Abstract
Neurodegenerative diseases have two general characteristics that are so fundamental we usually take them for granted. The first is that the pathology associated with the disease only affects particular neurons (‘selective neuronal vulnerability’); the second is that the pathology worsens with time and impacts more regions in a stereotypical and predictable fashion. The mechanisms underpinning selective neuronal and regional vulnerability have been difficult to dissect, but the recent application of whole-genome technologies, the development of mouse models that reproduce spatial and temporal features of the pathology, and the identification of intrinsic morphological, electrophysiological, and biochemical properties of vulnerable neurons are beginning to shed some light on these fundamental features of neurodegenerative diseases. Here we detail our emerging understanding of the underlying biology of selective neuronal vulnerability and outline some of the areas in which our understanding is incomplete.
The clinical manifestation of a particular neurodegenerative disease reflects the region of the brain and the specific population of cells within it that are affected. Why proteins that usually show widespread expression should accumulate in one set of cells but not in apparently similar neighboring cells is a fundamental question for the field. Addressing the basis of this selective neuronal vulnerability will not only help explain the molecular underpinnings of neurodegenerative diseases, but will also inform on the basic biology and complexity of neuronal sub-types that we currently have little appreciation for. In this review, we describe the neuropathology of several neurodegenerative diseases, focusing on cell types impacted, and we relate what is known about the cell types and pathways that may underlie their vulnerability. We propose that vulnerable neurons have a higher propensity to accumulate disease-related proteins and organelles due to their intrinsic anatomy and biochemistry. Because of this, vulnerable neurons can be considered to teeter on the brink of a ‘catastrophic cliff’. For many of the protein aggregation disorders, whether a neuron falls off the cliff is largely dependent on the solubility of the aggregation-prone protein and the efficiency of clearance mechanisms that keep misfolded or aggregated proteins in check. Correspondingly, genetic variability in the expression level of the deposited protein is important in pathogenesis, and risk factors for disease are often related to intrinsic or extrinsic protein-clearance pathways. Interacting with these variations in expression level and clearance mechanisms are the propagation properties of the deposited proteins, which contribute to the spread of induced neuronal failure. Concepts related to the biochemistry and anatomy of different cell types are discussed here in terms of why a particular cell may be predisposed to accumulate toxic proteins and degenerate, coupled with insight from genetics.
Types of neurons vulnerable to proteinopathy and degeneration
The major neurodegenerative diseases differ from each other not only in the type of pathological protein that accumulates but also in the regions impacted and the types of neurons that are vulnerable. Vulnerability usually refers to vulnerability to pathology, and in most cases, cells that accumulate pathology-associated proteins are also the cells that are lost as the result of cytotoxic events (for example, synaptic toxicity, cell-death-related signaling pathways, or neuroinflammation). For the purpose of this review, we refer to vulnerability as selective, but in many cases it is differential, with cells showing varying degrees of susceptibility.
Alzheimer’s disease.
The brain of patients with Alzheimer’s disease (AD) is characterized by the presence of amyloid plaques composed of β-amyloid (Aβ) and the presence of neurofibrillary tangles composed of misfolded, hyperphosphorylated tau protein. Amyloid deposition typically starts in the terminal fields of neurons expressing high levels of the gene encoding amyloid precursor protein (APP)1. However, selective loss of vulnerable neurons in early AD is more closely linked to tau pathology. Neurons that are vulnerable to the accumulation of pathological forms of tau and that are lost early in the disease mainly include large pyramidal neurons in layer II of the entorhinal cortex (EC), the subiculum, the CA1 region of the hippocampus2–5, corticopetal cholinergic neurons in the basal forebrain6,7, and noradrenergic neurons in the locus coeruleus8,9. Moreover, reelin-immunoreactive excitatory neurons in layer II of the EC5,10 and pyramidal neurons immunoreactive to SMI32 (nonphosphorylated medium and heavy neurofilament proteins)11,12 are particularly vulnerable in AD, whereas inhibitory neurons expressing calcium-binding proteins (parvalbumin, somatostatin, calbindin-D28k, and calretinin) are less vulnerable in AD or animal models of AD13–15. Dentate gyrus granule neurons, deeper parts of layer III and layers V and VI of EC, and cortical interneurons are relatively spared in early AD2,16,17. Interestingly, a comparison between rostral neurons (which express neuronal markers with forebrain cortical fates that are vulnerable in AD) and caudal neurons (which express classical markers of hindbrain and spinal cord and are relatively spared in AD) derived from induced pluripotent stem cells (iPSCs) of patients harboring an APP mutation revealed that both the generation of Aβ and the responsiveness of tau to Aβ were affected by neuronal cell type. Rostral neurons, which represent a mix of excitatory and inhibitory cells, were more sensitive than caudal (predominantly inhibitory) neurons, suggesting that cell-autonomous factors may, in part, dictate the pattern of selective regional vulnerability in human neurons in AD18.
Parkinson’s disease.
The motor manifestations of Parkinson’s disease (PD) are primarily linked to the selective and progressive loss of substantia nigra pars compacta (SNpc) dopaminergic neurons19. Interestingly, even within the SNpc there is a major loss (~90%) of dopaminergic neurons in the ventral tier, while dopaminergic neuron loss in the dorsal tier may be as little as 25%20,21. In contrast, the very similar dopaminergic neurons in the ventral tegmental area (VTA) demonstrate a much lower degree of degeneration19. Neuronal loss is apparent in a handful of other regions. For example, cholinergic neurons in the basal forebrain22 and in the pedunculopontine nucleus are lost, but not glutamatergic or GABAergic pedunculopontine nucleus neurons23. There is also a modest loss of glutamatergic neurons in the intralaminar nuclei of the thalamus and the basolateral amygdala24,25. Of note, GABAergic neurons, regardless of where they are, appear to be resistant to Lewy pathology20,26. Although nigral dopaminergic neurons have been the primary focus of PD research, they are not the first affected. Instead, neurons in the dorsal motor nuclei of the medulla oblongata, raphe nucleus, and locus coeruleus of the brainstem, as well as in the anterior olfactory nucleus, succumb first27,28. The loss of nigral dopaminergic neurons is followed by degeneration of neurons in the transentorhinal region, motor and sensory cortex, and prefrontal cortex17.
Amyotrophic lateral sclerosis.
Motor neurons (MNs) in spinal cord and in brainstem, as well as upper MNs in the motor cortex, are selectively vulnerable in amyotrophic lateral sclerosis (ALS). Among MNs, fast-fatigable MNs are particularly vulnerable in ALS, while slow MNs are least vulnerable19,29–31. Furthermore, the motor neurons of Onuf’s nucleus, as well as the oculomotor, trochlear, and abducens nerves, remain largely unaffected by cell loss even at late disease stages19,32–34. The degeneration of MNs in ALS is often initiated in, and progresses from, the lower to upper spinal cord, followed by loss of upper MNs in the cerebral cortex, although there is considerable variability among patients17,35.
Frontotemporal lobar degeneration.
Frontotemporal lobar degeneration (FTLD) affects the pregenual anterior cingulate cortex (ACC), extending back to midcingulate cortex, whereas AD involves posterior cingulate regions and spares the ACC. ACC and frontal insula deficits best differentiate behavioral variant (bv) FTLD from AD36,37. Large bipolar spindle-shaped von Economo neurons (VENs) in layer Vb of those regions have been shown to represent an early target in bvFTLD but not in AD. In bvFTLD, a 69% reduction in VENs was found after controlling for neighboring layer 5 neuron loss38. This VEN selectivity was seen even in patients with early-stage disease. In contrast to bvFTLD, late stage AD (Braak stage VI) showed no selective loss of ACC VENs. Tangles have yet to be seen in VENs39. Pick’s disease is a rare cause of FTLD in which pyramidal neurons in the hippocampus and granular neurons in the dentate fascia are particularly vulnerable40.
Huntington’s disease.
Huntington’s disease (HD) selectively affects medium spiny GABAergic neurons (MSNs) in the striatum, whereas large aspiny cholinergic interneurons and other striatal interneurons that express parvalbumin, calretinin, or nitric oxide synthase are relatively spared from degeneration in HD31,41,42. Even within MSNs, those neurons expressing D2-type dopamine receptors, metenkephalin, or neurotensin are particularly vulnerable in HD, whereas MSNs expressing predominantly D1-receptors, substance P, and dynorphin are relatively spared in the early stages of the disease31,41,43,44.
Primary and secondary selective neuronal vulnerability
There are potentially two types of selectively vulnerable cell types: those affected in the initial stage of the disease (primary vulnerable cells) and those affected later, in regions where the pathology has spread to (secondary vulnerable cells). For AD, PD, and ALS, the distribution of pathology at different stages of the disease has been mapped out using cross-sectional, postmortem immunohistochemistry analysis27,45–48. Pathology-mapping studies suggest that disease proteins accumulate in regions of primary vulnerability and spread (propagate) to regions of secondary vulnerability along anatomical connections48,49. Numerous studies suggest that the propagation of pathology, especially for AD and PD, occurs through a protein-templating mechanism whereby conformational ‘seeds’ of a pathological protein are transmitted from cell to cell in a prion-like manner50–54. Whether prion-like proteins propagate in the same way in the human brain has been difficult to validate, but the fact that affected regions are connected supports the idea. It will be interesting to determine whether the physiological factors that make a cell vulnerable to accumulating pathological proteins in primary areas are the same as those in cells in secondary areas that develop pathology later as a result of propagation.
conformational strains and selective vulnerability
One of the intriguing features of neurodegenerative diseases that are caused by the same protein is the observation of clinical diversity. For example, the ‘4R tauopathies’ (which include progressive supranuclear palsy, corticobasal degeneration, and argyrophilic grain disease) are all caused by the accumulation of a form of tau protein that contains four microtubule binding domain repeats (4R tau). The tau protein in 4R tauopathies can take on different conformations, impact different neuronal and non-neuronal cell types, accumulate in different areas of the brain, and cause different clinical manifestations55–57. Thus, the conformation of a given protein has been postulated to dictate the patterns of cell pathology, progression rate, and regional and neuronal vulnerability, but the basis of this structure-driven cellular vulnerability remains unknown. Recently, sophisticated techniques such as cryo-electron microscopy have been applied to elucidate the ultrastructure of both Aβ1−42 and tau filaments58–60, which, when combined with cellular vulnerability studies, may help explain why diseases such as the 4R tauopathies are so clinically diverse. Identifying whether the formation of particular strains is determined by genetic variants associated with particular cell types may help explain how conformational strains originate in the first place.
Potential mechanisms of selective neuronal vulnerability
Insight from genomics.
With whole-genome expression studies and the development of tissue-specific and cell-type-specific expression databases, the pathogenic loci identified by genetic analysis can be used as seeds to identify other genes that show similar expression patterns, and these can then be cross referenced with gene ontology databases to pull out networks of genes with related functions and expression profiles61,62. These networks can be systematically investigated to identify other loci genetically involved in disease pathogenesis. As examples, the TREM2 module of co-expression in response to Aβ deposition in transgenic mice includes many other AD genetic risk loci that are largely expressed by microglia63. Similarly, PD Mendelian and risk loci are involved in mitophagy (Table 1)64. For several neurodegenerative syndromes, many loci map to particular biochemical pathways and are expressed in particular cell types (Tables 1 and 2). Identifying genetic loci can lead to identification of critical and intrinsic pathways that are close to failure in the normal brain and that fail in the diseased brain, for example, the endosome–lysosome system in diseases in which pyramidal neurons are lost or RNA metabolism in motor neurons (Table 1).
Table 1 |.
Risk loci and pathway implicated for the major neurodegenerative diseases
| Disease | Gene associated with risk or disease-causing mutation | Pathways |
|---|---|---|
| AD | APP, PSEN1, PSEN2, APOE, CR1, CLU, BIN1, ABCA7, INPP50, CD2AP, EPHA1, MS4A6A, PICALM, CD33, HLA, PTK2B, SORL1, SLC24A4, DSG2, MEF2C, NME8, ZCWPW1, SP11, FERMT2, CASS4, TREM2, ABI3, PLCG2 | Lipid metabolism Innate immunity Endosome-lysosome Ubiquitin proteasome |
| PD | SNCA, PKRN, PINK1, DJ-1, LRRK2, ATP13A2, PLA2G6, FBX07, VPS35, DNAJC6, SYNJ1, DNAJC13, VPS13C, RAB39B, GBA, NUCKS1, ITPKB, SIPA1L2, ILR2, TMEM163, SCNA3A, STK39, SATB1, NCKIPSD, ALS1, CHMP2B, MCCC1, TMEM175, FAM200B, FAM47E, ANK2, ELOVL7, ZNF184, HLA, KLHL7, CTSB, MICU3, SORBS3, SH3GL2, FAM171A1, BAG3, DLG2, MIR4697, OGFOD2, GCH1, TMEM229B, GALLC, CCQ7, ZNF846 TOX3, ATP6VOA1, MAPT, SYT4, LSMT, DDGK1, COMT | Endosome-lysosome Inflammation (adaptive immunity) Mitophagy Dopamine metabolism Vesicle fusion |
| ALS | C9orf72, TARDBP (TDP-43), SOD1, FUS, KIF5A, DCTN1, MATR3, TIA1, CHCHD10, VCP, SQSTM1 (p62), OPTN, UBQLN2, TBK1, CCNF, MOBP, SCFD1, SARM, UNC13A, C21orf2 | Axonal transport Mitophagy DNA and RNA metabolism Autophagy and ubiquitin proteasome Toxic aggregation |
| FTD | C9orf72, GRN, MAPT, CHMP2B, CHCHD10, VCP, SQSTM1 (p62), OPTN, UBQLN2, TBK1, CCNF, HLA, TMEM106B, CTSC | Endosome and lysosome Autophagy and lysosomal pathway Mitochondrial damage Toxic aggregation Inflammation (adaptive immunity) |
| HD | HTT, MSH3, MTRNR2L2, DHFR | DNA mismatch repair |
Bold font indicates Mendelian genes, italics indicates risk loci, and bold italics indicates that the locus appears in both categories. FTD, frontotemporal dementia.
Table 2 |.
Regions and neurons vulnerable in neurodegenerative diseases
| Disease | Protein aggregates | Early-affected regions | Early vulnerable neurons |
|---|---|---|---|
| AD | Aβ42, Tau | LC, TEC, EC, BF, HP | Pyramidal neurons in EC-II & HP-CA1; cholinergic neurons in BF, noradrenergic neurons in LC |
| PD | α-synuclein | OB, DMV, SNpc | Dopaminergic neurons |
| ALS | TDP-43, SOD1, FUS, DPRs | MNC, SC, BS | Fast-fatigable motor neurons |
| bvFTLD | Tau, TDP-43, FUS | ACC, FI | VENs |
| PiD | Tau | HP, DG | Pyramidal in HP, granular neurons in DG |
| HD | Huntingtin | ST | MSNs |
PiD, Pick’s disease; Aβ42, Aβ peptide (1–42); Tau, microtubule-associated protein tau; TDP-43, TAR DNA-binding protein 43; FUS, RNA-binding protein fused in sarcoma; DPRs, dipeptide repeat proteins related to C9orf72; TEC, transentorhinal cortex; BF, basal forebrain; EC-II, entorhinal cortex layer II; CA1, Cornu Ammonis area 1 of hippocampus.
Mechanisms that have been linked to vulnerable cell populations.
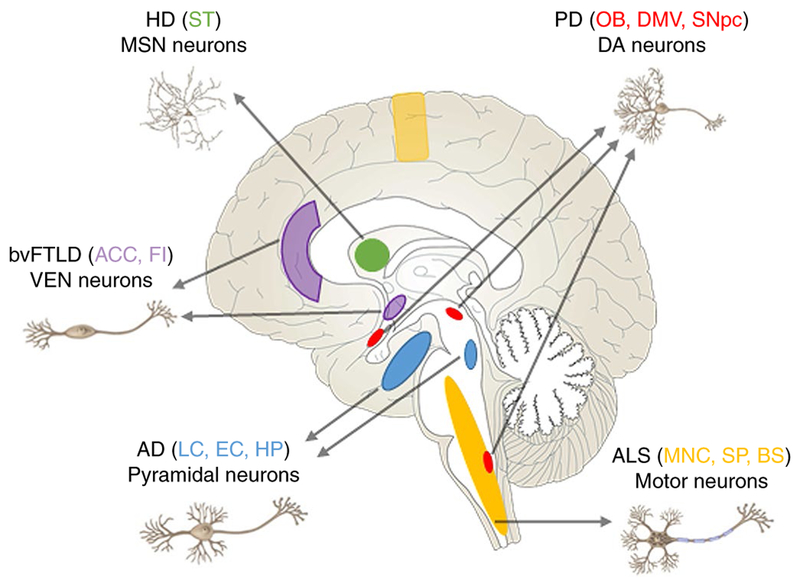
In the aging brain and in the context of external stressors (for example, peripherally produced cytokines), several pathways can fail, leading to neurodegeneration65. Why specific cells (Table 2 and Fig. 1) are selectively vulnerable to the breakdown of pathways that are critical in both vulnerable and resistant cells is not known. Explanation as to why will only be possible once neuronal subtypes have been better defined at a molecular level and we have a deeper understanding of their physiology.
Fig. 1 |. Regions and neurons that are vulnerable in neurodegenerative diseases.
Early-affected regions in different neurodegenerative diseases are indicated by different colors. LC, locus coeruleus; HP, hippocampus; OB, olfactory bulb; DMV, dorsal motor nucleus of the vagus; MNC, motor neocortex; SP, spinal cord; BS, brainstem; FI, frontal insula; DG, fascia dentata of the dentate gyrus; ST, striatum.
Protein supersaturation/metastable subproteome.
Cells go to great lengths to maintain proteins in a soluble state, as protein aggregation is associated with a wide variety of human diseases. The proteins most prone to aggregation are those whose cellular concentrations are high relative to their solubilities, i.e., proteins that are supersaturated. During stress, aging, and neurodegenerative disorders, cellular homeostasis of intrinsically supersaturated proteins becomes dysfunctional66–70. Proteins impacted in AD, PD, ALS, and HD are collectively known as the ‘metastable subproteome’, and they aggregate as plaques, tangles, Lewy bodies, and intracellular inclusions. Metastable proteins are inherently supersaturated, especially in neurons compared to in astrocytes and microglia, which may contribute to neuronal-specific vulnerability. Consistent with this, gene duplication events and haplotypes that lead to increased gene expression are associated with increased risk of disease71. While it is not known whether particular cell types within a population express different levels of a metastable protein, expression levels do differ between primary and secondary affected regions of the AD brain, suggesting a causal link between vulnerable cell populations and protein supersaturation70. It is notable that many risk alleles include genes involved in either lysosomal or ubiquitin proteasome system (UPS) function, which can directly affect the levels of supersaturated proteins. Thus, genetic risk data is consistent with the view that protein concentrations are critical, and being close to saturation puts a neuron at risk.
Protein homeostasis.
As mentioned previously, it seems likely that for each protein prone to misfolding, certain types of neurons are more affected by how that protein disrupts cellular protein homeostasis networks, and this may contribute to their vulnerability to a particular neurodegenerative disease29. Protein homeostasis genes are altered in pretangle-bearing neurons as neurofibrillary tangle (NFT) pathology spreads through the brain72,73, indicating disrupted protein homeostasis before clinical symptoms in AD. A gene co-expression analysis revealed that protein trafficking and clearance mechanisms, including specific branches of the endosomal–lysosomal systems and UPS, play a particular role in maintaining the homeostasis of the metastable subproteome associated with AD74. Moreover, a transcriptome-wide microarray analysis across more than 500 healthy brain tissues from the Allen Brain Atlas revealed a quantitative correlation between the histopatho-logical staging of the disease and the expression patterns of the proteins that co-aggregate in amyloid plaques and neurofibrillary tangles, together with those of the protein homeostasis components that regulate Aβ and tau. Because this expression signature was evident in healthy brains, the analysis provided an explanatory link between a tissue-specific environmental risk of protein aggregation and a corresponding vulnerability to AD70. Although these human-tissue-based findings are inherently correlative and cannot address causality, they can point the way for more detailed mechanistic approaches for understanding selective neuronal vulnerability in preclinical models.
In ALS and frontotemporal dementia, motor neurons and pyramidal neurons are vulnerable to overload of the UPS. Pyramidal neurons appear to be vulnerable to lysosomal failure in dementia with Lewy bodies as well as in frontotemporal dementia. It is interesting that cortical pyramidal neurons are susceptible to failure in either the lysosome system or the UPS. Both of these very general pathways are protein homeostasis mechanisms. Pyramidal cell death is associated with amyloid deposition and either tau tangle pathology (in AD) or with Lewy body (α-synuclein) pathology (in dementia with Lewy bodies). It is tempting to speculate that tangle pathology occurs when the UPS fails and that Lewy body pathology occurs when the lysosome system fails in the context of Aβ accumulation65. In HD, cell-type-specific differences in the ability to maintain proteostasis (for example, lower autophagic degradation capacity in vulnerable striatal neurons than in resistant cortical and even striatal interneurons) may contribute to selective vulnerability to toxic huntingtin protein75–78. Vulnerability may also involve neuron-specific combinations of dysfunction in cellular stress and proteostasis pathways, aggravated by advancing age, gene predisposition, and environmental factors.
Calcium homeostasis.
The lack of calcium-buffering proteins (for example, parvalbumin and calbindin D-28k) and disturbed cellular Ca2+ regulation is thought to play an important role in the vulnerability of neurons in AD, PD, and ALS. Neurons expressing calcium-buffering proteins are resistant, or less vulnerable, in the neocortex of AD patients3,13,14, the spinal cord and brainstem of ALS patients, the spinal cord of a mouse model of ALS3, and the striatum of HD patients42. Gene expression profiling studies in rats and mice demonstrate that calbindin transcripts are enriched in VTA dopaminergic neurons as compared to in SNpc dopaminergic neurons79,80. A similar expression pattern of calbindin was observed in human midbrain19,81. The lack of calcium-buffering proteins parvalbumin and calbindin D28k may render human motor neurons particularly vulnerable to calcium toxicity following glutamate receptor activation82.
CA1 neurons are selectively vulnerable to degeneration in AD, whereas CA3 neurons are less vulnerable, and dentate granule neurons do not degenerate17,83. Studies in transgenic mice (line 3xTg) suggest that excessive Ca2+ influx through L-type voltage-gated calcium channels (L-VGCC) may be one possible explanation for the selective vulnerability of CA1 neurons84.
SNpc dopaminergic neurons, selectively affected in PD, share a set of traits that may underlie their vulnerability. They have long, highly branched axons with an extraordinary number of transmitter release sites. This combination of features—broad spikes, pace-making, low intrinsic Ca2+ buffering, and cytosolic Ca2+ oscillations—is what appears to distinguish vulnerable neurons, and these features lead to Ca2+ overloading and mitochondrial oxidant stress and damage20,85. L-type Cav1.3 calcium channels, Kir6.2, K-ATP channels, and SK3 (the small-conductance calcium-activated potassium channel member) are molecular determinants for the electrophysiological differences between SNpc and VTA dopaminergic neurons, resulting in the selective vulnerability of SNpc neurons in PD19.
Among MNs, fast-fatigable MNs are particularly vulnerable in ALS. They exhibit the highest thresholds for excitation, firing rarely and in bursts29. Vulnerable MNs express low levels of cytosolic calcium-buffering proteins and are subjected to large and fast calcium fluxes across intracellular organelles such as mitochondria and smooth endoplasmic reticulum86. Large intracellular calcium fluxes and large numbers of neuromuscular junctions lead to particularly high energetic demands for function in these neurons29. Consistent with this notion, calcium dyshomeostasis and/or mitochondrial dysfunction have been implicated in many pathogenic processes in neurodegenerative diseases31,87–89.
Mitochondria and energy demand.
Large and long projection neurons in the EC and hippocampal CA1 are characterized by particularly high energy consumption and are vulnerable to decreased glucose and oxygen delivery through the vasculature and thus to energy deprivation90. Similarly, SNpc dopaminergic neurons are estimated to form much larger axonal arbors and a higher number of synapses than VTA dopaminergic neurons, which may result in the pronounced redistribution of mitochondria to their axonal terminals and the tremendous elevation of their energy demand, as well as their susceptibility to insults jeopardizing the neuronal energy supply91,92. Also, SNpc dopaminergic neurons were found to have a lower mitochondrial mass (i.e., higher level of mitochondrial DNA deletions) than VTA dopaminergic neurons, which might contribute to the selective vulnerability of SNpc over VTA93–95. In addition, SNpc neurons in PD appear to be close to a catastrophic failure in mitochondrial function. Why this should be so is not clear, but one possibility is that dopamine synthesis and metabolism lead to oxidative damage to mitochondria; this may contribute to their sensitivity and may be the reason that genes involved in mitophagy lead to their selective loss65,96.
Moreover, it has been suggested that projection neurons with sparsely myelinated axons would require prodigious energy expenditure to maintain axonal function and transport51. Such high energy demands would result in continuously high levels of oxidative stress and mitochondrial dysfunction that could increase neuron vulnerability to α-synuclein aggregation in PD. Consistent with this, projection neurons with long, thin axons that were only sparsely myelinated or unmyelinated were vulnerable to α-synuclein aggregates, whereas neurons with long but thickly myelinated axons with large diameters were resistant to the formation of such aggregates51. Intriguingly, a highly similar pattern can be noted in AD: sparsely myelinated temporal mesocortex exhibits tau aggregates first, whereas such pathology is last to appear in the heavily myelinated primary cortical fields97.
ALS-vulnerable motor neurons are large cells with long axonal processes, which lead to requirements for a high level of mitochondrial activity compared to other neuronal groups98. Motor neurons also have high perisomatic expression of the glutamate transporter protein (EAAT2) and very high expression of the cytosolic free-radical scavenging enzyme Cu/Zn superoxide dismutase (SOD1), which may render this cell group vulnerable in the face of genetic or post-translational alterations interfering with the function of these proteins82.
Striatal MSNs require high amounts of energy to maintain a hyperpolarized state, i.e., to remain electrophysiologically silent. This unique energy requirement of MSNs may contribute to their susceptibility to mitochondrial damage42. Another possibility for why MSNs are vulnerable to mitochondrial dysfunction may be associated with their low expression of the superoxide free-radical scavengers SOD1 and SOD2, which are enriched in resistant cholinergic interneurons42.
Neurotransmitters and neurotransmitter receptors.
The difference in several types of neurotransmitters and neurotransmitter receptors between subtypes of neurons has been proposed to explain selective neuronal vulnerability in neurodegenerative diseases. The expression profile and subunit composition of ionotropic glutamate receptors, especially NMDA receptors, may confer vulnerability in AD9,99,100. Previous studies demonstrate that GluN1 and GluN2B subunits of NMDA receptors are more susceptible to the effects of aging101 and the progression of AD pathology102, suggesting that there might be intrinsic and differential expression profiles of NMDA receptor subunits between vulnerable and resistant neurons. Further characterization of the expression profile of ionotropic glutamate receptor subunits will help us better understand the selective neuronal vulnerability in AD. The expression of metabotropic glutamate receptor 2 (mGluR2) is increased in a similar pattern to both the regional and cellular subtype of neuronal vulnerability to degeneration and neurofibrillary alterations103, while the differential expression of mGluRs (groups I and II) on specific neuronal populations might be responsible for selective neuronal degeneration in ALS104,105. SNpc neurons may be more vulnerable due to their production of reactive oxygen species, from dopamine and its metabolites, that eventually kill neurons106. Vulnerable neurons in the ventral SNpc exhibit increased expression of factors such as D2 dopamine autoreceptors, GIRK-2, lactotransferrin, and the dopamine transporter, coupled with a relative lack of neuroprotective elements, such as the dopamine vesicle transport protein and a number of trophic and growth factors21.
Differential expression of GABAA and glycine receptors29,107, as well as GluR282, in ALS-resistant versus ALS-vulnerable motor neurons may render human motor neurons particularly vulnerable to hyperexcitation. The function of VENs in FTLD are as yet unknown, but the finding that VENs express dopamine (D3), serotonin (1b/2b), and vasopressin receptors is of interest because the neurotransmitters involved with these receptors are related to the behavior problems observed in patients with bvFTLD108,109, suggesting that those neurotransmitter receptors might be responsible for the selective vulnerability of VENs in FTLD.
Similarly, glutamate excitotoxicity induced by activation of NMDA and AMPA receptors in the striatum is considered as one cause of the selective vulnerability of MSNs in HD42,110. The NR2B subunits of NMDA receptors are highly expressed in vulnerable MSNs, while the NR2D subunits are highly expressed in resistant striatal interneurons111,112. This differential expression pattern may explain the increased vulnerability of MSNs113. Moreover, increased expression of NR2B-subunit-containing extrasynaptic NMDA receptors in HD mouse striatum alters the balance between synaptic and extrasynaptic NMDA receptors activity, which may determine the vulnerability in HD42,114,115.
Synaptic transmission happens when a neurotransmitter activates its receptors on the postsynaptic neuron. Synapse-related markers (for example, synaptophysin and synaptotagmin-1) are substantially reduced in NFT-bearing hippocampal CA1 pyramidal neurons and basal forebrain cholinergic neurons in AD patients, compared to normal non-NFT-bearing neurons in aged-matched controls116. Altered expression of genes related to synaptic transmission and synaptic vesicle transport is found in the entorhinal cortex and hippocampus of AD brains, which suggest that there are dramatic synaptic changes in vulnerable neurons affected early in AD117.
Aging.
Age is the major risk factor for both AD and PD, the two most prevalent neurodegenerative diseases. It is also a major inter-actor with and the second most important determinant (after the CAG-repeat length) of HD onset. Selective neuronal vulnerability may, in part, be a consequence of mature or aged neurons being close to different catastrophic cliffs, depending on their function, history of stress exposure, and genetic predisposition, and this may explain why certain inclusions and aggregates preferentially injure certain types of neurons90. Aging has been shown to be associated with metabolic impairment, oxidative stress, perturbed neuronal calcium handling, and proteostasis dysfunction17,118–122, which could trigger and accelerate Aβ, tau, and α-synuclein pathologies, thereby setting the stage for disease-specific neuronal vulnerabilities and transneuronal propagation of the proteinopathies.
Several other cellular and molecular changes that occur during normal aging could render neurons vulnerable to degeneration. Age-related reduction in calbindin expression has been implicated in the selective vulnerability of basal forebrain cholinergic neurons and entorhinal cortex layer II neurons in AD, dopaminergic neurons in PD, and MSNs in HD17, suggesting that alterations in cellular Ca2+ homeostasis, especially the expression of calcium-binding proteins, might play an important role in selective vulnerability during aging. Alterations in numerous neurotransmitter (for example, dopamine) and neurotrophic factor (for example, brain-derived neurotrophic factor or BDNF) signaling pathways occur during normal aging, and many such changes are amplified in neurodegenerative disease17.
It is increasingly appreciated that synapses are the most vulnerable compartments of neurons. Differences among synapses in terms of structure, metabolism, and signaling mechanisms might therefore be determinants of selective vulnerability. Analysis of cell-type-specific aging-altered genes reveals enrichment of pathways associated with synaptosomes in downregulated neuron-specific genes123. Synaptic changes have also been identified in a study that examined the initial cell-type-specific transcriptional changes in a mouse model of ALS124.
Other factors.
Several other factors have been implicated in selective neuronal vulnerability, including neurotrophins and neurotrophin receptors, protein kinases, and phosphatases. For example, a major downregulation of neurotrophin receptors (for example, TrkA, TrkB, and TrkC) and neurotrophin genes (for example, Gdnf, Ngfb, and Ntf4) in CA1 pyramidal neurons and basal forebrain cholinergic neurons associated with tau pathology has been reported in mild cognitive impairment or early-stage AD compared to controls116,125. Moreover, the expression of pan-neurotrophin receptor p75(NTR) in basal forebrain cholinergic neurons has also been implicated as a vulnerability risk factor, probably due to the interaction with Aβ and with proapoptotic ligands that induce neuronal cytoskeletal abnormalities, leading to NFT formation in the basal forebrain cholinergic neurons of AD126,127. NFTs are also impacted by the activity of a wide range of protein kinases and phosphatases that are differentially expressed. For example, PRKCB and MAPK1 levels have been shown to be higher in highly vulnerable CA1 pyramidal neurons128, while DAPK1 is selectively activated in excitatory pyramidal neurons in the entorhinal cortical layer II of AD mice129. In addition, downregulation of protein phosphatase 1 and protein phosphatase 2 subunit mRNAs was observed in AD compared with normal control basal forebrain cholinergic neurons and CA1 neurons116. Although the exact role of these factors in selective vulnerability is unclear, tau aggregation and downstream death-related signaling pathways would be likely targets.
Developmental factors have also been implicated in selective vulnerability. For example, some of the transcription factors expressed during neurogenesis continue to be expressed in dopaminergic neurons during adulthood, but the expression pattern is different between SNpc and VTA dopaminergic neurons, which may mediate the relative vulnerability of the former in PD. Other transcription factors that seem to be differentially regulated between SNpc and VTA neurons include PITX3130,131, homeobox protein OTX2130, and DCC132. In addition, ASCL1 and LMX1B seem to be required for specification of many brainstem regions that are susceptible to degeneration in early PD131. The role of these transcription factors in selective vulnerability, however, needs to be validated in the future.
Taken together, determinants of selective neuronal vulnerability might include intrinsic morphological, electrophysiological, and biochemical properties, as well as exposure to stressors such as aging. It should be noted that these determinants are not likely to be independent, and they may play synergistic roles in the selective loss of particular neurons in vulnerable regions of the brain.
Future directions
The majority of studies that define selective vulnerability are based on end-stage pathology, and their assessments are based on neuronal loss, structural or visible changes from immunostaining, or histochemistry in case series. While critically important, this is a crude assessment of cell function and vulnerability, as remaining neurons that appear normal could have vastly altered electrical properties, synaptic connections, gene expression patterns, or other changes that are poorly measured using histology-based techniques. The recent development of novel techniques such as single-cell profiling and the development of cell-type-specific human iPSC-derived neurons will help elucidate the molecular mechanisms underlying selective cellular vulnerability, including at early stages of the disease.
Single cell profiling.
Our ability to discriminate between neuronal subtypes is currently rudimentary, and therefore defining the gene signature of different neurons (especially at the single-cell level) will be critical for understanding why particular subtypes of neurons are vulnerable in neurodegenerative diseases. Techniques such as laser-capture microdissection and bacterial artificial chromosome-translating ribosome-affinity purification (bacTRAP) methodology, combined with microarray19,116 or bulk RNA-seq124,133,134, have been employed to create gene expression profiles of different types of neurons in various brain regions that are vulnerable to or resistant in AD and other neurodegenerative diseases. Moreover, single-cell RNA-seq135,136 and single-nuclei RNA-seq techniques137,138 have been successfully applied to identify microglia and to distinguish between different subtypes of excitatory and inhibitory neurons in human postmortem brain tissues based on their unique transcriptomics. One caveat of using transcriptomics in postmortem tissue to identify key vulnerability factors is that changes in mid- or late-stage disease may be an attempt at compensation and may not reflect the signaling milieu that underlies the initial cell-type-specific vulnerability in early-stage disease. Thus, comparison to early-affected regions with much less pathology would be beneficial.
Of note, a novel single-cell RNA-seq method, fluorescent in situ RNA-sequencing (FISSEQ)139, has been developed to consider the cellular and spatial context of each RNA within a biological sample, which is absent in other single-cell or single-nucleus RNA-seq methods. If this method can be optimized for human fresh-frozen brain tissue—or ideally, fixed brain tissue—it will be a very powerful tool to identify the gene expression signature of vulnerable and resistant neurons in neurodegenerative diseases and will provide important insights for elucidating the mechanisms underlying selective vulnerability.
Exploring selective vulnerability using cell-type-specific human iPSCs-derived models.
Human iPSC-derived neurons have been extensively used to examine disease-relevant cellular and molecular phenotypes in a physiologically and genetically relevant context, potentially recapitulating the early stages of disease and allowing researchers to test therapeutic targets and small molecules in a human neuronal environment18,140–142. With the advent of approaches to differentiate human iPSCs into specific cell types, such as dopaminergic143–145, glutamatergic146, and GABAergic neurons147,148, we will be better able to explore which subtypes of neurons are vulnerable and why they are vulnerable to different proteins or different forms of the same protein, especially when these studies are combined with single-cell RNA-seq analysis and systems-biology approaches. However, a critical issue for these human iPSCs-derived models is the fact that without further manipulation (for example, overexpressing the progerin gene)149 they do not represent the mature or aged state in which neurodegeneration takes place. New approaches, such as direct conversion150, can recapitulate disease phenotype, cell type, and age, and they may have more utility for cell-based studies of selective vulnerability.
In conclusion, recent efforts to use genetics to identify cell-type-specific genetic risk factors and the cell populations affected, coupled with molecular approaches that can better define afflicted cells in the diseased brain, have advanced our understanding of the pathogenic processes associated with selective cellular and regional vulnerability. Considerable effort, however, is now needed to develop new techniques that can identify cell signatures in postmortem human brain tissue and identify relevant disease-associated pathways in complex biological systems.
Acknowledgements
The authors thank D. Dickson for critical reading of the manuscript. K.E.D. acknowledges the support of NIH (NS074874 and AG056151), Cure Alzheimer’s Fund, the Tau Consortium, and the Brightfocus Foundation. This work was also supported by a grant from the NIH (AG056673) and the Alzheimer’s Association (AARF-17–505009) to H.J.F.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roberts GW, Nash M, Ince PG, Royston MC & Gentleman SM On the origin of Alzheimer’s disease: a hypothesis. Neuroreport 4, 7–9 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Hyman BT, Van Hoesen GW, Damasio AR & Barnes CL Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225, 1168–1170 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Morrison BM, Hof PR & Morrison JH Determinants of neuronal vulnerability in neurodegenerative diseases. Ann. Neurol 44 (Suppl 1), S32–S44 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Morrison JH & Hof PR Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog. Brain Res 136, 467–486 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Stranahan AM & Mattson MP Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010, 108190, 10.1155/2010/108190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehouse PJ et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239 (1982). [DOI] [PubMed] [Google Scholar]
- 7.Davies P & Maloney AJ Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2, 1403 (1976). [DOI] [PubMed] [Google Scholar]
- 8.Bondareff W, Mountjoy CQ & Roth M Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Neurology 32, 164–168 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Greenamyre JT & Young AB Excitatory amino acids and Alzheimer’s disease. Neurobiol. Aging 10, 593–602 (1989). [DOI] [PubMed] [Google Scholar]
- 10.Chin J et al. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J. Neurosci 27, 2727–2733 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison JH et al. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer’s disease. Brain Res. 416, 331–336 (1987). [DOI] [PubMed] [Google Scholar]
- 12.Bussière T et al. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J. Comp. Neurol 463, 281–302 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Hof PR, Nimchinsky EA, Celio MR, Bouras C & Morrison JH Calretinin-immunoreactive neocortical interneurons are unaffected in Alzheimer’s disease. Neurosci. Lett 152, 145–148 (1993). [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto N & Emson PC Demonstration of neurofibrillary tangles in parvalbumin-immunoreactive interneurones in the cerebral cortex of Alzheimer-type dementia brain. Neurosci. Lett 128, 81–84 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Fu H et al. Tau pathology induces excitatory neuron loss, grid cell dysfunction, and spatial memory deficits reminiscent of early Alzheimer’s disease. Neuron 93, 533–541.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarter M & Bruno JP The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur. J. Neurosci 15, 1867–1873 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP & Magnus T Ageing and neuronal vulnerability. Nat. Rev. Neurosci 7, 278–294 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muratore CR et al. Cell-type dependent Alzheimer’s disease phenotypes: probing the biology of selective neuronal vulnerability. Stem Cell Rep. 9, 1868–1884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brichta L & Greengard P Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Front. Neuroanat 8, 152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surmeier DJ, Obeso JA & Halliday GM Parkinson’s disease is not simply a prion disorder. J. Neurosci 37, 9799–9807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Double KL Neuronal vulnerability in Parkinson’s disease. Parkinsonism Relat. Disord 18 (Suppl 1), S52–S54 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Jellinger KA Post mortem studies in Parkinson’s disease–is it possible to detect brain areas for specific symptoms? J. Neural Transm. Suppl 56, 1–29 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Halliday GM et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann. Neurol 27, 373–385 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Henderson JM, Carpenter K, Cartwright H & Halliday GM Degeneration of the centré median-parafascicular complex in Parkinson’s disease. Ann. Neurol 47, 345–352 (2000). [PubMed] [Google Scholar]
- 25.Harding AJ, Stimson E, Henderson JM & Halliday GM Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125, 2431–2445 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Kingsbury AE et al. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov. Disord 25, 2508–2515 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Braak H et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Walker DG et al. Changes in properties of serine 129 phosphorylated α -synuclein with progression of Lewy-type histopathology in human brains. Exp. Neurol 240, 190–204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena S & Caroni P Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Boillée S, Vande Velde C & Cleveland DW ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Roselli F & Caroni P From intrinsic firing properties to selective neuronal vulnerability in neurodegenerative diseases. Neuron 85, 901–910 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Alexianu ME et al. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann. Neurol 36, 846–858 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Kihira T, Yoshida S, Yoshimasu F, Wakayama I & Yase Y Involvement of Onuf’s nucleus in amyotrophic lateral sclerosis. J. Neurol. Sci 147, 81–88 (1997). [DOI] [PubMed] [Google Scholar]
- 34.von Lewinski F & Keller BU Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 28, 494–500 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Mills KR The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain 126, 2558–2566 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Varrone A et al. Voxel-based comparison of rCBF SPET images in frontotemporal dementia and Alzheimer’s disease highlights the involvement of different cortical networks. Eur. J. Nucl. Med. Mol. Imaging 29, 1447–1454 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Rabinovici GD et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am. J. Alzheimers Dis. Other Demen 22, 474–488 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann. Neurol 60, 660–667 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Seeley WW Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr. Opin. Neurol 21, 701–707 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickson DW, Kouri N, Murray ME & Josephs KA Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J. Mol. Neurosci 45, 384–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galvan L, André VM, Wang EA, Cepeda C & Levine MS Functional differences between direct and indirect striatal output pathways in Huntington’s disease. J. Huntingtons Dis 1, 17–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morigaki R & Goto S Striatal vulnerability in Huntington’s disease: neuroprotection versus neurotoxicity. Brain Sci. 7, E63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiner A et al. Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. USA 85, 5733–5737 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richfield EK, Maguire-Zeiss KA, Vonkeman HE & Voorn P Preferential loss of preproenkephalin versus preprotachykinin neurons from the striatum of Huntington’s disease patients. Ann. Neurol 38, 852–861 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 46.Brettschneider J et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol 74, 20–38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josephs KA et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 127, 441–450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braak H & Del Tredici K Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J. Parkinsons Dis 7 s1, S71–S85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braak H et al. Amyotrophic lateral sclerosis–a model of corticofugal axonal spread. Nat. Rev. Neurol 9, 708–714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker LC & Jucker M Neurodegenerative diseases: expanding the prion concept. Annu. Rev. Neurosci 38, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brettschneider J, Del Tredici K, Lee VM & Trojanowski JQ Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat. Rev. Neurosci 16, 109–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goedert M, Eisenberg DS & Crowther RA Propagation of tau aggregates and neurodegeneration. Annu. Rev. Neurosci 40, 189–210 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Mudher A et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun 5, 99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jucker M & Walker LC Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spina S et al. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain 131, 72–89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghetti B et al. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol 41, 24–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker LC Proteopathic strains and the heterogeneity of neurodegenerative diseases. Annu. Rev. Genet 50, 329–346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt M et al. Peptide dimer structure in an Aβ (1–42) fibril visualized with cryo-EM. Proc. Natl. Acad. Sci. USA 112, 11858–11863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gremer L et al. Fibril structure of amyloid-β (1–42) by cryo-electron microscopy. Science 358, 116–119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzpatrick AWP et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langfelder P & Horvath S WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gamazon ER et al. A gene-based association method for mapping traits using reference transcriptome data. Nat. Genet 47, 1091–1098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matarin M et al. A genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Rep. 10, 633–644 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Ryan BJ, Hoek S, Fon EA & Wade-Martins R Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem. Sci 40, 200–210 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Hardy J Catastrophic cliffs: a partial suggestion for selective vulnerability in neurodegenerative diseases. Biochem. Soc. Trans 44, 659–661 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM & Vendruscolo M Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 5, 781–790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciryam P, Kundra R, Morimoto RI, Dobson CM & Vendruscolo M Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol. Sci 36, 72–77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciryam P et al. A transcriptional signature of Alzheimer’s disease is associated with a metastable subproteome at risk for aggregation. Proc. Natl. Acad. Sci. USA 113, 4753–4758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciryam P et al. Spinal motor neuron protein supersaturation patterns are associated with inclusion body formation in ALS. Proc. Natl. Acad. Sci. USA 114, E3935–E3943 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freer R et al. A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. Sci. Adv 2, e1600947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hardy J Expression of normal sequence pathogenic proteins for neurodegenerative disease contributes to disease risk: ‘permissive templating’ as a general mechanism underlying neurodegeneration. Biochem. Soc. Trans 33, 578–581 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Tiernan CT et al. Protein homeostasis gene dysregulation in pretangle-bearing nucleus basalis neurons during the progression of Alzheimer’s disease. Neurobiol. Aging 42, 80–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson NR et al. Evidence for sortilin modulating regional accumulation of human tau prions in transgenic mice. Proc. Natl. Acad. Sci. USA 114, E11029–E11036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kundra R, Ciryam P, Morimoto RI, Dobson CM & Vendruscolo M Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, E5703–E5711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsvetkov AS et al. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat. Chem. Biol 9, 586–592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guedes-Dias P et al. Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol. Dis 90, 51–57 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Le Grand JN et al. Specific distribution of the autophagic protein GABARAPL1/GEC1 in the developing and adult mouse brain and identification of neuronal populations expressing GABARAPL1/GEC1. PLoS One 8, e63133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fimia GM et al. Ambra1 regulates autophagy and development of the nervous system. Nature 447, 1121–1125 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Chung YH et al. Decreased expression of calretinin in the cerebral cortex and hippocampus of SOD1G93A transgenic mice. Brain Res. 1035, 105–109 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Greene JG, Dingledine R & Greenamyre JT Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in Parkinsonism. Neurobiol. Dis 18, 19–31 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Mendez I et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 128, 1498–1510 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw PJ & Eggett CJ Molecular factors underlying selective vulnerability of motor neurons to neurodegeneration in amyotrophic lateral sclerosis. J. Neurol 247 (Suppl 1), I17–I27 (2000). [DOI] [PubMed] [Google Scholar]
- 83.Simonian NA & Hyman BT Functional alterations in neural circuits in Alzheimer’s disease. Neurobiol. Aging 16, 305–309 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Wang Y & Mattson MP L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD micein an age-dependent manner. Neurobiol. Aging 35, 88–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sulzer D Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30, 244–250 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Kanning KC, Kaplan A & Henderson CE Motor neuron diversity in development and disease. Annu. Rev. Neurosci 33, 409–440 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Lin MT & Beal MF Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Chan CS, Gertler TS & Surmeier DJ Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 32, 249–256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirsch EC, Jenner P & Przedborski S Pathogenesis of Parkinson’s disease. Mov. Disord 28, 24–30 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Mattsson N, Schott JM, Hardy J, Turner MR & Zetterberg H Selective vulnerability in neurodegeneration: insights from clinical variants of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 87, 1000–1004 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Surmeier DJ, Guzman JN & Sanchez-Padilla J Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium 47, 175–182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bolam JP & Pissadaki EK Living on the edge with too many mouths to feed: why dopamine neurons die. Mov. Disord 27, 1478–1483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bender A et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet 38, 515–517 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Bender A et al. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J. Neurol 255, 1231–1235 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Kraytsberg Y et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet 38, 518–520 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Schapira AH et al. The Royal Kings and Queens Parkinson’s Disease Research Group. Mitochondrial function in Parkinson’s disease. Ann. Neurol 32 (Suppl), S116–S124 (1992). [DOI] [PubMed] [Google Scholar]
- 97.Braak H & Braak E Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 92, 197–201 (1996). [DOI] [PubMed] [Google Scholar]
- 98.Richter C, Park JW & Ames BN Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 85, 6465–6467 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi DW Ionic dependence of glutamate neurotoxicity. J. Neurosci 7, 369–379 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morrison JH Differential vulnerability, connectivity, and cell typology. Neurobiol. Aging 14, 51–54 (1993)discussion 55–56. [DOI] [PubMed] [Google Scholar]
- 101.Magnusson KR, Brim BL & Das SR Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front. Aging Neurosci. 2, 11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishizen-Eberz AJ et al. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol. Dis 15, 80–92 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Lee HG et al. Aberrant expression of metabotropic glutamate receptor 2 in the vulnerable neurons of Alzheimer’s disease. Acta Neuropathol. 107, 365–371 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Laslo P, Lipski J & Funk GD Differential expression of Group I metabotropic glutamate receptors in motoneurons at low and high risk for degeneration in ALS. Neuroreport 12, 1903–1908 (2001). [DOI] [PubMed] [Google Scholar]
- 105.Tomiyama M et al. Expression of metabotropic glutamate receptor mRNAs in the human spinal cord: implications for selective vulnerability of spinal motor neurons in amyotrophic lateral sclerosis. J. Neurol. Sci 189, 65–69 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Götz J, Schonrock N, Vissel B & Ittner LM Alzheimer’s disease selective vulnerability and modeling in transgenic mice. J. Alzheimers Dis 18, 243–251 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Lorenzo LE, Barbe A, Portalier P, Fritschy JM & Bras H Differential expression of GABAA and glycine receptors in ALS-resistant vs. ALS-vulnerable motoneurons: possible implications for selective vulnerability of motoneurons. Eur. J. Neurosci 23, 3161–3170 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Nitrini R Selective vulnerability of von Economo neurons in frontotemporal dementia. Dement. Neuropsychol 2, 164 (2008). [PMC free article] [PubMed] [Google Scholar]
- 109.Seeley WW et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis. Assoc. Disord 21, S50–S57 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Zeron MM et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron 33, 849–860 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB Jr. & Young AB NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J. Neurosci 15, 5297–5307 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Küppenbender KD et al. Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J. Comp. Neurol 419, 407–421 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Han I, You Y, Kordower JH, Brady ST & Morfini GA Differential vulnerability of neurons in Huntington’s disease: the role of cell type-specific features. J. Neurochem 113, 1073–1091 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okamoto S et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med 15, 1407–1413 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milnerwood AJ et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 65, 178–190 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Ginsberg SD, Che S, Counts SE & Mufson EJ Single cell gene expression profiling in Alzheimer’s disease. NeuroRx 3, 302–318 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang WS et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol. Genomics 33, 240–256 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor RC & Dillin A Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol 3, a004440 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Labbadia J & Morimoto RI The biology of proteostasis in aging and disease. Annu. Rev. Biochem 84, 435–464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaushik S & Cuervo AM Proteostasis and aging. Nat. Med 21, 1406–1415 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Wojda U, Salinska E & Kuznicki J Calcium ions in neuronal degeneration. IUBMB Life 60, 575–590 (2008). [DOI] [PubMed] [Google Scholar]
- 122.Squier TC Oxidative stress and protein aggregation during biological aging. Exp. Gerontol 36, 1539–1550 (2001). [DOI] [PubMed] [Google Scholar]
- 123.Soreq L et al. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18, 557–570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun S et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc. Natl. Acad. Sci. USA 112, E6993–E7002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ginsberg SD et al. Selective decline of neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons and hippocampus proper: correlation with cognitive performance and neuropathology in mild cognitive impairment and Alzheimer’s disease. Hippocampus (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fombonne J, Rabizadeh S, Banwait S, Mehlen P & Bredesen DE Selective vulnerability in Alzheimer’s disease: amyloid precursor protein and p75(NTR) interaction. Ann. Neurol 65, 294–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perez SE et al. Rac1b increases with progressive tau pathology within cholinergic nucleus basalis neurons in Alzheimer’s disease. Am. J. Pathol 180, 526–540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerschütz A et al. Neuron-specific alterations in signal transduction pathways associated with Alzheimer’s disease. J. Alzheimers Dis 40, 135–142 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Shu S et al. Selective degeneration of entorhinal-CA1 synapses in Alzheimer’s disease via activation of DAPK1. J. Neurosci 36, 10843–10852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Giovannantonio LG et al. Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability. Dev. Biol 373, 176–183 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Oliveira MAP, Balling R, Smidt MP & Fleming RMT Embryonic development of selectively vulnerable neurons in Parkinson’s disease. NPJ Parkinsons Dis. 3, 21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Osborne PB, Halliday GM, Cooper HM & Keast JR Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience 131, 671–681 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Nichterwitz S et al. Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat. Commun 7, 12139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McKeever PM et al. Cholinergic neuron gene expression differences captured by translational profiling in a mouse model of Alzheimer’s disease. Neurobiol. Aging 57, 104–119 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Keren-Shaul H et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lake BB et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352, 1586–1590 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Habib N et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee JH et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silva MC et al. Human iPSC-derived neuronal model of tau-A152T frontotemporal dementia reveals tau-mediated mechanisms of neuronal vulnerability. Stem Cell Rep. 7, 325–340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C et al. Scalable production of iPSC-derived human neurons to identify tau-lowering compounds by high-content screening. Stem Cell Rep. 9, 1221–1233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Imamura K et al. Calcium dysregulation contributes to neurodegeneration in FTLD patient iPSC-derived neurons. Sci. Rep 6, 34904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swistowski A et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28, 1893–1904 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roessler R, Boddeke E & Copray S Induced pluripotent stem cell technology and direct conversion: new possibilities to study and treat Parkinson’s disease. Stem Cell Rev. 9, 505–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sundberg M et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 31, 1548–1562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun AX et al. Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells. Cell Rep. 16, 1942–1953 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Yang N et al. Generation of pure GABAergic neurons by transcription factor programming. Nat. Methods 14, 621–628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller JD et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13, 691–705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Victor MB et al. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat Neurosci. 21, 341–352 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]