Abstract
Fungal aetiology of keratitis/corneal ulcer is considered to be one of the leading causes of ocular morbidity, particularly in developing countries including India. More importantly, Fusarium and Aspergillus are reported commonly implicating corneal ulcer and against this background the present work was undertaken so as to understand the current epidemiological trend of the two fungal keratitis. During the project period, a total of 500 corneal scrapings were collected from suspected mycotic keratitis patients, of which 411 (82.2%) were culture positive for bacteria, fungi, and parasites. Among fungal aetiologies, Fusarium (216, 52.5% of 411) and Aspergillus (68, 16.5% of 411) were predominantly determined. While the study revealed a male preponderance with both the fungal keratitis , it further brought out that polyene compounds (natamycin and amphotericin B) and azoles were active, respectively, against Fusarium spp. and Aspergillus spp. Additionally, 94.1% of culture proven Fusarium keratitis and, respectively, 100% and 63.6% of A. flavus and A. fumigatus were confirmed by multiplex PCR. The sensitivity of the PCR employed in the present study was noted to be 10 fg/μl, 1 pg/μl, and 300 pg/μl of DNA, respectively, for Fusarium, A. flavus, and A. fumigatus. Alarming fact was that Fusarium and Aspergillus regionally remained to be the common cause of mycotic keratitis and the Fusarium isolates had a higher antifungal resistance than Aspergillus strains against most of the test drugs.
1. Introduction
Blindness due to corneal infections is a serious problem next to cataract [1] and fungal infections of the cornea have emerged as a major eye disease globally. The corneal infection of fungal etiology is very common and comprising at least 50% of all culture positive cases in India [2]. However, the prevalence rate varies from one country to the other and also from one population to another within the same country [3, 4]. In South India, the dominance of fungal keratitis, particularly of Fusarium and Aspergillus, is prevalent more than a decade and have been documented in many literatures [5–8]. Species of Fusarium and Aspergillus are widespread in nature being causative agents of important diseases of major crops as well as immunocompromised humans [9] and have been considered as important pathogens in eye infections, especially keratitis [2, 10]. In India, Fusarium and Aspergillus species are being isolated from corneal ulcers in large numbers [6, 8, 11–14], irrespective of the geographical location.
The importance of fungal keratitis gained momentum in 2005 following the outbreak of fungal keratitis among contact lens wearers in many developed countries [15, 16]. The fact that the outcome of fungal keratitis is worse than that of bacterial keratitis must be underscored [17] due to poor response to the therapy as well as the limited availability of antifungal agents [18]. The diagnosis and treatment of fungal keratitis is one of the most difficult problems encountered by ophthalmologists. Further, the prognoses of the fungal keratitis worsen with inadvertent antifungal agents and recalcitrant course of the disease [2]. Although voriconazole and other triazoles have broad-spectrum activity against causative fungal isolates, clinically no single drug was found to be effective against fungal keratitis. Also, Fusarium spp. are completely tolerant to itraconazole and caspofungin [19].
Accurate identification of the aetiological agent of fungal keratitis is of great importance in order to administer appropriate treatment [10, 20]. Though conventional culture methods are often useful, it takes more time for sufficient growth and subsequent identification of the causative agent [2]. The use of molecular techniques offers a significant reduction in time required for precise diagnosis of such infections [20, 21]. Also, the scarcity of region-specific antifungal susceptibility data, the limited availability of commercially available antifungal drugs, and the lack of response lead to corneal blindness in a high number of infected patients. Therefore, due to the magnitude of the fungal keratitis in Tamilnadu, India, a survey of local antifungal susceptibility pattern, and exploring a suitable, rapid diagnostic method is of paramount importance.
Hence, the present study was designed for the rapid detection fungal pathogens causing keratitis by multiplex polymerase chain reaction (PCR) and also to evaluate the efficacy of multiplex PCR against routine culture method. Further, the study also presents data on regional prevalence of fungal keratitis and minimum inhibitory concentration [4] values of routinely used antifungal agents against Fusarium and Aspergillus isolated from corneal ulcers. The paper not only provides information on the current incidence of Fusarium/Aspergillus keratitis but also gives valuable information on drug susceptibilities so as to help the ophthalmologists to initiate appropriate antifungal regimen against fungal keratitis.
2. Materials and Methods
2.1. Patients
A total of 500 (including repeat specimens) corneal scraping specimens were collected between June 2010 and January 2011 from clinically suspected patients with mycotic keratitis who attended Cornea services at Aravind Eye Hospital, Coimbatore, after obtaining ethical clearance (Institutional Review Board, Aravind Medical Research Foundation, Madurai, India).
2.2. Collection of Specimens
Corneal scraping was performed under aseptic condition by an ophthalmologist using a sterile Kimura's spatula and a portion was inoculated on 5% sheep blood agar (SBA), chocolate agar (CA), and potato dextrose agar (PDA). Additionally, the remaining specimen was smeared on two clear glass slides to observe the presence of fungal filaments microscopically using 10% KOH wet mount and Gram staining. Corneal scrapings that revealed fungal filaments in direct microscopy were considered for the study. Repeat corneal scraping was done to collect specimen for PCR assay. The collected specimens were placed in 400 μl of lysis buffer (0.5M Tris HCL, 0.5M EDTA, 3% SDS, 1% β-mercaptoethanol) and were stored at −20°C until further processing.
2.3. Identification of Fusarium spp. and Aspergillus spp. by Culture Method
All fungal isolates were identified based on the standard culture techniques followed by microscopy after lacto-phenol cotton blue staining. The identified isolates were preserved in 0.85% saline at 4°C.
2.4. Determination of Minimum Inhibitory Concentration (MIC)
The MICs of seven different antifungal agents, namely, amphotericin B (Himedia, Mumbai, India), itraconazole (Sigma-Aldrich, St. Louis, MO, USA), natamycin (Sigma-Aldrich, St. Louis, MO, USA), voriconazole (Aurolab, Madurai, India), ketoconazole (Himedia, Mumbai, India), econazole (Aurolab, Madurai, India) and clotrimazole (Aurolab, Madurai, India) were determined in accordance with the guidelines of Clinical and Laboratory Standards Institute (CLSI) [22]. The MIC values were defined as the lowest concentrations of antimicrobials that inhibit the visible growth of an isolate. The MIC50 and MIC90 values were defined as the MICs required to inhibit the growth of 50% and 90% of the isolates from a given species, respectively [23].
A. flavus ATCC 204304 was included as a quality control strain in all the batches of MIC analysis. The antifungal agents were prepared in order to achieve the dilution ranges in the order of 8 μg/ml - 0.015 μg/ml (amphotericin B, econazole, voriconazole, and clotrimazole), 32 μg/ml - 0.06 μg/ml (itraconazole), 16 μg/ml - 0.03 μg/ml (ketoconazole), and 128 μg/ml - 0.25 μg/ml (natamycin).
2.5. DNA Extraction
DNA was extracted from infected corneal tissue scrapings using Qiagen DNA extraction kit (Hilden, Germany), as per the manufacturer instructions. The concentration of extracted DNA was determined by nanophotometer (Implen, Munich, Germany). The DNA was stored at −20°C (Sanyo, Osaka, Japan) until further use.
2.6. Polymerase Chain Reaction
The extracted DNA was initially subjected to the first round of PCR using universal fungal primers (ITS1 and ITS4, internal transcribed spacer region). The first round amplicons were subsequently subjected for multiplex PCR using Fusarium and Aspergillus specific primers [24].
For each reaction in the first round PCR, a cocktail comprising 10 μl of DNA extract with 5 μl of 10 × PCR buffer (mixed with 1.5 mM magnesium chloride), 1 μl of dNTPs mix (200 μM each dNTPs), 20 pm/μl of each primer, and 0.5 U of Taq DNA polymerase amounting to a total volume of 50 μl was prepared. The ITS primers (Sigma, St Louis, MO, USA) used were 5′-TCCGTAGGTGAACCTGCGG-3′ (F) and 5′-TCCTCCGCTTATTGATATGC-3′ (R). The reaction was run in gradient thermocycler (Eppendorf, Hamburg Germany) involving initial denaturation at 95°C for 5 min, followed by 34 cycles in series of denaturation at 95°C for 30s, annealing at 54°C for 1 min, and extension at 72°C for 1 min, with a final step of extension at 72°C for 6 min and final holding at 4°C.
Multiplex PCR cocktail was prepared as described above. The specific primers (Sigma, St Louis, MO, USA) used for identification of Fusarium spp., A. flavus, and A. fumigatus were CAACTCCCAAACCCCTGTGA (F) & GCGACGATTACCAGTAACGA (R), CCGCCGGAGACACCACGAAC (F) & TGGGCAGCAATGACGCTCGG (R), and TTGTGTGTTGGGCCCCCGTC (F) & AAAGTTGGGTGTCGGCTGGCG (R), respectively. The amplicons were subjected to agarose gel electrophoresis in 1.5% agarose (Sigma, St. Louis, MO, USA) for 20-25 min at 80V (GeneI, Bangalore, India) along with 100 bp (Sigma, St. Louis, MO, USA) molecular marker. The DNA bands were visualized, analyzed, and documented using gel documentation system (Vilber Lourmat, France).
3. Results
Of the 500 corneal scrapings collected during the study period, the culture revealed that 411 (82.2% of 500) were positive for fungi, bacterial, and mixed etiologies (Table 1). Of 402 ocular specimens (97.8% of 411), 6 (1.4% of 411) and 3 (0.72% of 411) were identified to be due to fungal, bacterial, and mixture of bacterial and fungal causes, respectively. Further, 10% KOH and Gram staining revealed that 96.1% (449 of 467) and 94.7% (473 of 499) correlated with culture findings in the detection of fungi from corneal scrapings. Number of Fusarium (n=216) keratitis cases occurred more in males (134, 62%) than among females (82, 38%). The age group affected with Fusarium keratitis ranged from 21 to 70 years and particularly, 66 patients (30.5% of 216) belonged to 41-50 years and 50 (23.1% of 216) belonged to 51-60 years.
Table 1.
Microbial etiologies of corneal ulcer isolates during the study period.
| Fungi | Incidence | Percentage (n = 411) |
|---|---|---|
| Fusarium spp. | 216 | 52.5 |
| A. flavus | 48 | 11.6 |
| A. fumigatus | 11 | 2.6 |
| A. terreus | 5 | 1.2 |
| A. niger | 3 | 0.7 |
| A. tamarii | 1 | 0.2 |
| Bipolaris spp. | 22 | 5.3 |
| Curvularia spp. | 12 | 2.9 |
| Exserohilum spp. | 11 | 2.6 |
| Cladosporium spp. | 4 | 0.9 |
| Aureobasidium spp. | 3 | 0.7 |
| Exophiala spp. | 2 | 0.4 |
| Lasiodiplodia spp. | 1 | 0.2 |
| Pseudallescheria sp. | 1 | 0.2 |
| C. albicans | 1 | 0.2 |
| Alternaria sp. | 1 | 0.2 |
| Scedosporium spp. | 1 | 0.2 |
| S. apiospermum | 1 | 0.2 |
| Unidentified dematiaceous fungi (UID) | 21 | 5.1 |
| Unidentified hyaline fungi(UIH) | 37 | 9 |
|
| ||
| Bacteria | ||
|
| ||
| S. aureus | 1 | 0.2 |
| Pseudomonas spp. | 1 | 0.2 |
| Nocardia spp. | 1 | 0.2 |
| CoNS | 1 | 0.2 |
| S. viridans | 1 | 0.2 |
| Citrobacter spp. | 1 | 0.2 |
|
| ||
| Mixed infection | ||
|
| ||
| CoNS + Fusarium spp. | 1 | 0.2 |
| S. pneumoniae + Bipolaris spp. | 1 | 0.2 |
| Citrobacter spp. + Bipolaris spp. | 1 | 0.2 |
| Total | 411 | 100 |
CoNS: coagulase negative Staphylococcus spp.
Similarly, Aspergillus keratitis was confirmed predominantly among males (55.8% of 68). The age group affected with Aspergillus keratitis ranged from 31 to 50 years (31, 45.5% of 68). A. flavus (48, 11.6% of 411) was the predominant species among the identified Aspergillus identified during the study period. Bipolaris spp. (22, 5.3% of 411), Curvularia spp. (12, 2.9% of 411), and Exserohilum spp. (11, 2.6% of 411) were the other fungi isolated during the study period.
3.1. Minimum Inhibitory Concentration (MIC)
In this study, a total of 200 Fusarium and 67 Aspergillus isolates (47 A. flavus, 11 A. fumigatus, 5 A. terreus, 3 A. niger, and 1 A. tamarii) were included to determine the minimum inhibitory concentrations / MIC50 and MIC90 of routine antifungal drugs. Overall, the isolates of Fusarium spp. required higher concentrations (Tables 2 and 3) of specific antifungal drug than Aspergillus spp. in order to be inhibited. Most of the Aspergillus isolates were inhibited by amphotericin-B at a concentration of ≥ 1 μg/ml. More interestingly, natamycin acted against 65% (130, n = 200) of the Fusarium isolates at a concentration of 16 μg/ml while majority of Aspergillus spp. were inhibited at ≥ 32 μg/ml. A notable observation was with itraconazole activity, where 92.5% (185, n = 200) Fusarium spp. were susceptible only at a concentration of ≥ 32 μg/ml while 100% (n = 67) of the Aspergillus isolates were completely inhibited at ≤ 1 μg/ml. Similar MIC patterns were observed with econazole, clotrimazole, and ketoconazole where Fusarium isolates had higher MICs compared to Aspergillus spp.
Table 2.
Minimum inhibitory concentration (μg/ml) of antifungal agents against Fusarium spp. (n=200).
| Amphotericin B | ||||
| MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
| 8 – 0.125 | 77 (38.5%) | 123 (61.5%) | 1 | 1 |
|
| ||||
| Natamycin | ||||
| MIC range | ≤8 μg/ml | ≥16 μg/ml | MIC50 | MIC90 |
| 64 – 2 | 70 (35%) | 130 (65%) | 16 | 32 |
|
| ||||
| Itraconazole | ||||
| MIC range | ≤16 μg/ml | ≥32 μg/ml | MIC50 | MIC90 |
| 32 – 4 | 15 (7.5%) | 185 (92.5%) | 32 | 32 |
|
| ||||
| Voriconazole | ||||
| MIC range | ≤4 μg/ml | ≥8 μg/ml | MIC50 | MIC90 |
| 8 – 1 | 101 (50.5%) | 99 (49.5%) | 4 | 8 |
|
| ||||
| Econazole | ||||
| MIC range | ≤4 μg/ml | ≥8 μg/ml | MIC50 | MIC90 |
| 8 – 2 | 38 (19%) | 162 (81%) | 8 | 8 |
|
| ||||
| Clotrimazole | ||||
| MIC range | ≤4 μg/ml | ≥8 μg/ml | MIC50 | MIC90 |
| 8 – 0.5 | 145 (72.5%) | 55 (27.5%) | 4 | 8 |
|
| ||||
| Ketoconazole | ||||
| MIC range | ≤8 μg/ml | ≥16 μg/ml | MIC50 | MIC90 |
| 16 – 2 | 35 (17.5%) | 165 (82.5%) | 16 | 16 |
Table 3.
Minimum inhibitory concentration (μg/ml) of antifungal agents against Aspergillus spp.
| Amphotericin B | |||||
|
| |||||
| Isolates | MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 8 – 0.25 | 21 (44.7%) | 26 (53.4%) | 1 | 2 |
| A. fumigatus (n = 11) | 4 – 0.25 | 5 (45.4%) | 6(54.5%) | 1 | 4 |
| A. terreus (n = 5) | 2 – 0.5 | 2 (40%) | 3 (60%) | 1 | 2 |
| A. niger (n = 3) | 0.5 – 0.25 | 3 (100%) | - | 0.5 | 0.5 |
| A. tamarii (n = 1) | NA | 1 (100%) | - | NA | NA |
|
| |||||
| Natamycin | |||||
|
| |||||
| Isolates | MIC range | ≤16 μg/ml | ≥32 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 64 – 16 | 18 (38.2%) | 29 (61.7%) | 32 | 64 |
| A. fumigatus (n = 11) | 64 – 16 | 7 (63.6%) | 4 (36.3%) | 16 | 32 |
| A. terreus (n = 5) | 32 – 16 | 1 (20%) | 4 (80%) | 32 | 32 |
| A. niger (n = 3) | 32 – 8 | 2 (66.7%) | 1 (33.4%) | 16 | 32 |
| A. tamarii (n = 1) | NA | - | 1 (100%) | NA | NA |
|
| |||||
| Itraconazole | |||||
|
| |||||
| Isolates | MIC range | ≤0.25 μg/ml | ≥0.5 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 1 – 0.25 | 25 (53.1%) | 22 (46.8%) | 0.25 | 0.5 |
| A. fumigatus (n = 11) | 0.5 – 0.25 | 5 (45.4%) | 6 (54.5%) | 0.5 | 0.5 |
| A. terreus (n = 5) | NA | - | 5 (100%) | 0.5 | 0.5 |
| A. niger (n = 3) | 0.5 – 0.25 | 2 (66.7%) | 1 (33.4%) | 0.25 | 0.5 |
| A. tamarii (n = 1) | NA | 1 (100%) | - | NA | NA |
|
| |||||
| Voriconazole | |||||
|
| |||||
| Isolates | MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 4 – 0.25 | 37 (78.7%) | 10 (21.2%) | 0.5 | 1 |
| A. fumigatus (n = 11) | 4 – 0.25 | 6 (54.5%) | 5 (45.4%) | 0.5 | 1 |
| A. terreus (n = 5) | 1 – 0.5 | 3 (60%) | 2 (40%) | 0.5 | 1 |
| A. niger (n = 3) | 1 – 0.25 | 2 (66.7%) | 1 (33.4%) | 0.5 | 1 |
| A. tamarii (n = 1) | NA | - | 1 (100%) | NA | NA |
|
| |||||
| Econazole | |||||
|
| |||||
| Isolates | MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 2 – 0.25 | 37 (78.7%) | 10 (21.2%) | 0.5 | 1 |
| A. fumigatus (n = 11) | 1 – 0.25 | 5 (45.4%) | 6 (54.5%) | 1 | 1 |
| A. terreus (n = 5) | 1 – 0.5 | 2 (40%) | 3 (60%) | 0.5 | 1 |
| A. niger (n = 3) | 2 – 0.25 | 1 (33.4%) | 2 (66.7%) | 2 | 2 |
| A. tamarii (n = 1) | NA | - | 1 (100%) | NA | NA |
|
| |||||
| Clotrimazole | |||||
|
| |||||
| Isolates | MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 1 – 0.125 | 31 (65.9%) | 16 (34%) | 0.5 | 1 |
| A. fumigatus (n = 11) | 1 – 0.125 | 8 (72.7%) | 3 (27.2%) | 0.5 | 1 |
| A. terreus (n = 5) | 1 – 0.5 | 1 (20%) | 4 (80%) | 1 | 1 |
| A. niger (n = 3) | 1 – 0.5 | 1 (33.4%) | 2 (66.7%) | 1 | 1 |
| A. tamarii (n = 1) | NA | 1 (100%) | - | NA | NA |
|
| |||||
| Ketoconazole | |||||
|
| |||||
| Isolates | MIC range | ≤0.5 μg/ml | ≥1 μg/ml | MIC50 | MIC90 |
|
| |||||
| A. flavus (n = 47) | 8 – 0.5 | 14 (29.7%) | 33 (70.2%) | 1 | 4 |
| A. fumigatus (n = 11) | 4 – 0.125 | 4 (36.3%) | 7 (63.6%) | 1 | 2 |
| A. terreus (n = 5) | 4 – 1 | - | 5 (100%) | 2 | 4 |
| A. niger (n = 3) | 4 – 0.5 | 1 (33.4%) | 2 (66.7%) | 1 | 4 |
| A. tamarii (n = 1) | NA | - | 1 (100%) | NA | NA |
NA: not applicable.
3.2. PCR Study
A total of 473 corneal scrapings which were culture positive for Fusarium spp. (205), A. flavus (46), A. fumigatus (11), A. terreus (4), A. tamarii (1), Bipolaris spp. (22), Exserohilum spp. (10), Curvularia spp. (12), Cladosporium spp. (4), Aureobasidium spp. (3), Exophiala spp. (2), Lasiodiplodia sp. (1), Pseudallescheria sp. (1), Alternaria sp. (1), Scedosporium spp. (2), UID (20), and UIH (36) were included for PCR. Additionally, 86 culture negative corneal scrapings along with 2 mixed infections and 4 bacterial positive specimens were also included for PCR analysis.
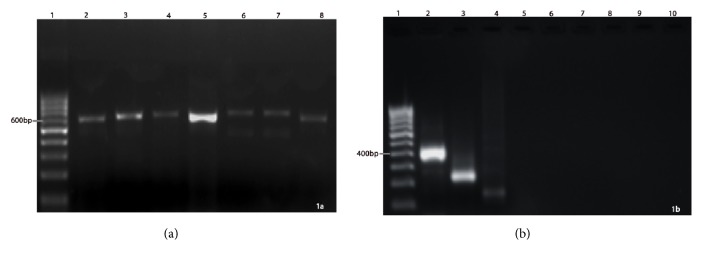
All the primers specifically amplified the target region. The specific primers of ITS 1 & 4 (1st round), Fusarium spp., A. flavus, and A. fumigatus after PCR and upon electrophoresis produced amplicons of approximately 600 bp, 400 bp, 250 bp, and 150 bp, respectively (Figure 1(a)). In addition, other fungal culture positive corneal scrapings such as Bipolaris, Curvularia, Exserohilum, etc. could not be amplified in 2nd round multiplex PCR (Figure 1(b)). All the PCR positive specimens (Fusarium, A. flavus, A. fumigatus) were further confirmed with culture identification to ensure the specificity.
Figure 1.
First and second round of PCR amplifications (a) Uniplex PCR with ITS1 & ITS4 primers: Lane 1 - 100 bp ladder, lane 2 - Fusarium sp. (∼600 bp), lane 3 - A. flavus, lane 4 - A. fumigatus, lane 5 - Bipolaris spp., lane 6 - Exerohilum sp., lane 7 - Alternaria sp., lane 8 - Curvularia sp. (b) Multiplex PCR with species specific primers: Lane 1 - 100 bp marker, Lane 2 - Fusarium sp., Lane 3 - A. flavus, Lane 4 - A. fumigatus, Lane 5 - Bipolaris sp., Lane 6 - Exerohilum sp., Lane 7 - Alternaria sp., Lane 8 - Curvularia sp. Lane 9 - UID, Lane 10 - UID.
3.3. Sensitivity of the PCR
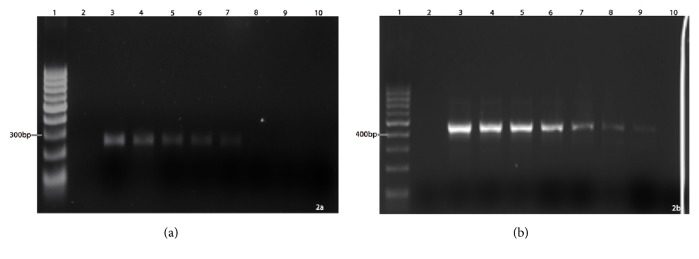
To determine the minimum amount of fungal DNA that could be detected by the established PCR assay, variable quantities (ranging from 10 ng/μl to 300 fg/μl) of Fusarium and Aspergillus genomic DNA were used as DNA template (Figure 2(a)) and it was found that the best optimized PCR conditions could amplify Fusarium DNA as less as 10 fg /μl (Figure 2(b)). Similarly, A. flavus DNA could be amplified as low as 1 pg/μl. However, the PCR was noted to be less sensitive towards the detection of A. fumigatus DNA as it required a minimum DNA concentration of at least 300 pg/μl for amplification and detection (Figure 3).
Figure 2.
Determination of PCR sensitivity and specificity (a) A. flavus: Lane 1 - 100 bp marker, lane 2 - negative control, Lane 3 - 10 ng, Lane 4 - 1 ng, Lane 5 - 100 pg, lane 6 - 10 pg, lane 7 - 1 pg, lane 8 - 100 fg, lane 9 - 10 fg, lane 10 - 1 fg. (b) Fusarium sp.: Lane 1 - 100 bp marker, lane 2 - negative control, Lane 3 - 10 ng, Lane 4 - 1 ng, Lane 5 - 100 pg, lane 6 - 10 pg, lane 7 - 1 pg, lane 8 - 100 fg, lane 9 - 10 fg, lane 10 - 1 fg.
Figure 3.
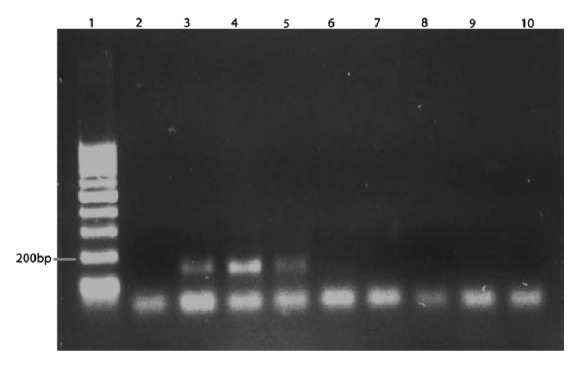
PCR sensitivity for A. fumigatus. Lane 1 - 100 bp marker, lane 2 - negative control, lane 3 - 30 ng, lane 4 - 3 ng, lane 5 - 300 pg, lane 6 - 30 pg, lane 7 - 3 pg, lane 8 - 300 fg, lane 9 - 30 fg, lane 10 - 3 fg.
3.4. PCR-First Round of Amplification with ITS Primers
In the 1st round of PCR amplification, using the universal fungal primers (ITS1 and ITS4), PCR products (550-600 bp) were generated from 347 (73.3%) corneal specimens. Of 347, 337 (97.1%) specimens were already confirmed to be fungal culture positive. An increase in the total number of positive cases through the applications of PCR under the project indicated the obvious and inevitable requirement of such techniques in routine diagnostic procedures. Also, the accuracy of the PCR detection was superior as most (16%, 76 of 473) of the PCR negative cases (ITS1 and ITS4) were also negative for conventional culture primarily. However, the DNA from 50 (10.5% of 473) corneal scrapings which were actually identified to be culture positive failed to amplify ITS 1 & 4 by PCR.
3.5. PCR-Second Round of Amplification with Fungal Species Specific Primers
The findings upon second round of amplification using exclusive primers for each genus/species were diverse. Of the 205 corneal scrapings that were positive for Fusarium spp. culture, only 193 (94.1% of 205) were reconfirmed as Fusarium spp. (Figure 1(b)). Similarly, 46 (100%) A. flavus and 7 (63.6% of 11) A. fumigatus were confirmed with PCR. No amplification of other fungal DNA was observed for which specific primers were not used (but their DNA could be amplified in the first round using universal fungal primers for ITS). More remarkably, of the 86 culture negative corneal scrapings, 9 (10.4% of 86) of them showed positive for Fusarium spp. in PCR which indicated that the PCR primers could identify even those specimens/cases which were reported to be negative in culture and that the primers could amplify minimum quantity of Fusarium DNA in culture negative cases also.
4. Discussion
Rapid identification of fungal pathogens and instilling of appropriate antifungal agents are key factors of a successful fungal keratitis management and the present study focused on the two features with special reference to Fusarium spp. and Aspergillus spp. The study included only fungal positive specimens identified through direct microscopy to evaluate the PCR specificity of rapid detection. The incidences of fungal keratitis reported were highly variable across the Indian states: southern and western India with 36.7% [8] and 36.3% [8, 25], northern (7.3%), northeastern (25.6%), and eastern India (26.4%) [26–28]. The present study revealed a direct microscopic sensitivity of 10% KOH and gram staining 96.1% and 94.7%, respectively, from corneal scrapings and were in accordance with Bibhudutta et al., 2011 [28]. Similarly, Bharathi et al. [8] reported 99.23% and 88.73% sensitivity in KOH wet mount and Gram staining, respectively. In another study, giemsa stain (75%) and Gram stain (55.5%) were used for the detection of fungal filaments [29].
Similar to other studies [8, 30], male patients (60.5%) were dominant with Fusarium and Aspergillus keratitis, than females (39.4%). Gonzales et al. [31] and Srinivasan et al. [6] reported the ratio of male to female with corneal ulcer as 1.6 to 1. Fusarium and Aspergillus keratitis were majorly (94%) confirmed in middle aged (21-70 years) individuals with a focused predominance in 41-50 years (28.8%).
Middle age group was noted to be highly vulnerable for fungal keratitis in Madurai [6] region with 31-60 and Nepal [32] with 21 -50 years. In this study, male patients were at a higher risk (55.8%) for Aspergillus keratitis, though our previous assessment [23] brought out 60% with 1.5:1 male to female ratio. In this study, Fusarium spp. (52.5%) followed by Aspergillus spp. (16.5%) were predominantly responsible for mycotic keratitis. Similar findings were observed in other parts of Southern India [6, 8] western India [25, 30], and eastern India [28]. Also, the prevalence of Aspergillus keratitis was found to be consistent with our previous findings [23]. However, Aspergillus spp. had been the dominant aetiology in fungal keratitis followed by Fusarium spp. in parts of northern [26] and eastern India [4]. In addition, the fungal keratitis aetiology greatly varied from country to country [10]. Candida spp. with incidence rates of 60.6% and 32.7% were observed in London [33] and Melbourne [18], respectively. Acremonium spp. (40%) were the most predominant fungal isolate in Paraguay [34]. Antifungal susceptibility testing and MIC determination procedures have very significant role in terms of successful management of fungal keratitis patients. However, the limited availability of commercial antifungal agents especially in the form of eye drops made the therapy more complicated [10]. In this study, Fusarium spp. required higher concentration of antifungal agents to inhibit the growth when compared to Aspergillus spp. except for amphotericin B and natamycin. In a similar investigation, amphotericin-B and natamycin were reported with significant activity against Fusarium spp. [35]. Exactly, 90% of the Fusarium spp. were sensitive at 1 μg/ml while 90% of A. flavus, A. fumigatus, A. terreus, and A. niger were sensitive at 2, 4, 2, and 0.5 μg/ml, respectively [36]. However, an assessment by Lalitha et al. [19] and Isabel et al. [35] reported MIC90 at 4 μg/ml and 4.62 μg/ml, respectively, against Fusarium spp. with amphotericin-B. The MIC90 of Aspergillus spp. observed in the present study was similar to the study by Lalitha et al. [19]. Natamycin, though the drug of choice against filamentous fungi [37], because of its poor penetration, is effective only in nonsevere superficial keratitis [13]. Fusarium spp. were more sensitive to natamycin than Aspergillus spp. In this study, 90% of the Fusarium and Aspergillus strains were inhibited at 32 μg/ml and 64 μg/ml, respectively, and the findings were similar to the previous assessments [19]. The resistant pattern of Fusarium spp. against itraconazole in this study was clearly evident from other reports [19, 35] though the MIC values showed variations. On the contrary, Aspergillus was significantly sensitive against itraconazole with consistent findings to our previous work [23] as well as with other investigators [19, 38]. Similar to itraconazole, other agents such as voriconazole, econazole, clotrimazole, and ketoconazole were relatively effective against Aspergillus spp. Likewise, higher drug concentrations were required to inhibit Fusarium spp. indicating that the tested azole drugs were ineffective. Eduardo et al. 2008 [39] and Lalitha et al. 2007 [19] reported MIC90 of voriconazole as 4 μg/ml and the similar range was found in the present study (8 μg/ml). Eduardo et al. concluded that F. solani tends to be more resistant to certain azoles [39]. However, in case of Aspergillus spp., highest MIC was noted against voriconazole (1 μg/ml), which was similar to the data published previously [19, 23]. In case of ketoconazole, the MIC90 and MIC50 of Fusarium spp. were noted as 16 μg/ml, while among Aspergillus spp. A. flavus, A. terreus and A. niger had a highest MIC90 of 4 μg/ml. Isabel et al. 1997 [35] reported higher MIC90 (>51.20 mg/l) against Fusarium. On the contrary, Theresa et al. 2006 [36] reported higher MIC percentile value of ketoconazole in A. niger and A. terreus when compared to other filamentous fungi. In general, the isolates of Fusarium spp. showed more resistance than Aspergillus spp. Most of the Fusarium isolates required higher concentration of drugs to get inhibited. Further, most of the Aspergillus isolates were sensitive to amphotericin-B.
All the test PCR primers (ITS 1 & 4) amplified the target region of Fusarium spp., A. flavus, and A. fumigatus representing 600 bp, 400 bp, 250 bp, and 150 bp, respectively, after fractionation and were confirmed with suitable positive (fungal culture positive corneal scraping with Bipolaris spp., Curvularia spp., Exserohilum spp., etc.) and negative controls. The PCR identified Fusarium, A. flavus, and A. fumigatus were subsequently determined to be culture positive. Though a prompt identification of fungal causative agent is the most important task behind every successful management of mycotic keratitis, the issue never has been completely redressed. The impediments of rapid identification could be possibly due to the less than a minimum quantity of the samples and cross contamination of conjunctival flora. Therefore, studies on rapid identification of fungi directly from corneal scrapings are very limited. Hence, the present study evaluated direct identification of major fungal agents such as Fusarium and Aspergillus directly from corneal scraping using multiplex PCR. The specificity of the PCR primers was determined by evaluation of positive control using known fungal isolates and negative control (bacterial DNA) and subsequent observation of culture positive cases. PCR confirmed positivity for all the positive control specimens while no bands in negative controls. The specimens positive for Fusarium spp. and Aspergillus spp. in PCR analyses were also identified to be culture positive in respective microbiological media.
Ferrer et al. 2001 [21] reported high specificity of PCR upon amplification of ITS of the fungal genome isolated from ocular infection when DNA isolated from human leukocytes and bacterial DNA were used as a negative control. Zunaina et al. 2008 [40] used 18S rRNA segment for direct identification of fungal pathogens from corneal scrapings, in which the specificity (94.7%) was confirmed by sequencing of amplified DNA fragment. The PCR sensitivity of the present study for Fusarium spp., A. flavus, and A. fumigatus was 10 fg, 1 pg, and 300 pg/micro liter of DNA, respectively. Other investigators reported the sensitivity of A. versicolor genome with 100 fg [40]. In a significant difference from our findings, A. fumigatus sensitivity was also reported up to 1 fg upon semi-nested PCR [21]. A positive PCR detection of the fungal pathogens was reported by Emma et al. 2000 [41] from a single patient which was culture negative. However, in the present study, PCR detected and amplified fungal DNA from 10 patients. Ferrer et al. 2011 [42] asserted that fungal PCR must be added as the screening diagnosis since PCR not only proved to be an effective rapid method for the diagnosis of fungal keratitis but was also sensitive compared to staining and culture methods of investigation. To the best of the literature survey, the present study was the first attempt to identify the species of Fusarium and Aspergillus directly from corneal scrapings of culture proven fungal keratitis cases. The outcomes of the preliminary assessment were highly specific and sensitive in detecting Fusarium and Aspergillus. An extended scientific evaluation and optimization is being suggested as to apply PCR in vitro assays for a routine as well as rapid diagnostic applications.
Acknowledgments
This study was supported by the Indian Council of Medical Research (IRIS ID: 2008-03140), New Delhi, India. The authors of this study were also supported by the Deanship of Scientific Research at Majmaah University, Kingdom of Saudi Arabia, under Project No. 38/128.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Whitcher J. P., Srinivasan M., Upadhyay M. P. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas P. A. Current perspectives on ophthalmic mycoses. Clinical Microbiology Reviews. 2003;16(4):730–797. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith G. T. H., Taylor H. R. Epidemiology of corneal blindness in developing countries. Journal of Refractive Surgery. 1991;7(6):436–439. [PubMed] [Google Scholar]
- 4.Basak S. K., Mohanta A., Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, Eastern India. Indian Journal of Ophthalmology. 2005;53(1):17–22. doi: 10.4103/0301-4738.15280. [DOI] [PubMed] [Google Scholar]
- 5.Savitri S., Srinivasan M., Celine G. The Current Status Of Fusarium Species In Mycotic Keratitis In South India. Indian Journal of Medical Microbiology. 1993;11(2):140–147. [Google Scholar]
- 6.Srinivasan M., Gonzales C. A., George C. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. British Journal of Ophthalmology. 1997;81(11):965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasudevan R., Jeya M. Prevalence of bacterial and fungal keratitis in and around Chidambaram. Journal of TNOA. 2006:44–25. [Google Scholar]
- 8.Bharathi M. J., Ramakrishnan R., Vasu S., Meenakshi R., Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian Journal of Ophthalmology. 2003;51(4):315–321. [PubMed] [Google Scholar]
- 9.De Lucca A. J. Harmful fungi in both agriculture and medicine. Revista Iberoamericana de Micología. 2007;24(1):3–13. [PubMed] [Google Scholar]
- 10.Manikandan P., Dóczi I., Kocsubé S., Varga J., Német T., Antal Z. In: Aspergillus in the genomic era. Varga J., Samson R., editors. The Netherlands: Wageningen Academic Publishers; 2008. [Google Scholar]
- 11.Bharathi M. J., Ramakrishnan R., Vasu S., Meenakshi Palaniappan R. Aetiological diagnosis of microbial keratitis in South India - a study of 1618 cases. Indian Journal of Medical Microbiology. 2002;20(1):19–24. [PubMed] [Google Scholar]
- 12.Dóczi I., Gyetvai T., Kredics L., Nagy E. Involvement of Fusarium spp. in fungal keratitis. Clinical Microbiology and Infection. 2004;10(9):773–776. doi: 10.1111/j.1469-0691.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan M. Fungal keratitis. Current Opinion in Ophthalmology. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bharathi M. J., Ramakrishnan R., Meenakshi R., Mittal S., Shivakumar C., Srinivasan M. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. British Journal of Ophthalmology. 2006;90(10):1271–1276. doi: 10.1136/bjo.2006.096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang D. C., Grant G. B., O'Donnell K., et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. The Journal of the American Medical Association. 2006;296(8):953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 16.Khor W.-B., Aung T., Saw S.-M., et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. Journal of the American Medical Association. 2006;295(24):2867–2873. doi: 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- 17.Liesegang T. J., Forster R. K. Spectrum of microbial keratitis in South Florida. American Journal of Ophthalmology. 1980;90(1):38–47. doi: 10.1016/S0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 18.Bhartiya P., Daniell M., Constantinou M., Islam F. M. A., Taylor H. R. Fungal keratitis in Melbourne. Clinical & Experimental Ophthalmology. 2007;35(2):124–130. doi: 10.1111/j.1442-9071.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 19.Lalitha P., Shapiro B. L., Srinivasan M., et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. JAMA Ophtalmology. 2007;125(6):789–793. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 20.Manikandan P., Vismer H. F., Kredics L., et al. Corneal ulcer due to Neocosmospora vasinfecta in an immunocompetent patient. Medical Mycology. 2008;46(3):279–284. doi: 10.1080/13693780701625149. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer C., Colom F., Frasés S., Mulet E., Abad J. L., Alió J. L. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. Journal of Clinical Microbiology. 2001;39(8):2873–2879. doi: 10.1128/JCM.39.8.2873-2879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition. CLSI document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute, 2008
- 23.Manikandan P., Varga J., Kocsubé S., et al. Epidemiology of Aspergillus keratitis at a tertiary care eye hospital in South India and antifungal susceptibilities of the causative agents. Mycoses. 2013;56(1):26–33. doi: 10.1111/j.1439-0507.2012.02194.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamel A., Ibrahim N., Mohmed A., Mohmed S., Joseph A. PCR identification of Fusarium genus based on nuclear ribosomal-DNA sequence data. African Journal of Biotechnology. 2003;2(4):82–85. [Google Scholar]
- 25.Deshpande S. D., Koppikar G. V. A study of mycotic keratitis in Mumbai. Indian Journal of Pathology and Microbiology. 1999;42(1):81–87. [PubMed] [Google Scholar]
- 26.Chander J., Sharma A. Prevalence of fungal corneal ulcers in Northern India. Infection. 1994;22(3):207–209. doi: 10.1007/BF01716706. [DOI] [PubMed] [Google Scholar]
- 27.Saha S., Banerjee D., Khetan A., Sengupta J. Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman J Ophthalmol. (3) 2009;2:114–118. doi: 10.4103/0974-620X.57310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rautaraya B., Sharma S., Kar S., Das S., Sahu S. K. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern india. BMC Ophthalmology. 2011;11(1, article 39) doi: 10.1186/1471-2415-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khairallah S. H., Byrne K. A., Tabbara K. F. Fungal keratitis in Saudi Arabia. Documenta Ophthalmologica. 1992;79(3):269–276. doi: 10.1007/BF00158257. [DOI] [PubMed] [Google Scholar]
- 30.Deorukhkar S., Katiyar R., Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: A five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singapore Medical Journal. 2012;53(4):264–267. [PubMed] [Google Scholar]
- 31.Gonzales C. A., Srinivasan M., Whitcher J. P., Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiology. 1996;3(3):159–166. doi: 10.3109/09286589609080122. [DOI] [PubMed] [Google Scholar]
- 32.Upadhyay M. P., Karmacharya P. C. D., Koirala S., et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. American Journal of Ophthalmology. 1991;111(1):92–99. doi: 10.1016/S0002-9394(14)76903-X. [DOI] [PubMed] [Google Scholar]
- 33.Galarreta D. J., Tuft S. J., Ramsay A., Dart J. K. G. Fungal keratitis in London: Microbiological and clinical evaluation. Cornea. 2007;26(9):1082–1086. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 34.Laspina F., Samudio M., Cibils D. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefe's Archive for Clinical and Experimental Ophthalmology. 2004;242(3):204–209. doi: 10.1007/s00417-003-0808-4. [DOI] [PubMed] [Google Scholar]
- 35.Pujol I., Guarro J., Gené J., Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. Journal of Antimicrobial Chemotherapy. 1997;39(2):163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 36.Therese K. L., Bagyalakshmi R., Madhavan H. N., Deepa P. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates. Indian Journal of Medical Microbiology. 2006;24(4):273–279. doi: 10.4103/0255-0857.29386. [DOI] [PubMed] [Google Scholar]
- 37.O'Day D. M. Selection of appropriate antifungal therapy. Cornea. 1987;6(4):238–245. doi: 10.1097/00003226-198706040-00002. [DOI] [PubMed] [Google Scholar]
- 38.Arikan S., Lozano-Chiu M., Paetznick V., Nangia S., Rex J. H. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. Journal of Clinical Microbiology. 1999;37(12):3946–3951. doi: 10.1128/jcm.37.12.3946-3951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfonso E. C. Genotypic identification of Fusarium species from ocular sources: comparison to morphologic classification and antifungal sensitivity testing (an AOS thesis) Transactions of the American Ophthalmological Society. 2008;106:227–239. [PMC free article] [PubMed] [Google Scholar]
- 40.Embong Z., Wan Hitam W. H., Yean C. Y., et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmology. 2008;8 doi: 10.1186/1471-2415-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaeger E. E. M., Carroll N. M., Choudhury S., et al. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. Journal of Clinical Microbiology. 2000;38(8):2902–2908. doi: 10.1128/jcm.38.8.2902-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer C., Alió J. L. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. Journal of Ophthalmic Inflammation and Infection. 2011;1(1):15–22. doi: 10.1007/s12348-011-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.