]. Therefore, the degree of ICaL block is the primary determinant of the difference in negative inotropic effects among antiarrhythmic agents. In the present study, the class I antiarrhythmic agents also blocked ICaL with different IC50 and Hill coefficient values, suggesting the binding of the class I agents to L-type Ca2+ channels with different kinetics. iCell cardiomyocytes may also be useful for evaluation of blocking actions of class I agents on human ventricular L-type Ca2+ channels. As shown in Table 2, the IC50 values of ICaL inhibitions by pirmenol, mexiletine and pilsicainide in iCell cardiomyocytes were close to their clinical concentrations (2.08–6.08 μM); the class I antiarrhythmic agents may impair the cardiac contraction by significantly blocking ICaL in the clinical setting. Taken together, the combined inhibition of INa and ICaL may explain an adverse effect, impaired contractility, of class I antiarrhythmic agents on human ventricular myocytes.
4.4. Limitation of study
There are several limitations of this study. In the present study, we used only two lots for iCell cardiomyocytes. Therefore, the differences in electrophysiological properties of iCell cardiomyocytes between the present study and previous reports may be attributed to the difference in their lots. In this study, we intended to evaluate possible inhibitory effects of class I antiarrhythmic agents on contractile functions of human ventricular myocytes via exploring their blockade of Na+ and L-type Ca2+ channels; thus, we just studied their effects on both INa and ICaL using iCell cardiomyocytes as ventricular cells selected by MYH6 expression. However, effects of the class I antiarrhythmic agents on ion channels should be tested using atrial-type cardiomyocytes as well, because these agents are used not only for patients with ventricular arrhythmias but also for those with supraventricular arrhythmias. This is the future project.
5. Conclusion
We examined electrophysiological properties, mRNA expression of ion channels as well as Ca handling proteins, and responses to class I antiarrhythmic agents of the iCell cardiomyocyte that is the hiPSC-derived cardiac myocyte. We found that the mRNA expression profile of Nav1.5, Cav1.2, Kir2.1 and HCN1 was similar to that in human adult ventricular tissues, although iCell cardiomyocytes showed a diversity of AP waveforms, i.e., ventricular-, SAN- and atrial-type APs, and expressed INa, ICaL, IKr, If and IK1, but not ICaT or IKs. It is the subject in a future study to determine whether the diversity of their AP waveforms is attributable to impaired expressions of some proteins such as minK and MiRP. This study has for the first time demonstrated that INa in iCell cardiomyocytes is significantly blocked by the class Ia antiarrhythmic agent pirmenol and class Ic agent pilsicainide at clinical concentrations, while the effect of the class Ib agent mexiletine on INa is relatively weak with the IC50 higher than clinical concentrations. ICaL in iCell cardiomyocytes was significantly blocked by all the class I agents in the range of their clinical concentrations.
Acknowledgements
This study was partially supported by Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (17K08539 to Y.S., 17K09580 to I.H.).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2018.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ma J., Guo L., Fiene S.J., Anson B.D., Thomson J.A., Kamp T.J. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamargo J., Valenzuela C., Delpón E. New insights into the pharmacology of sodium channel blockers. Eur Heart J. 1992;13:2–13. doi: 10.1093/eurheartj/13.suppl_f.2. Suppl F. [DOI] [PubMed] [Google Scholar]
- 3.The cardiac arrhythmia pilot study. The caps investigators. Am J Cardiol. 1986;57:91–95. doi: 10.1016/0002-9149(86)90958-6. [DOI] [PubMed] [Google Scholar]
- 4.Investigators CAPSC Effects of encainide, flecainide, imipramine and moricizine on ventricular arrhythmias during the year after acute myocardial infarction: the caps. Am J Cardiol. 1988;61:501–509. doi: 10.1016/0002-9149(88)90754-0. [DOI] [PubMed] [Google Scholar]
- 5.Investigators CASTC Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 6.Echt D.S., Liebson P.R., Mitchell L.B., Peters R.W., Obias-Manno D., Barker A.H. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 7.Sogo T., Morikawa K., Kurata Y., Li P., Ichinose T., Yuasa S. Electrophysiological properties of iPS cell-derived cardiomyocytes from a patient with long QT syndrome type 1 harboring the novel mutation M437V of KCNQ1. Regenerative Therapy. 2016;4:9–17. doi: 10.1016/j.reth.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J., Li G.R., Fermini B., Nattel S. Properties of sodium and potassium currents of cultured adult human atrial myocytes. Am J Physiol. 1996;270:H1676–H1686. doi: 10.1152/ajpheart.1996.270.5.H1676. [DOI] [PubMed] [Google Scholar]
- 9.Sakakibara Y., Furukawa T., Singer D.H., Jia H., Backer C.L., Arentzen C.E. Sodium current in isolated human ventricular myocytes. Am J Physiol. 1993;265:H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- 10.Ivashchenko C.Y., Pipes G.C., Lozinskaya I.M., Lin Z., Xiaoping X., Needle S. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype. Am J Physiol Heart Circ Physiol. 2013;305:H913–H922. doi: 10.1152/ajpheart.00819.2012. [DOI] [PubMed] [Google Scholar]
- 11.Beuckelmann D.J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 12.Iost N., Virág L., Opincariu M., Szécsi J., Varró A., Papp J.G. Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 1998;40:508–515. doi: 10.1016/s0008-6363(98)00204-1. [DOI] [PubMed] [Google Scholar]
- 13.Jost N., Virág L., Bitay M., Takács J., Lengyel C., Biliczki P. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 14.Konarzewska H., Peeters G.A., Sanguinetti M.C. Repolarizing K+ currents in nonfailing human hearts. Similarities between right septal subendocardial and left subepicardial ventricular myocytes. Circulation. 1995;92:1179–1187. doi: 10.1161/01.cir.92.5.1179. [DOI] [PubMed] [Google Scholar]
- 15.Schaffer P., Pelzmann B., Bernhart E., Petra L., Heinrich M., Bruno R. Repolarizing currents in ventricular myocytes from young patients with tetralogy of Fallot. Cardiovasc Res. 1999;43:332–343. doi: 10.1016/s0008-6363(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 16.Virág L., Iost N., Opincariu M., Jeno S., Janosc S., Gabor B. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 17.Sanguinetti M.C., Curran M.E., Zou A., Shen J., Spector P.S., Atkinson D.L. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 18.Nerbonne J.M. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koumi S., Backer C.L., Arentzen C.E., Sato R. beta-Adrenergic modulation of the inwardly rectifying potassium channel in isolated human ventricular myocytes. Alteration in channel response to beta-adrenergic stimulation in failing human hearts. J Clin Invest. 1995;96:2870–2881. doi: 10.1172/JCI118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikawa K., Bahrudin U., Miake J., Igawa O., Kurata Y., Nakayama Y. Identification, isolation and characterization of HCN4-positive pacemaking cells derived from murine embryonic stem cells during cardiac differentiation. Pacing Clin Electrophysiol. 2010;33:290–303. doi: 10.1111/j.1540-8159.2009.02614.x. [DOI] [PubMed] [Google Scholar]
- 21.Sartiani L., Bettiol E., Stillitano F., Mugelli A., Cerbai E., Jaconi M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cell. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan Williams E.M. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984;24:129–147. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 23.Hammill S.C., Shand D.G., Routledge P.A., Hindman M.C., Baker J.T., Pritchett E.L. Pirmenol, a new antiarrhythmic agent: initial study of efficacy, safety and pharmacokinetics. Circulation. 1982;65:369–375. doi: 10.1161/01.cir.65.2.369. [DOI] [PubMed] [Google Scholar]
- 24.Kihara Y., Inoko M., Hatakeyama N., Momose Y., Sasayama S. Mechanisms of negative inotropic effects of class Ic antiarrhythmic agents: comparative study of the effects of flecainide and pilsicainide on intracellular calcium handling in dog ventricular myocardium. J Cardiovasc Pharmacol. 1996;27:42–51. doi: 10.1097/00005344-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y., Kamibayashi M., Yamashita Y., Imai T., Tanaka H., Nakahara T. Evidence for the possible involvement of Ca2+ entry blockade in the relaxation by class I antiarrhythmic drugs in the isolated pig coronary smooth muscle. Naunyn-Schmiedeberg’s Arch Pharmacol. 2002;365:56–66. doi: 10.1007/s00210-001-0495-9. [DOI] [PubMed] [Google Scholar]
- 26.Qu Y., Vargas H.M. Proarrhythmia risk assessment in human induced pluripotent stem cell-derived cardiomyocytes using the maestro MEA platform. Toxicol Sci. 2015;147:286–295. doi: 10.1093/toxsci/kfv128. [DOI] [PubMed] [Google Scholar]
- 27.Gibson J.K., Yue Y., Bronson J., Palmer C., Numann R. Human stem cell-derived cardiomyocytes detect drug-mediated changes in action potentials and ion currents. J Pharmacol Toxicol Methods. 2014;70:255–267. doi: 10.1016/j.vascn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Hondeghem L.M., Katzung B.G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- 29.Kodama I., Toyama J., Takanaka C., Yamada K. Block of activated and inactivated sodium channels by class-I antiarrhythmic drugs studied by using the maximum upstroke velocity (Vmax) of action potential in Guinea-pig cardiac muscles. J Mol Cell Cardiol. 1987;19:367–377. doi: 10.1016/s0022-2828(87)80582-5. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K., Makita N., Sunami A., Sakurada H., Shirai N., Yokoi H. Unexpected mexiletine responses of a mutant cardiac Na+ channel implicate the selectivity filter as a structural determinant of antiarrhythmic drug access. Mol Pharmacol. 2004;66:330–336. doi: 10.1124/mol.66.2.330. [DOI] [PubMed] [Google Scholar]
- 31.Desaphy J.F., Dipalma A., Costanza T., Bruno C., Lentini G., Franchini C. Molecular determinants of state-dependent block of voltage-gated sodium channels by pilsicainide. Br J Pharmacol. 2010;160:1521–1533. doi: 10.1111/j.1476-5381.2010.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheu S.S., Blaustein M.P. Sodium/calcium exchange and regulation of cell calcium and contractility in cardiac muscle, with a note about vascular smooth muscle. The heart and cardiovascular system. 1986;1:509–536. [Google Scholar]
- 33.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.