Abstract
Objective:
To characterize disparities in childhood health outcomes by socioeconomic status (SES) and ethnicity in a mixed rural-urban US community.
Methods:
This was a retrospective population-based study of children ≤18 years of age residing in Olmsted County, Minnesota, in 2009. The prevalence rates of childhood health outcomes were determined using ICD9 codes. SES was measured using HOUSES (HOUsing-based SocioEconomic Status index) derived from real property data. Adjusting for age and sex, logistic regression models were used to examine the relationship between HOUSES, ethnicity, and prevalence of childhood health outcomes, considering an interaction between HOUSES and ethnicity. Odds ratios were calculated using the lowest SES quartile and non-Hispanic whites as the reference groups.
Results:
Of 31,523 eligible children, 51% were male and 86% were non-Hispanic white. Overall, lower SES was associated with higher prevalence of bronchiolitis, urinary tract infection, asthma, mood disorder, and accidents/adverse childhood experiences (physical and sexual abuse) in a dose-response manner (P<.04). Prevalence rates of all childhood conditions considered except for epilepsy were significantly different across ethnicities (P<.002). Racial disparities for asthma and mood disorder were greater among higher SES.
Conclusion:
Significant health disparities are present in a predominately affluent, non-Hispanic white, mixed rural-urban community. SES modifies disparities by race/ethnicity in clinically less overt conditions. Interpretation of future health disparity research should account for the nature of disease.
Keywords: Health disparities, children, socioeconomic status, ethnicity
INTRODUCTION
Associations between poverty, ethnicity, and poor health outcomes in children are well documented in the extant literature. Investigations utilizing large national databases have uncovered socioeconomic and ethnic disparities in a broad range of childhood health outcomes, including obesity1,2, asthma 2-4, diabetes5, hypertension6, obstructive sleep apnea7, cancer 8-12, infections 13-16, congenital heart disease 11,17, and critical illness 18. The American Academy of Pediatrics released a policy statement acknowledging child health to be strongly influenced by race, ethnicity, and socioeconomic status (SES) 19. As the population of children and adolescents in the United States continues to increase in both ethnic diversity and socioeconomic disadvantage, understanding the mechanism of how ethnicity and SES impact health disparities is important to improve health equity19-21.
The majority of literature regarding pediatric health disparities has been based in inner cities, and little is known regarding health disparities within a mixed rural-urban community like Olmsted County, Minnesota, where community-level factors such as easy access to health care services (about one third of the population working in the health care industry), higher health insurance coverage, and low air pollution may potentially mitigate health disparities. In addition, while previous studies are informative, methodological challenges abound. For example, individual SES measures such as maternal education and income are often unavailable in commonly used clinical datasets 22. Thus, many studies use neighborhood-level SES measures based on recent census information as a proxy for individual-level SES23,24; however, the discordance between individual and neighborhood-level SES measures is significant, resulting in misclassification (20-35%) of individual SES 23,24. To overcome the absence of SES measures in commonly used data sources, we previously developed and validated the individual-level housing-based SES index HOUSES (HOUsing-based SocioEconomic Status) 22 Furthermore, there is scant information on health disparities derived from population-based studies utilizing medical records instead of self-report. The present retrospective, population-based study sought to address these limitations and advance current understanding of pediatric health disparities using an innovative individual-level SES measure and comprehensive medical records in a self-contained health care environment.
We hypothesized that health disparities across SES and race/ethnicity exist for childhood health outcomes in our study setting, and the magnitude of racial disparities will differ by SES for clinically less overt conditions. To test this hypothesis, we specifically assessed the prevalence of clinically distinct conditions reflecting different levels of health care access and literacy. The results will have important health policy implications by providing insight into whether health disparities observed in inner cities occur in a mixed rural-urban setting and whether SES modifies the effect of race/ethnicity on disparities in childhood health outcomes.
PATIENTS AND METHODS
Study Setting and Population:
Olmsted County, Minnesota, is a mixed rural-urban county located 90 miles southeast of Minneapolis, Minnesota 25. In the 2010 United States Census, the population of Olmsted County was 86% non-Hispanic white, 5% black, 5% Asian, and 4% Hispanic 26. Poverty levels in Olmsted County were 8%, well below national (14%) and state levels (11%), with median family income significantly higher than the national average ($66,252 vs $53,046 between 2009-2013) 26. Furthermore, Olmsted County is not a Medically Underserved Area; a large proportion (27%) of residents work in health care, and 95% of adult residents have health insurance 27-29. Air quality in Olmsted County, Minnesota is significantly cleaner than metropolitan cities such as Minneapolis according to the Minnesota Air Pollution Control Agency 30. Olmsted County, Minnesota, is an ideal setting to conduct population-based epidemiologic research such as this because 98% of medical care received by county residents is delivered through Mayo Clinic, Olmsted Medical Center, and their affiliated health care facilities. The Rochester Epidemiology Project (REP) has been continuously funded since 1966 and has electronically indexed all inpatient and outpatient episodes of almost all Olmsted County residents including children (95%). For this study, we utilized the REP census to identify all children ≤18 years who resided in Olmsted County in 2009, excluding only those individuals without research authorization (<5%). The Institutional Review Boards at Mayo Clinic and Olmsted Medical Center approved this study.
Childhood Diseases:
Detailed identification algorithms for each disease were previously described31. Briefly, ICD9 codes for common acute and chronic conditions among children were extracted from the medical records of Olmsted County residents participating in the REP from January 1, 2005 through December 31, 2009 (5-year prevalence). We chose clinically distinct conditions reflecting different levels of clinical overtness and health care access and literacy for identification, allowing us to examine the nature of disparities in relation to SES and race/ethnicity. These conditions include 1) acute infectious diseases (bronchiolitis, pneumonia, urinary tract infections), 2) chronic diseases (asthma, epilepsy, mood disorders), and 3) accidents (accident in home and accidental poisoning) and adverse childhood experiences (child physical abuse and child sexual abuse). These ICD9 codes were first grouped into clinical classification codes (CCCs) proposed by the Agency for Healthcare Research and Quality-Healthcare Cost and Utilization Project 32,33. The prevalence of each CCC was measured using April 1, 2009 with a 5-year capture time frame. While adverse childhood experiences include a broad range of early childhood maltreatment exposures (household mental illness, alcoholism, drug abuse, etc)12,34, this study examined only physical and sexual abuse.
Individual SES as measured by HOUSES:
SES of the study population in 2009 was measured using the validated individual-level SES measure HOUSES. The development, initial testing, and validation of this index have been previously reported 22. Briefly, the addresses of eligible study participants were geocoded - matched to real property data of individual housing units from the county assessor’s office. Principle components factor analysis identified four real property variables (housing value, square footage of housing unit, number of bedrooms, and number of bathrooms) that were then formulated into a standardized HOUSES index score through summation of the z-score for each variable. The higher the HOUSES (z-score), the higher the SES. A broad range of health outcomes in both adults and children have been previously reported to be associated with HOUSES which include risk of low birth weight, obesity, smoking exposure at home, asthma control status, pneumococcal disease, postmyocardial infarction mortality, and rheumatoid arthritis 22,35-38.
Other variables:
In 2010, 15% of the population in both Olmsted County and Minnesota as a whole were minority 39. For this study, we categorized self-reported race/ethnicity into 4 groups: non-Hispanic white, black, Asian, and Hispanic as recommended by the National Institutes of Health 40,41. In line with a previous report, we grouped the other/unknown category with the non-Hispanic white category under the presumption that most of the patients in the other/unknown category were non-Hispanic white (86% of the Olmsted County’s population self-reported white in the 2010 census) 26,31.
Statistical Analysis:
Descriptive statistics were used to summarize socio-demographic characteristics and prevalence of each pediatric outcome. The raw HOUSES scores (z-score) were collapsed into four groups using quartiles [Q1 (lowest) to Q4 (highest)]. Distribution of demographic characteristics and prevalence of childhood conditions by HOUSES quartiles were first compared univariately using Chi-Square and Kruskal-Wallis tests (Table 1). To assess main effects of HOUSES index and race/ethnicity for each pediatric outcome, logistic regression models were applied, adjusting for age and gender (Table 2 and Table 3 [left column]). As the reference group, Q1 (the lowest quartile) for the HOUSES index and non-Hispanic white were used for each main effect model. Similar analysis for HOUSES was performed only for non-Hispanic whites (ie, single largest racial/ethnic group) to demonstrate the association of HOUSES on the prevalence of each pediatric outcome controlling for age and gender (right column of Table 3). To assess potential interactive effect between the HOUSES and race/ethnicity for each pediatric outcome, logistic regression models were used by testing an interaction term between binary HOUSES (above or below the median) and race/ethnicity, adjusting for age and gender. In this interaction analysis, binary HOUSES category (instead of quartiles) was used for improving statistical power for relatively rare outcomes such as epilepsy. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were estimated for each combination of HOUSES and race/ethnicity groups using non-Hispanic whites with HOUSES index above the median HOUSES. P<.05 was considered statistically significant, and all statistical analyses utilized the R statistical software package (version 3.4.2).
Table 1.
Sociodemographic Characteristics, Prevalence of Childhood Conditions, and Association between Variables and HOUSES as a Continuous Scale and Quartilesa, b, c, d, e
| Variable | Summary Statisticsc |
HOUSESb | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) (n=31523) |
Q1 (lowest)e
(n= 7881) |
Q2 (n= 7884) |
Q3 (n= 7879) |
Q4 (highest) (n=7879) |
P valued |
||
| Age in years, median (IQR)a | 7 (3, 12) | 5 (2, 11) | 7 (3, 12) | 7 (3, 12) | 9 (5, 13) | <.001 | |
| Gender | .96 | ||||||
| Male | 16045 (51%) | 0.19 (−2.00, 2.69) | 4026 (51%) | 4021 (51%) | 3993 (51%) | 4005 (51%) | |
| Female | 15478 (49%) | 0.20 (−1.97, 2.70) | 3855 (49%) | 3863 (49%) | 3886 (49%) | 3874 (49%) | |
| Ethnicity | <.001 | ||||||
| Non-Hispanic White | 26970 (86%) | 0.40 (−1.48, 2.99) | 5522 (70%) | 6943 (88%) | 7113 (90%) | 7392 (94%) | |
| Black | 1954 (6%) | −4.22 (−5.15, −0.14) | 1179 (15%) | 400 (5%) | 292 (4%) | 83 (1%) | |
| Asian | 1506 (5%) | −0.05 (−2.81, 2.08) | 454 (6%) | 350 (4%) | 374 (5%) | 328 (4%) | |
| Hispanic | 1093 (3%) | −4.88 (−4.97, −0.68) | 726 (9%) | 191 (2%) | 100 (1%) | 76 (1%) | |
| Prevalence of childhood conditions | |||||||
| Acute condition | |||||||
| Bronchiolitis | 3018 (10%) | −0.39 (−2.62, 1.70) | 900 (11%) | 855 (11%) | 716 (9%) | 547 (7%) | <.001 |
| Pneumonia | 3274 (10%) | 0.00 (−2.32, 2.31) | 899 (11%) | 846 (11%) | 794 (10%) | 735 (9%) | <.001 |
| Urinary tract infection | 1487 (5%) | −0.05 (−2.27, 2.21) | 421 (5%) | 375 (5%) | 368 (5%) | 323 (4%) | .004 |
| Chronic condition | |||||||
| Asthma | 3429 (11%) | 0.17 (−2.05, 2.70) | 872 (11%) | 857 (11%) | 841 (11%) | 859 (11%) | .89 |
| Epilepsy | 211 (1%) | −0.58 (−2.75, 1.84) | 65 (1%) | 55 (1%) | 49 (1%) | 42 (1%) | .14 |
| Mood disorder | 1855 (6%) | 0.39 (−1.50, 2.98) | 386 (5%) | 482 (6%) | 474 (6%) | 513 (7%) | <.001 |
| Accidents and Adverse Childhood Experiences | |||||||
| Any of accidents or adverse childhood experience | 1017 (3.6%) | −0.82 (−3.81, 1.11) | 350 (4%) | 274 (3%) | 200 (2%) | 173 (2%) | <.001 |
| Accident in home | 864 (3%) | −0.78 (−3.53, 1.17) | 291 (4%) | 240 (3%) | 179 (2%) | 154(2%) | <.001 |
| Accidental poisoning | 83 (0.3%) | −1.06 (−4.44, 0.52) | 37 (0.5%) | 19 (0.2%) | 16 (0.2%) | 11 (0.1%) | <.001 |
| Physical abuse | 22 (<0.1%) | −2.15 (−4.97, −0.18) | 11 (0.1%) | 7 (<0.1%) | 2 (<0.1%) | 2 (<0.1%) | .02 |
| Sexual abuse | 48 (0.2%) | −1.77 (−4.23, 0.55) | 21 (0.3%) | 14 (0.2%) | 6 (<0.1%) | 7 (<0.1%) | .01 |
IQR: interquartile range
HOUSES: HOUsing-based index of SocioEconomic Status
Data are presented as No. (percentage) of patients unless indicated otherwise
P value from Kruskal Wallis test (Age) or Chi-square test (all other variables) using quartiles HOUSES
Q1 is lowest quartile, Q4 is highest quartile
Table 2.
Association Between HOUSES and Prevalence of Childhood Conditions in Overall Cohort and Among Non-Hispanic Whites
| Overall Study Cohort OR (95% CI) [P Value] | Non-Hispanic White Subset OR (95% CI) [P Value] | |
|---|---|---|
| Bronchiolitis | ||
| Q1 | 1.0 (Reference) [P<.001] | 1.0 (Reference) [P<.001] |
| Q2 | 1.01 (0.92, 1.12) | 0.99 (0.88, 1.11) |
| Q3 | 0.85 (0.77, 0.95) | 0.82 (0.73, 0.92) |
| Q4 | 0.70 (0.62, 0.78) | 0.69 (0.61, 0.78) |
| Pneumonia | ||
| Q1 | 1.0 (Reference) [P=.07] | 1.0 (Reference) [P=.08] |
| Q2 | 0.97 (0.88, 1.07) | 0.95 (0.85, 1.07) |
| Q3 | 0.91 (0.83, 1.01) | 0.88 (0.79, 0.99) |
| Q4 | 0.88 (0.79, 0.98) | 0.88 (0.78, 0.99) |
| Urinary tract infection | ||
| Q1 | 1.0 (Reference) [P<.001] | 1.0 (Reference) [P<.001] |
| Q2 | 0.86 (0.74, 0.99) | 0.83 (0.71, 0.97) |
| Q3 | 0.83 (0.72, 0.96) | 0.81 (0.69, 0.96) |
| Q4 | 0.70 (0.60, 0.82) | 0.66 (0.56, 0.78) |
| Asthma | ||
| Q1 | 1.0 (Reference) [P=.008] | 1.0 (Reference) [P=.11] |
| Q2 | 0.92 (0.83, 1.02) | 0.92 (0.82, 1.03) |
| Q3 | 0.88 (0.80, 0.98) | 0.90 (0.80, 1.01) |
| Q4 | 0.84 (0.76, 0.93) | 0.87 (0.77, 0.97) |
| Epilepsy | ||
| Q1 | 1.0 (Reference) [P=.10] | 1.0 (Reference) [P=.04] |
| Q2 | 0.83 (0.58, 1.19) | 0.73 (0.49, 1.09) |
| Q3 | 0.74 (0.50, 1.07) | 0.68 (0.45, 1.01) |
| Q4 | 0.62 (0.41, 0.91) | 0.53 (0.35, 0.82) |
| Mood Disorder | ||
| Q1 | 1.0 (Reference) [P=.03] | 1.0 (Reference) [P<.001] |
| Q2 | 1.04 (0.90, 1.20) | 0.90 (0.77, 1.05) |
| Q3 | 0.96 (0.83, 1.11) | 0.81 (0.70, 0.95) |
| Q4 | 0.85 (0.74, 0.99) | 0.72 (0.62, 0.84) |
| Accidents and Adverse Childhood Experiences | ||
| Q1 | 1.0 (Reference) [P<.001] | 1.0 (Reference) [P<.001] |
| Q2 | 0.79 (0.67, 0.92) | 0.78 (0.65, 0.94) |
| Q3 | 0.57 (0.48, 0.68) | 0.57 (0.46, 0.69) |
| Q4 | 0.50 (0.41, 0.60) | 0.54 (0.44, 0.66) |
OR adjusted for age and gender and p value from likelihood ratio test
Table 3:
Association Between Ethnicity and Prevalence of Childhood Conditionsa
| Black | Asian | Hispanic | P Valueb | |
|---|---|---|---|---|
| Bronchiolitis | 1.08 (0.93, 1.26) | 0.75 (0.62, 0.91) | 1.23 (1.01, 1.48) | .002 |
| Pneumonia | 1.31 (1.14, 1.50) | 1.18 (1.00, 1.38) | 1.17 (0.97, 1.41) | <.001 |
| Urinary tract infection | 0.81 (0.63, 1.02) | 0.64 (0.46, 0.85) | 1.32 (1.00, 1.71) | <.001 |
| Asthma | 1.80 (1.58, 2.03) | 0.96 (0.80, 1.14) | 0.95 (0.76, 1.16) | <.001 |
| Epilepsy | 0.92 (0.49, 1.59) | 1.00 (0.50, 1.80) | 1.25 (0.59, 2.30) | .92 |
| Mood Disorder | 0.50 (0.38, 0.65) | 0.48 (0.34, 0.65) | 0.99 (0.73, 1.31) | <.001 |
| Accidents and Adverse Childhood Experiences | 1.47 (1.16, 1.83) | 0.75 (0.53, 1.05) | 1.97 (1.50, 2.54) | <.001 |
Data are presented as OR (95% CI) with non-Hispanic white as reference group models adjusted for age and gender
P-value from likelihood ratio test comparing all ethnic groups
RESULTS
Characteristics of Study Population:
There were 31,523 eligible study participants. The median age of the cohort was 7 years (interquartile range, IQR 3 to 12 years). Half of the cohort was male with 86% non-Hispanic white, 6% black, 5% Asian, and 3% Hispanic. The 5-year prevalence rates of the following health outcomes during the study period were as follows: bronchiolitis (10%), pneumonia (10%), urinary tract infection (UTI; 5%), asthma (11%), epilepsy (0.7%), mood disorder (6%), accident in home (3%), accidental poisoning (0.3%), child physical abuse (<0.1%), and child sexual abuse (0.2%).
SES and Prevalence of Childhood Conditions:
In the overall cohort, SES as measured by HOUSES was inversely associated with prevalence of bronchiolitis (P<.001), UTI (P<.001), asthma (P=.008), mood disorder (P=.03), and accidents/adverse childhood experiences (P<.001) when controlling for age and gender (Table 2). In the non-Hispanic white cohort, the results after adjusting for age and gender were similar to those for the overall cohort except the prevalence of asthma which became not associated with HOUSES (P=.11) while the prevalence of epilepsy was now inversely associated with HOUSES (P=.04) (Table 2). After adjusting for age, gender, and race/ethnicity, the association results of HOUSES for the whole cohort virtually remain unchanged (data not shown) except asthma and mood disorder compared to the results from non-Hispanic white cohort (see an interaction analysis below).
Ethnicity and Prevalence of Childhood Conditions:
Except for epilepsy (P=.92), prevalence rates of all childhood conditions considered were significantly different across ethnicities (all P<.002 for comparing 4 race/ethnicity groups) (Table 3). Compared to non-Hispanic whites, black children were more likely to have pneumonia [OR=1.31 (1.14, 1.50)], asthma [OR=1.80 (1.58, 2.03)], and accidents and adverse childhood experiences [OR=1.47 (1.16, 1.83)] and less likely to have mood disorder [OR=0.50 (0.38, 0.65)]. Children of Asian descent were less likely to have bronchiolitis [OR=0.75 (0.62, 0.91)], UTI [OR=0.64 (0.46, 0.85)], and mood disorder [OR=0.48 (0.34, 0.65)] than non-Hispanic white children but more likely to have pneumonia [OR=1.18 (1.00, 1.38)]. Children of Hispanic descent were more likely to have bronchiolitis [OR=1.23 (1.01, 1.48)], UTI [OR=1.32 (1.00, 1.71)], and accidents and adverse childhood experiences [OR 1.97 (1.50, 2.54)].
Interaction between SES and Ethnicity in Prevalence of Childhood Conditions:
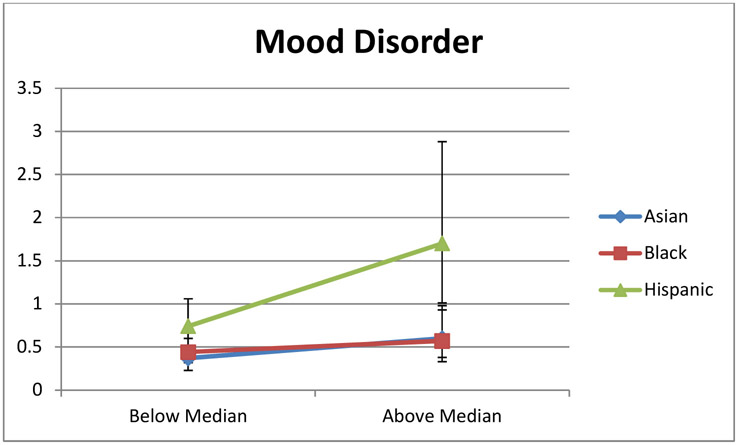
To examine whether the magnitude of racial disparities will differ by SES, we assessed interactions between SES (above or below the median HOUSES) and ethnicity in relation to prevalence of each health outcome examined. While there were no interaction between SES and ethnicity in relation to bronchiolitis, pneumonia, UTI, epilepsy, and accidents/adverse childhood experiences, SES did modify the effect of ethnicity on both asthma (P=0.03 for interaction) and mood disorder (P=0.03 for interaction) (Table 4). Compared to non-Hispanic white children with HOUSES index above median HOUSES, SES significantly increased the odds ratio for asthma prevalence in black children and adolescents from 1.62 (1.40, 1.88) for children with lower SES (HOUSES below the median) to 2.36 (1.83, 3.04) for those with higher SES (HOUSES above the median) (Table 4 and Figure 1A). While non-Hispanic white children had an attenuated risk of asthma with higher SES (compared to those with lower SES; Table 2), black children presented persistently higher prevalence of asthma even with higher SES (Table 4, Figure 1A). On the contrary, the odds ratio for prevalence of mood disorder in Hispanic children (compared to non-Hispanic white children above the median) significantly increased from 0.74 (0.52, 1.06) for children with lower SES (HOUSES below the median) to 1.70 (1.01, 2.88) for those with higher SES (HOUSES above the median) (Table 4 and Figure 1B).
Table 4:
Association Between Ethnicity, HOUSES, and Odds of Asthma and Mood Disorder in Children
| Black | Asian | Hispanic | Interaction P Value | |
|---|---|---|---|---|
| Asthma | .03 | |||
| HOUSES below Median | 1.62 (1.40, 1.88) | 0.99 (0.78, 1.26) | 0.99 (0.79, 1.23) | |
| HOUSES above Median | 2.36 (1.83, 3.04) | 0.91 (0.70, 1.18) | 0.61 (0.34, 1.09) | |
| Mood Disorder | .03 | |||
| HOUSES below Median | 0.44 (0.32, 0.60) | 0.37 (0.23, 0.60) | 0.74 (0.52, 1.06) | |
| HOUSES above Median | 0.57 (0.33, 0.98) | 0.60 (0.38, 0.93) | 1.70 (1.01, 2.88) |
OR (95% CI) and P value adjusted for age and gender
Figure 1A.
Interaction between HOUSES, Ethnicity, and Odds of Asthma. Data are presented as age and sex adjusted OR (95% CI) with non-Hispanic white as reference group. (Blue line – Asian; Red line – Black; and Green line – Hispanic).
Figure 1B.
Interaction between HOUSES, Ethnicity, and Odds of Mood Disorder. Data are presented as age and sex adjusted OR (95% CI) with non-Hispanic white as reference group. (Blue line – Asian; Red line – Black; and Green line – Hispanic).
DISCUSSION
Our study results revealed significant disparities in childhood health outcomes in a mixed rural-urban community of the United States despite the existence of community-level factors presumed to mitigate health disparities. While SES did not modify the effect of ethnicity on prevalence of more clinically overt conditions which often require immediate care (infections, epilepsy, and accidents), SES widened the disparities in asthma and mood disorder, clinically less overt diseases where disease recognition is often dependent on health care access or health literacy.
While it is widely accepted that children in inner cities living in low SES households have health disadvantages, to our knowledge this is the first population-based study using medical records and an individual SES measure to demonstrate significant health disparities among children living in a mixed rural-urban community. Among the over 30,000 children living in Olmsted County, Minnesota, in 2009, higher proportions of non-Hispanic white children were in the lowest quartiles of SES. After adjusting for age and gender, children in the lowest quartiles of SES had increased prevalence of health outcomes examined in this present study; however, health disparities across ethnicity seem to be inconsistent and contextual. While this study is consistent with the extant literature showing more adverse health outcomes in children with low SES compared to high SES 2,11,20, there are a few important noteworthy findings. First, SES as measured by HOUSES is a more robust determinant of the disease burden reflected in prevalence of childhood health outcomes than ethnicity in our study setting. Second, although clinically more overt health conditions are less dependent on health care access and literacy in identification of health conditions overall, adverse effects of lower SES on disease burden have a dose-responsive relationship which is independent of clinical overtness and other factors (age and gender). Finally, community-level factors perceived to mitigate health disparities such as access to health care services, higher health insurance coverage, low air pollution, and higher family income have little influence on the magnitude of health disparities examined in our present study across SES and ethnicity. Our study highlights that in capturing adverse effects of SES, housing-based SES measure such as HOUSES index will be a crucial tool for future health disparities research, especially capturing the emerging lower SES groups in rural or suburban populations given the epidemiology of child poverty in the United States is shifting with the fastest and largest increases in poverty since 2008 occurring in the suburbs 21.
Another aim of our present work was to examine whether an increase of SES reduces health disparities in the disease burden across ethnicity. There was no interaction between SES and ethnicity in the burden of clinically more overt diseases; however, an increase of SES widened the disparities in asthma prevalence between black and non-Hispanic white children (Figure 1A) and the disparities in prevalence of mood disorder between Hispanic and non-Hispanic white children (Figure 1B). These results may suggest a higher SES may not necessarily pose a mitigating effect on disparities across ethnicity. Also, the effect modification on health disparities across ethnicity by SES depends on the nature of health outcomes and certain race/ethnic background. For example, while disparities in clinical overt diseases (eg, UTI) across ethnicity might be less dependent on changes of SES, disparities in clinically less overt diseases (eg, mood disorder and perhaps asthma) might be more influenced by changes in SES among Hispanic children. This is conceptually supported by a recent Finnish study that examined prostate cancer risk by SES and found that patients with higher SES had an increased incidence of low-moderate risk prostate cancer and decreased incidence of advanced prostate cancer, supporting a notion that patients with higher SES may identify a disease in an early stage when it is clinically less overt 42. Finally, as our study used ICD codes to measure prevalence of health outcomes, prevalence of health outcomes by ICD codes may represent both risk of disease and detection of disease. Therefore, it is difficult to know which constructs (eg, exposure to risk factors vs. health care access) ethnicity represents as it interacts with SES in clinically less overt diseases. There is limited data available addressing whether SES modifies the effect of ethnicity on the disease burden in children using distinct clinical conditions. A population-based, prospective study in an urban community found black children were twice as likely as whites to be readmitted for asthma and >40% of the disparity could be explained by SES and hardships measured by self-reported income, caregiver educational attainment, and hardships 43. While supportive of our hypothesis that SES modifies the reported adverse effect of non-Hispanic white ethnicity on prevalence of childhood health outcomes, no studies using similar methodologies to our study are available to our knowledge for direct comparison of our study results.
The strengths of this study include population-based design, self-contained health care environment, and comprehensive identification of diseases based on documented physician-diagnoses obtained from the REP, a county-wide medical record linkage system, and objectively measured SES based on individual real property data. Since address information was utilized for HOUSES, the possibility exists for future geospatial analysis for hot spots for diseases of interest and their corresponding targeted interventions. The main limitation of our study is the inability to verify and validate the ICD9 codes; however, using ICD9 codes stored in electronic medical records to estimate prevalence rates may be an inexpensive and still valuable way to monitor population health over time31. Our study has inherent limitations as a retrospective, crosssectional study. Finally, given the unique epidemiological advantages of our study setting, generalizability of our study findings to other mixed rural-urban settings may be limited. However, given the unique strengths of the present study and paucity of pediatric population-based health disparity studies based on the comprehensive medical records and validated, individual-level SES measures, the study findings are an important addition to the current literature.
Conclusion
In conclusion, significant health disparities are present in both clinically more and less overt health outcomes across SES and ethnicity among children living in a mixed rural-urban community. While health disparities across ethnicity are inconsistent and contextual, the association between SES and the burden of adverse health outcomes is dose-dependent with SES interacting with ethnicity for clinically less overt conditions. Interpretation of research findings on health disparities in the future should account for disease nature, and HOUSES is useful for identifying vulnerable children in the community.
What’s New:
It is well documented that US children living in low SES households in inner cities are at risk of poor health outcomes. This study reveals significant disparities in health outcomes also exist among children living in a predominately affluent mixed rural-urban community.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases (R21 AI101277), The National Heart, Lung and Blood Institute (R01 HL126667) and the Scholarly Clinician Award from the Mayo Foundation. Also, this study was made possible using the resources of the Rochester Epidemiology Project which is supported by the National Institute on Aging of the 21 National Institutes of Health under Award Number R01AG034676.
Abbreviations:
- SES
Socioeconomic status
- HOUSES
HOUsing-based SocioEconomic Status index
- ICD-9
International Classification of Diseases, Ninth Revision
- REP
Rochester Epidemiology Project
- UTI
Urinary Tract Infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Young Juhn is the Principal Investigator of the Innovative Asthma Research Methods Award from Genentech which has no relationship with the work presented in this manuscript. The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Human Subjects:
The Institutional Review Board at Mayo Clinic reviewed and approved this study.
REFERENCES
- 1.Datar A, Chung PJ. Changes in Socioeconomic, Racial/Ethnic, and Sex Disparities in Childhood Obesity at School Entry in the United States. JAMA Pediatr. July 2015;169(7):696–697. [DOI] [PubMed] [Google Scholar]
- 2.Seith DKC. Who are America's Poor Children? Examining Health Disparities by Race and Ethnicity. National Center for Children in Poverty. 2011. [Google Scholar]
- 3.Hughes HK, Matsui EC, Tschudy MM, Pollack CE, Keet CA. Pediatric Asthma Health Disparities: Race, Hardship, Housing, and Asthma in a National Survey. Acad Pediatr. November 19 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill TD, Graham LM, Divgi V. Racial disparities in pediatric asthma: a review of the literature. Curr Allergy Asthma Rep. February 2011;11(1):85–90. [DOI] [PubMed] [Google Scholar]
- 5.Borschuk AP, Everhart RS. Health disparities among youth with type 1 diabetes: A systematic review of the current literature. Fam Syst Health. September 2015;33(3):297–313. [DOI] [PubMed] [Google Scholar]
- 6.Kaczmarek M, Stawinska-Witoszynska B, Krzyzaniak A, Krzywinska-Wiewiorowska M, Siwinska A. Who is at higher risk of hypertension? Socioeconomic status differences in blood pressure among Polish adolescents: a population-based ADOPOLNOR study. Eur J Pediatr. November 2015;174(11):1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. May 2011;158(5):789–795 e781. [DOI] [PubMed] [Google Scholar]
- 8.Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA Pediatr. December 1 2015;169(12):1096–1104. [DOI] [PubMed] [Google Scholar]
- 9.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988-2011: A population-based observational study. Pediatr Blood Cancer. October 2015;62(10):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petridou ET, Sergentanis TN, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. March 2015;26(3):589–597. [DOI] [PubMed] [Google Scholar]
- 11.Flores G, Committee On Pediatric R. Technical report--racial and ethnic disparities in the health and health care of children. Pediatrics. April 2010;125(4):e979–e1020. [DOI] [PubMed] [Google Scholar]
- 12.Holman DM, Ports KA, Buchanan ND, et al. The Association Between Adverse Childhood Experiences and Risk of Cancer in Adulthood: A Systematic Review of the Literature. Pediatrics. November 2016;138(Suppl 1):S81–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedaghat AR, Cunningham MJ, Ishman SL. Regional and socioeconomic disparities in emergency department use of radiographic imaging for acute pediatric sinusitis. Am J Rhinol Allergy. Jan-Feb 2014;28(1):23–28. [DOI] [PubMed] [Google Scholar]
- 14.Spicer JO, Thomas S, Holst A, Baughman W, Farley MM. Socioeconomic and racial disparities of pediatric invasive pneumococcal disease after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. February 2014;33(2):158–164. [DOI] [PubMed] [Google Scholar]
- 15.Yousey-Hindes KM, Hadler JL. Neighborhood socioeconomic status and influenza hospitalizations among children: New Haven County, Connecticut, 2003-2010. Am J Public Health. September 2011;101(9):1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DF, Boss EF. Racial/ethnic and socioeconomic disparities in the prevalence and treatment of otitis media in children in the United States. Laryngoscope. November 2010;120(11):2306–2312. [DOI] [PubMed] [Google Scholar]
- 17.Kucik JE, Nembhard WN, Donohue P, et al. Community socioeconomic disadvantage and the survival of infants with congenital heart defects. Am J Public Health. November 2014;104(11):e150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein D, Reibel M, Unger JB, et al. The effect of neighborhood and individual characteristics on pediatric critical illness. J Community Health. August 2014;39(4):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng TL, Goodman E, Committee on Pediatric R. Race, ethnicity, and socioeconomic status in research on child health. Pediatrics. January 2015;135(1):e225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Policy statement--health equity and children's rights. Pediatrics. April 2010;125(4):838–849. [DOI] [PubMed] [Google Scholar]
- 21.Poverty and Child Health in the United States. Pediatrics. March 9 2016. [DOI] [PubMed] [Google Scholar]
- 22.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. October 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narla NP, Pardo-Crespo MR, Beebe TJ, et al. Concordance between Individual vs. Area-Level Socioeconomic Measures in an Urban Setting. J Health Care Poor Underserved. November 2015;26(4):1157–1172. [DOI] [PubMed] [Google Scholar]
- 24.Pardo-Crespo MR, Narla NP, Williams AR, et al. Comparison of individual-level versus area-level socioeconomic measures in assessing health outcomes of children in Olmsted County, Minnesota. J Epidemiol Community Health. April 2013;67(4):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingram DD, Franco SJ. 2013 NCHS Urban-Rural Classification Scheme for Counties. Vital Health Stat 2. April 2014(166):1–73. [PubMed] [Google Scholar]
- 26. United States Census. 2010 https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF.
- 27.HRSA Data Warehouse. United States Department of Health and Human Services. http://datawarehouse.hrsa.gov/tools/analyzers/MuaSearchResults.aspx.
- 28. Olmsted County, Minnesota Community Health Needs Assessment. 2013 https://www.co.olmsted.mn.us/OCPHS/reports/Pages/CommunityHealthNeedsAssessment.aspx.
- 29.Health Resources and Services Administration, U.S. Department of Health and Human Services. http://muafind.hrsa.gov/index.aspx.
- 30.Minnesota Pollution Control Agency. https://www.pca.state.mn.us/air.
- 31.St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. January 2013;88(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey HC-120, Appendix 3: Clinical Classification Code to ICD-9-CM Code Crosswalk. . MEPS website. http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h120/h120_icd9codes.shtml. Accessed February 7, 2011.
- 33.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project: Clinical Classifications Software (CCS) for ICD-9-CM. HCUP website. . HCUP website. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Updated August 30, 2012. Accessed February 1, 2012.
- 34.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. May 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 35.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. April 2013;141(4):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang DW, Manemann SM, Gerber Y, et al. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. Int J Environ Res Public Health. November 2014;11(11):11597–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJ. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ open. 2015;5(4):e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Census Bureau, 2010. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Accessed Sep 8, 2014.
- 40.Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM. Racial and ethnic disparities among enrollees in Medicare Advantage plans. The New England journal of medicine. December 11 2014;371(24):2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes 2015; http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html#sthash.OD1zPDgj.dpuf.
- 42.Kilpelainen TP, Talala K, Raitanen J, et al. Prostate Cancer and Socioeconomic Status in the Finnish Randomized Study of Screening for Prostate Cancer. Am J Epidemiol. October 24 2016. [DOI] [PubMed] [Google Scholar]
- 43.Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. March 2014;133(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]