Abstract
The objective of this study was to examine the effects of Jackyakgamcho-tang (JGT) on acute colitis. GC/MS-based metabolomics and NGS-based metagenomics were applied to investigate the alteration of metabolites and microbiota in an acute colitis model. The severity of acute colitis symptoms was alleviated by JGT treatment. Induction of colitis and JGT treatment changed compositions of gut microbiota and inflammatory cytokine levels (TNF-α and IL-6). They also substantially change metabolites (i.e., lactic acid, linoleic acid, monostearin, and palmitoylglycerol). In addition, some clear correlations were observed among metabolites, cytokine, and microbiota. This study highlights the applicability of metabolomics and metagenomics study for evaluating anti-inflammatory effects of a new functional herbal medicine as a therapeutic agent for acute colitis.
1. Introduction
Inflammatory bowel disease (IBD) is a multifactorial disease that is difficult to identify the cause of onset [1]. Clinical features of IBD include abdominal pain, hemorrhagic diarrhea, and weight loss [2]. Some drugs such as 5-aminosalicylate and corticosteroid are used in the treatment of IBD, but long-term use of these drugs reduces efficacy and causes side effects. Recently, gut microbiome profiling studies have revealed that the occurrence and recovery of IBD are associated with changes of gut microbiota [3]. It has been also reported that gut microbiome is involved in the regulation of the host's metabolism. For example, nondigestible carbohydrates are fermented by gut bacteria to produce metabolites that can act as inflammatory modulators and signaling molecules [4].
Treatment of IBD by natural products (herbal medicine) can increase treatment effects with reduced side effects [5]. Substances such as andrographolide [6], andrographolide sulfonate [7], baicalin [8], curcumin [9], and baicalein [10] in natural products exhibit efficacy in treating IBD. Jackyakgamcho-tang (JGT) consists of Glycyrrhiza uralensis and Paeonia lactiflora. It is a Korean traditional herbal drug known to be effective for pain accompanied by muscle spasms. It contains various bioactive components with antioxidative, anti-inflammatory, antiviral, and neuroprotective effects [11, 12]. In Korean medicine, it is often prescribed for abdominal pain. However, effects of JGT against IBD-related recovery have not been fully studied [13–15].
Metabolomics involves comprehensive analysis of small-molecule metabolome within an organism or biological system [16]. Integral and systematic studies of metabolomics approaches are expected to provide new research methods for the study of Korean medicine. The purpose of this study was to examine the role of JGT in healing acute colitis. Next generation sequencing-based metagenomics and GC/MS-based metabolomics were employed in a profiling mode to reveal changes in microbial community structure and key metabolites.
2. Materials and Methods
2.1. HPLC Analysis of Major Components in JGT
A quantitative analysis of ten major compounds in JGT was performed with a Prominence LC-20A (Shimadzu, Kyoto, Japan) equipped with a photo-diode array detector. The marker components were separated on a Phenomenex Gemini C18 column (250 × 4.6 mm, 5 μm, Torrance, CA, USA) at column oven temperature of 40°C. The mobile phase consisted of 0.1% (v/v) aqueous formic acid and acetonitrile. Gradient flow was as follows: 8–55% B for 0–40 min, 55-78% B for 40-43 min, 78% B for 43-46 min, and 78-8% B for 46-50 min. The flow rate was at 1.0 mL/min. Chemical structures of these 10 reference standard compounds for JGT analysis are provided in Figure S1.
2.2. Animals
Male Sprague Dawley rats weighing approximately 240-250 g were obtained from Samtako Bio Korea (Osan, Korea). These rats were kept in standard laboratory conditions with a room temperature of 24 ± 1°C, humidity of 65 ± 5%, and a controlled light/dark cycle (12/12 h). The rat experiments in the present study were performed in compliance with the guidelines of the Ethics Committee of Dongshin University with approval number of 2017-05-02. Rats were randomly assigned to four groups (n = 6/group): (1) vehicle control, (2) acetic acid-induced colitis, (3) colitis induced plus treatment with JGT at 150 mg/kg, and (4) colitis induced plus treatment with JGT at 300 mg/kg.
2.3. Induction of Acute Colitis and JGT Treatment
Acute colitis was induced according to the sequence described by Ghasemi-Pirbaluti et al. [1]. Briefly, rats were fasted for 24 h before colitis induction with ad libitum access to water. For acute colitis induction, 1 mL of acetic acid (3% v/v in normal saline) was infused into the rectum with an 8-cm long tube under anesthesia using 2.5% of isoflurane. The rats were positioned head-down for 30 s to avoid expelling the solution. JGT treatment was administered through oral intake of 150 mg/kg (JGT 150) or 300 mg/kg (JGT 300) of JGT in drinking water. JGT was treated for 3 days from the colitis induction day and the first day was administered 4 hours after colitis induction. These doses of JGT were chosen based on existing clinical dose (Hanpoong Pharmaceutical Company, Seoul, Korea). The quality control data about JGT was provided in Table S1. The vehicle control group was treated with 1 mL of saline. All experimental groups were provided free access to laboratory chow and water for 3 days. The samples for the analyses were obtained at 3 days after the induction of colitis. The detailed experimental design is illustrated in Figure S2.
2.4. Histopathological Analysis
Distal portions of the colon were embedded in paraffin, sliced (6-μm in thickness), and stained with hemotoxylin and eosin (H&E). Inflammation and histological damage were observed under a light microscope (Nikon 80i, Japan) in a blinded manner.
2.5. Cytokine Quantification
Serum concentrations of cytokines TNF-α and IL-6 as indicators of inflammation were measured by ELISA kit (Invitrogen, USA). ELISA plates were read at 450 nm using a SpectraMax plate reader (M2, Molecular Devices, USA).
To measure the cDNA level of several cytokines such as IFN-γ, TNF-α, and IL-6, total RNA was extracted from the intestine using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and 100 ng RNA was used for the reaction. The RT-PCR cycles consisted of denaturation at 95°C for 5 s and annealing/extension at 65°C for 30 s for 40 cycles.
2.6. Analyses of Microbiota in Feces
Metagenomic DNA was extracted from each feces sample of rats using a PowerSoil® DNA Isolation kit (Cat. No. 12888, MO BIO). The concentration of obtained DNA and DNA quality were assessed using PicoGreen and Nanodrop, respectively. After checking the quality of metagenomic DNA by gel electrophoresis, 16S rRNA genes were amplified using 16S V3-V4 primers. DNA sequencing was then performed using MiSeq platform (Illumina, San Diego, CA, USA). Data were processed using Quantitative Insights Into Microbial Ecology (QIIME) v1.8 analysis pipeline [17].
2.7. Metabolic Analysis
Freeze dried serum and feces samples (100 mg) were ultrasonicated with 300 μL of solvent [methanol:water (7:3)] at 4°C for 30 min. Then, samples were centrifuged and lyophilized for derivatization. Sample derivatization and GC/MS analysis protocols were the same as described in our previous study [18].
2.8. Data Processing and Multivariate Analyses
GC/MS data were pretreated using XCMS web software (https://xcmsonline.scripps.edu) for noise removal, baseline correction, and alignment. Feature intensities were normalized according to the intensity of methyl stearate (internal standard) prior to multivariate statistical analyses. GC/MS preprocessing data files were imported into SIMCA-P (ver. 14.0) software package (Umetrics, Umea, Sweden) for multivariate analyses
3. Results
3.1. HPLC Analysis of JGT
Optimized HPLC-PDA method was used for quantitative determination of the ten major compounds in JGT composed of Paeonia lactiflora and Glycyrrhiza uralensis. As a result, these compounds (gallic acid, oxypaeoniflorin, albiflorin, paeoniflorin, liquiritin, benzoic acid, lsoliquiritin, ononin, benzoylpaeoniflorin, and glycyrrhizin) were eluted within 40 min (5.10, 11.35, 14.84, 15.88, 18.45, 21.49, 23.11, 24.08, 29.73, and 39.31 min, respectively). Representative HPLC-PDA chromatograms of the standard mixture and JGT sample are displayed in Figure S3. Based on the above results, amounts of these 10 compounds in JGT ranged from 0.34 mg/g to 30.94 mg/g (Table S2).
3.2. Clinical Symptoms
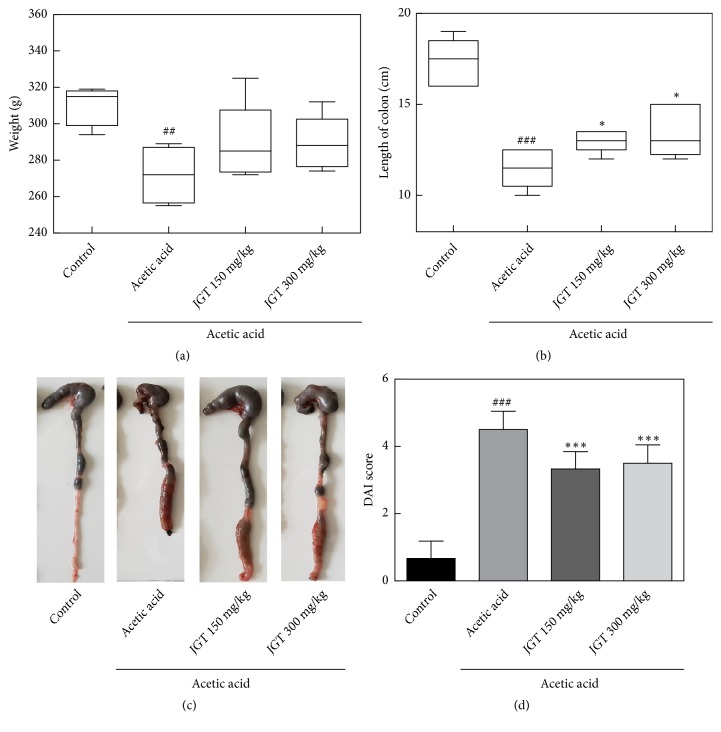
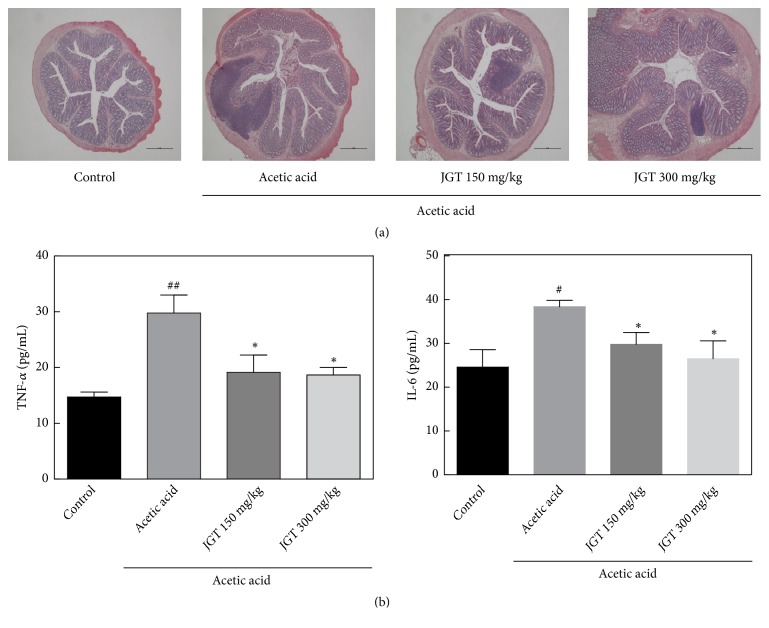
To assess effects of JGT on acute colitis, changes in body weight, length of large intestine, and histopathological signs in colon tissues were determined (Figure 1). The weight of rats that had colitis induced by acetic acid was decreased on the 3rd day of colitis induction from their baseline body weight. Treatment with JGT slightly prevented such weight loss (Figure 1). Similarly, the symptoms of colitis were alleviated by JGT treatment. The shortening of colon length was found in colitis-induced group (12.3 ± 0.7 cm vs. 17.3 ± 0.6 cm, p < 0.05). Colon shortening is known to be positively associated with colonic inflammation from acute colitis [19]. Disease activity index (DAI) score was calculated by using body weight loss, stool consistency, and blood in stool scores. DAI score of the control group was 0.67. It was increased to 4.29 in colitis-induced group. Significant decrease in DAI score was observed in groups receiving either JGT 150 mg/kg (p < 0.001) or JGT 300 mg/kg (p < 0.01) when compared to colitis-induced group. Acetic acid-induced rats exhibited acute colitis with severe inflammation for histopathological examinations of colons (Figure 2(a)). However, treatment with JGT (150 mg/kg or 300 mg/kg) reduced cell infiltrate and crypt damage, indicating that JGT treatment could decrease the severity of acetic acid-induced acute colitis.
Figure 1.
(a) Weight, (b) colon length, and (c) colon tissue pictures of rats treated with acetic acid and Jackyakgamcho-tang (JGT). (d) Disease activity index (DAI) value was calculated by using body weight loss, stool consistency, and blood in stool scores. Significant difference at #p < 0.05, ##p < 0.01, and ###p < 0.001 compared to the control group. Significant difference at ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p <0.001 compared to acetic acid-induced acute colitis group.
Figure 2.
Effect of JGT on inflammation in acute colitis rat gut tissue. (a) Representative images by H&E staining displaying colon segments on the day of sacrifice. (b) Levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Significant difference at #p < 0.05, ##p < 0.01, and ###p < 0.001 compared to the control group. Significant difference at ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p <0.001 compared to acetic acid-induced acute colitis group.
3.3. Inflammatory Cytokines
The pathogenesis of acute colitis is based on complicated cytokine-mediated signaling pathways [20]. Recent studies have demonstrated that most pathways are induced by intestinal T-cell activation via inflammatory mediators such as TNF-α, IL-1β, IL-6, IL-10, IL-12, and IL-23 [21–24]. Effects of JGT on cytokines' production are presented in Figure 2(b). Levels of TNF-α and IL-6 in the acute colitis group were noticeably higher than those in the control group. However, administration of JGT to colitis-induced rats significantly decreased levels TNF-α and IL-6, indicating reduced inflammation. Although some data are not significant, JGT administration slightly suppressed the levels of TNF-α, IL-6, and IFN-γ in the gut (Figure S4).
3.4. Metabolic Profiling
Representative GC/MS total ion current chromatograms from these four groups are shown in Figure S5. A total of 29 metabolites were identified in the serum and feces samples based on RI value, GC/MS library, and other researchers' data (Table S3). A multivariate analysis was conducted using features and normalized mass intensity obtained from the GC/MS (Figure 3). Supervised PLS-DA was used to obtain a clear separation among groups [25].
Figure 3.
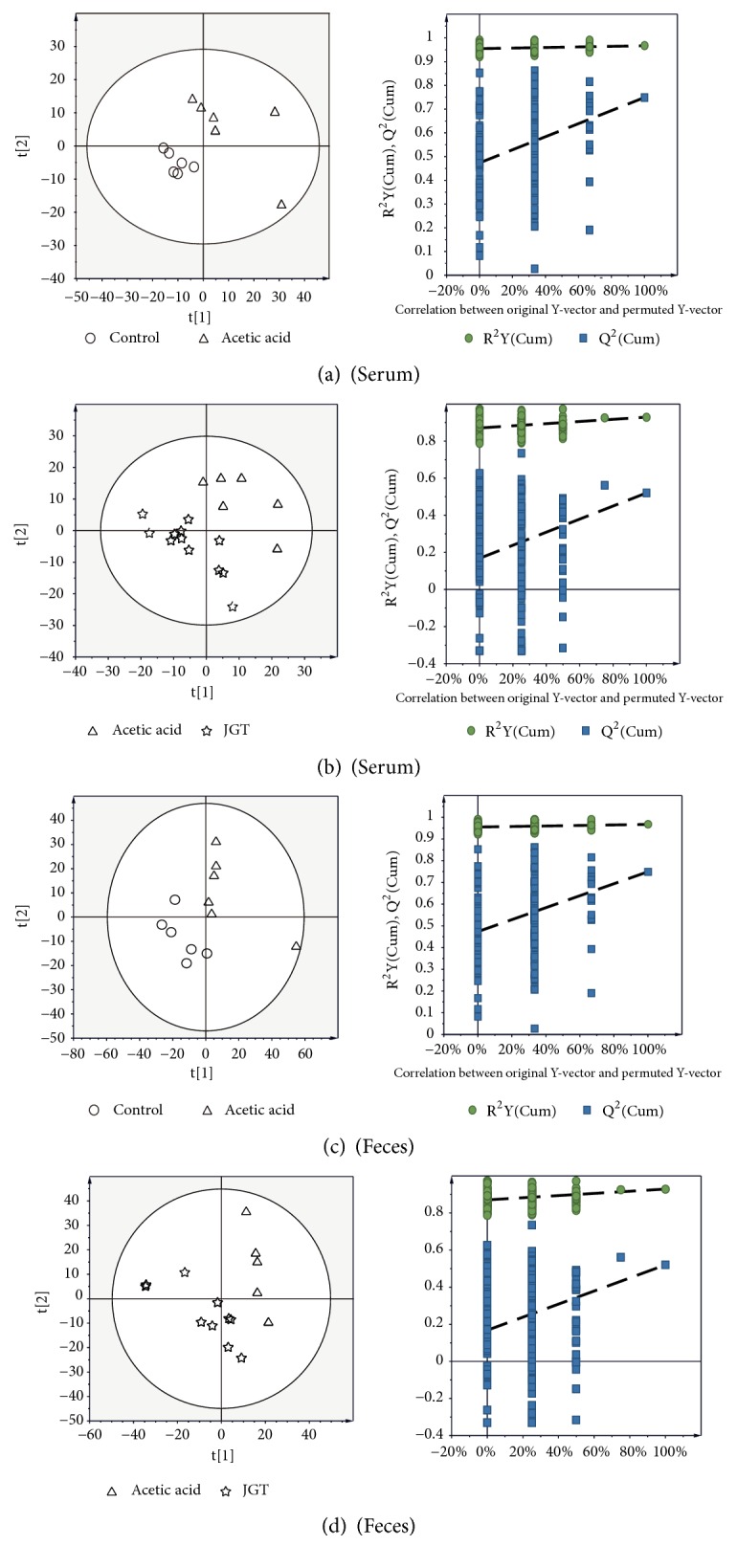
PLS-DA scores plots for control, acetic acid-induced acute colitis, and JGT treated groups derived from GC-MS data of serum (a, b) and feces (c, d) samples. These PLS-DA models were validated by a permutation test (n = 200).
PLS-DA score plots of serum and feces samples displayed a clear separation among groups, with high cumulative R2X, R2Y, and Q2 values. Low values of Q2 intercept in the permutation test indicated the robustness of models, demonstrating a low risk of overfitting [26]. These results indicated that metabolites of serum and feces could be changed by colitis induction and JGT treatment.
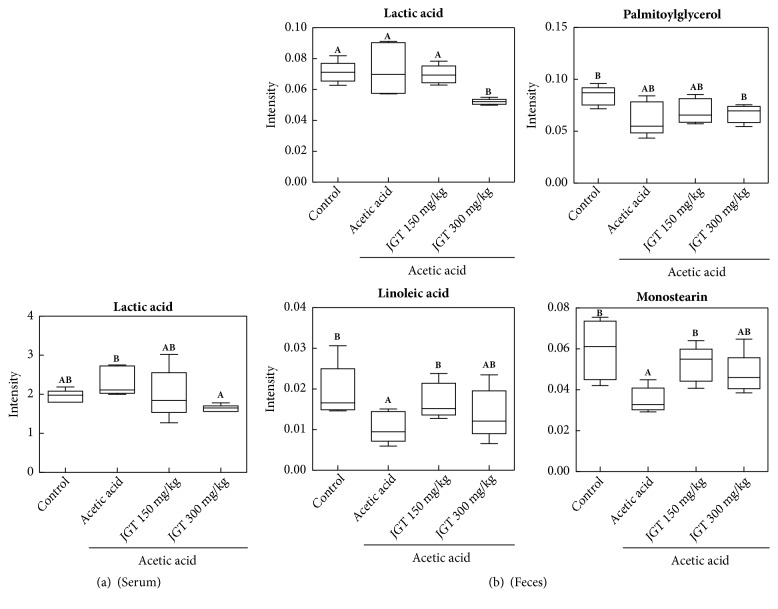
To find metabolites responsible for the classification, the parameter of variable importance in projection (VIP) was determined. Based on VIP greater than 1.0 from PLS-DA with p < 0.05 in two-tailed Student's t-test, a total of four metabolites were identified as variables contributing to the separation of samples in the PLS-DA score plot (Figure 4 and Table S4). The acute colitis group was characterized by higher serum levels of lactic acid together with lower levels of linoleic acid, monostearin, and palmitoylglycerol in feces compared to the control group. However, samples obtained in the JGT treatment groups exhibited opposite metabolites patterns except for palmitoylglycerol compared to the acute colitis group.
Figure 4.
Box plots of identified metabolites that contributed to the discriminating PLS-DA model (VIP > 1, p < 0.05) in (a) serum and (b) feces. Peak intensities of selected mass ions from GC/MS data were used for quantification. Data were based on six replicates (n = 6). For each compound, the statistical outcome was summarized within the figure (one-way ANOVA). Alphabet represents significant difference between samples (p < 0.05).
3.5. Gut Microbiota Changes
A metagenomics approach was used to analyze diversity of the microbial community after colitis induction and treatment with JGT. To analyze microbial richness and diversity in feces samples according to colitis induction, rarefaction curves at 97% similarity levels were calculated. Microbial richness and diversity of 16S rRNA libraries are shown in Table S5. Chao 1 richness values of feces samples were 327.8, 299.9, 271.9, and 298.5 for control, acetic acid, JGT 150, and JGT 300 groups, respectively. Simpson's diversity values of feces samples were 0.94, 0.83, 0.92, and 0.74 for control, acetic acid, JGT 150, and JGT 300 groups, respectively. Operational taxonomic units (OTUs) in control samples were also higher than those in other samples, implying more bacterial diversities in feces of control rats. Microbial community analysis results are shown in Figure S6. Bacteroidetes (35.5%) and Firmicutes (33.2%) were the two most abundant phylums in feces of the control group. However, Bacteroidetes was less abundant in rats with colitis induction or treatment with JGT compared to that in the control sample. Verrucomicrobia became the main component in microbial communities of colitis-induced rats except for those that received JGT 150 treatment.
3.6. Correlations among Metabolites, Gut Microbiota, and Inflammatory Cytokines
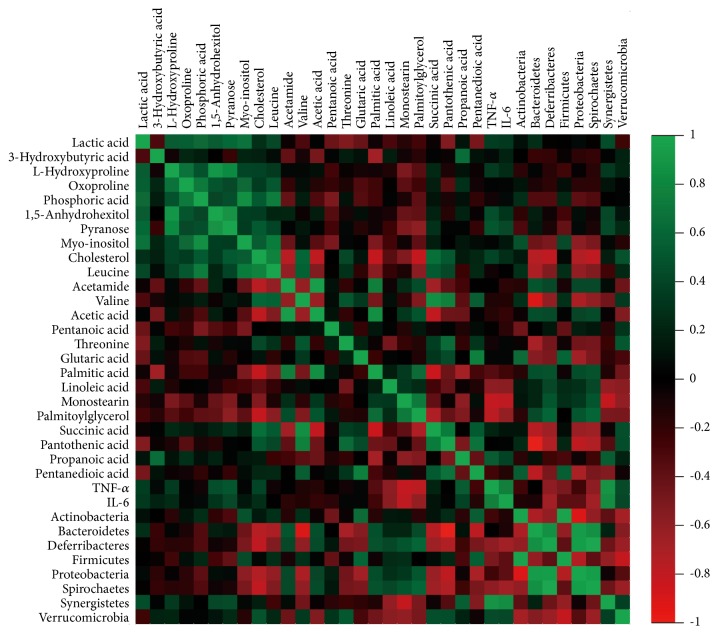
To explore functional relationships among altered gut microbiota, disturbed metabolites in serum and feces, and changed inflammatory cytokine levels (TNF-α and IL-6), three correlation matrixes were formed based on Pearson's correlation coefficients (Figure 5). Some variables were clearly correlated (r > 0.8 or r < -0.8). Hydroxyproline was positively correlated with 1, 5-anhydrohexitol and pyranose (r = 0.90 and r = 0.83, respectively). Acetic acid and valine were also positively correlated with palmitic acid (r = 0.85) and succinic acid (r = 0.85). Inflammatory cytokine levels (TNF-α and IL-6) displayed strong correlations with increased levels of Synergistetes (r = 0.88 and r = 0.84, respectively).
Figure 5.
Integration of variable correlations. The heat map derived from correlations among metabolites of serum and feces, inflammatory cytokines, and gut microbiota phyla.
4. Discussion
Recently, the use of herbal remedies has emerged as an alternative treatment for colitis [27–29]. Li et al. [30] have reported that YunNan BaiYao (YNBY), an amalgamation of various herbs, can significantly decrease disease progression of DSS- and TNBS-induced colitis with reduced levels of inflammatory cytokines such as TNF-α, IL-12, IFN-γ, and IL-17 in the colon and serum. In another study, Huang et al. [29] have reported the anti-inflammatory effect of Wedelia chinensis extracts in a colitis mouse model. As a Korean traditional herbal medicine, JGT is composed of Paeonia lactiflora and Glycyrrhiza uralensis whose leaf and stem tissues are commonly used as traditional medicinal herbs in many Asian countries. It is known to be effective for pain accompanied by muscle spasms. In this study, various bioactive compounds, including gallic acid, paeoniflorin, and glycyrrhizin, were detected in JGT. These compounds have been reported to possess anti-inflammatory, antioxidant, and immune functions [31, 32]. Pandurangan et al. [12] have reported that the administration of gallic acid can significantly (p < 0.05) suppress expression levels of inflammatory cytokines in DSS-induced colitis mice. In this study, JGT was effective in protecting against acetic acid-induced acute colitis. Its inhibition on TNF-α and IL-6 expression was observed in serum of test rats treated with JGT (Figure 2(b)). The expression of TNF-α was highly correlated with the development of colitis [33]. Therefore, the antiacute colitis activity of JGT may be regulated by its inhibition effect on TNF-α expression.
In the current study, multivariate statistical approaches were used to analyze metabolites present in the serum and feces samples. The PLS-DA model revealed a clear separation between not only the control and acetic acid-induced colitis groups, but also acetic acid-induced colitis and JGT treatment groups. These results suggest that metabolites of the serum and feces can be changed by colitis induction and JGT treatment. The acute colitis group was characterized by higher levels of lactic acid in the serum compared to control and JGT treatment groups. Several studies have reported that the incidence of colitis is related to the change in lactic acid concentrations. Song et al. [34] have found that levels of lactic acid are increased in inflammatory intestinal mucosa of IBD patients. The damage of intestinal endotheliocytes can increase the permeation of lactic acid produced by microorganisms in the intestine. Vernia et al. [35] have reported that lactic acid level is increased in feces water of severe colitis subjects. Another mechanism of increased lactic acid in colitis may be caused by changes in microorganisms in the intestine. Increased intraluminal oxygen concentration due to profuse bleeding favors facultative anaerobic strains such as lactobacilli and streptococci known to be lactic acid producers [35, 36]. Increased concentrations of lactic acid may also result from disruption of colonic mucosal cell lining and mesenchymal polysaccharides exposure to intraluminal bacteria [36]. Lactic acid has been reported to accumulate in feces from individuals who are suffering from ulcerative colitis. Scharff et al. [37] reported that eighteen of the 42 ischemic colitis patients (42%) had an abnormally elevated serum lactic acid level. According to the findings of Gilshtein et al. [38], the only laboratory factor found as a significant risk factor for mortality was increased lactic acid levels.
There are controversial studies on the relationship between linoleic acid contents and colitis. Some studies have reported the contribution of linoleic acid to inflammation [39, 40]. On the contrary, linoleic acid has been reported to have potential to inhibit inflammatory responses by lowering the production of inflammatory cytokines such as TNF-α and IL-1β [41]. In addition, linoleic acids can be transformed into transfatty acids and conjugated linoleic acids (CLA) by microorganisms in the intestine [42–44]. Several studies have reported that CLA and conjugated linolenic acid (CLNA) can suppress colonic inflammation and upregulate colonic peroxisome proliferator-activated receptor (PPAR) γ expression [45–48].
A combination of metagenomics and metabolomics can improve our understanding of the human gut microbiome and possibly provide a new strategy for the diagnosis and treatment of disease. In this study, bacterial diversities in the feces of the colitis group were lower compared to those of the control. A disbalance of commensal microbiota with decreased diversity and altered metagenome and metabolome might be linked to colitis [49–51]. There are many reports on the relationship between colonic microbial community and metabolites. Weir et al. [52] have reported that a mucin-degrading species (Akkermansia muciniphila) is about 4-fold higher while butyrate-producing species are underrepresented in colorectal cancer samples, although there are no significant differences in overall gut microbiome. Gut microorganisms can synthesize a wide range of lipids from short chain fatty acids (SCFAs) such as acetate, butyrate, and propionate to polyunsaturated fatty acids (PUFAs) involved in regulation of apoptosis and immune response [46, 53–55]. SCFAs are usually synthesized by gut bacteria from nondigestible polysaccharides [56]. Gut microbiome and its metabolites (SCFAs) can affect the immune system by regulating differentiation of resident mucosal immune cells [57]. Previous studies have demonstrated that butyrate can affect the induction of differentiation of colonic regulatory T cells [58]. PUFAs such as punicic and eleostearic acids have also received attention for colitis treatment [59]. The correlation between metabolites and gut bacteria associated with colitis is likely increased from changes in metabolites of microbes [60]. Duncan et al. [61] isolated 9 bacteria strains able to utilize lactate and produce butyrate from fecal samples. The lower quantity of lactate-utilizing butyrate-producing bacteria such as Clostridium coccoides could explain the low levels of SCFAs and greater abundance of lactate seen in active colitis [61, 62]. However, it is difficult to identify clear mechanisms involved in the change of metabolites. More precise studies are required to determine the relationship between colonic microorganisms and metabolites.
Acknowledgments
This research was supported by a grant (HI17C1858) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea.
Contributor Information
Chang-Su Na, Email: nakugi@hanmail.net.
Hong-Seok Son, Email: hsson@dsu.ac.kr.
Data Availability
The metabolomics data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
All authors contributed to this work, prepared the manuscript, and approved this version of the paper.
Supplementary Materials
Supplementary Figure 1. Chemical structures of the ten marker compounds in Jackyakgamcho-tang. Supplementary Figure 2. Schematic diagram of acute colitis induction and treatment. Supplementary Figure 3. Representative HPLC chromatograms of the (A) standard mixture and (B) Jackyakgamcho-tang sample at UV wavelengths 230 (I), 250 (II), 255 (III), 270 (IV), 275 (V), and 360 (VI) nm. Supplementary Figure 4. RT-PCR analysis of mucosal cytokine expression in acute colitis. Supplementary Figure 5. Representative GC-MS total ion current (TIC) chromatograms of (A) serum and (B) feces samples. Supplementary Figure 6. Microbial community analysis results. Supplementary Table 1. Quality control data of Jackyakgamcho-tang. Supplementary Table 2. Amounts of the ten marker compounds in Jackyakgamcho-tang by HPLC (n=3). Supplementary Table 3. Identified metabolites in serum and feces samples. Supplementary Table 4. Metabolic changes by acetic acid-induced colitis and Jackyakgamcho-tang administration in serum and feces. Supplementary Table 5. Microbial richness and diversity of 16S rRNA libraries based on 97% identity OTUs from airborne bacteria collected with groups, respectively.
References
- 1.Ghasemi-Pirbaluti M., Motaghi E., Bozorgi H. The effect of menthol on acute experimental colitis in rats. European Journal of Pharmacology. 2017;805:101–107. doi: 10.1016/j.ejphar.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Derikx L. A., Dieleman L. A., Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Practice & Research Clinical Gastroenterology. 2016;30(1):55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Nie Y., Xiao H., et al. TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Scientific Reports. 2016;6(1) doi: 10.1038/srep34680.32834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes E., Li J. V., Athanasiou T., Ashrafian H., Nicholson J. K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends in Microbiology. 2011;19(7):349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Guo B.-J., Bian Z.-X., Qiu H.-C., Wang Y.-T., Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Annals of the New York Academy of Sciences. 2017;1401(1):37–48. doi: 10.1111/nyas.13414. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Tan X. F., Nguyen V. S., et al. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis. Molecular & Cellular Proteomics. 2014;13(3):876–886. doi: 10.1074/mcp.M113.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Guo W., Guo L., et al. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. International Immunopharmacology. 2014;20(2):337–345. doi: 10.1016/j.intimp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W., Jin Z., Yu J., et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. International Immunopharmacology. 2016;35:119–126. doi: 10.1016/j.intimp.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto K., Hanai H., Tozawa K., et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123(6):1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 10.Dou W., Mukherjee S., Li H., et al. Alleviation of gut inflammation by Cdx2/PXR pathway in a mouse model of chemical colitis. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0036075.e36075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q., Chen X-G S., Huang H. Q., Chen Z. L. Effect of Glycyrrhiza flavonoids on amounts of immunocytes in S180 tumor-bearing mice. Journal of Traditional Chinese Medicine. 2007;12, article no 029 [Google Scholar]
- 12.Pandurangan A. K., Mohebali N., Norhaizan M. E., Looi C. Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Design, Development and Therapy. 2015;9:3923–3934. doi: 10.2147/DDDT.S86345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang C. K., Chun H. S. Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Bioscience, Biotechnology, and Biochemistry. 2012;76(3):536–543. doi: 10.1271/bbb.110842. [DOI] [PubMed] [Google Scholar]
- 14.Li P.-P., Liu D.-D., Liu Y.-J., et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. Journal of Ethnopharmacology. 2012;141(1):290–300. doi: 10.1016/j.jep.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z., Lin J., Huo R., et al. Total glucosides of paeony attenuated functional maturation of dendritic cells via blocking TLR4/5 signaling in vivo. International Immunopharmacology. 2012;14(3):275–282. doi: 10.1016/j.intimp.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson J. K., Lindon J. C., Holmes E. “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1991;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 17.Caporaso J. G., Kuczynski J., Stombaugh J., et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo S.-H., Park S.-E., Kim E.-J., Lee K.-I., Na C.-S., Son H.-S. A GC-MS based metabolomics approach to determine the effect of salinity on Kimchi. Food Research International. 2018;105:492–498. doi: 10.1016/j.foodres.2017.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Han F., Zhang H., Xia X., et al. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. The Journal of Immunology. 2015;194(4):1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- 20.Sato S., Chiba T., Nakamura S., Matsumoto T. Changes in cytokine profile may predict therapeutic efficacy of infliximab in patients with ulcerative colitis. Journal of Gastroenterology and Hepatology. 2015;30(10):1467–1472. doi: 10.1111/jgh.13008. [DOI] [PubMed] [Google Scholar]
- 21.Rogler G., Andus T. Cytokines in inflammatory bowel disease. World Journal of Surgery. 1998;22(4):382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 22.Atreya R., Mudter J., Finotto S., et al. blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nature Medicine. 2000;6:583–588. doi: 10.1038/nm1110-1341. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M., Yoshizaki K., Kishimoto T., Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. The Journal of Immunology. 2000;164(9):4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 24.Kai Y., Takahashi I., Ishikawa H., et al. Colitis in mice lacking the common cytokine receptor γ chain is mediated by IL-6-producing CD4+ T cells. Gastroenterology. 2005;128(4):922–934. doi: 10.1053/j.gastro.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Wu H., Liu T., Ma C., et al. GC/MS-based metabolomic approach to validate the role of urinary sarcosine and target biomarkers for human prostate cancer by microwave-assisted derivatization. Analytical and Bioanalytical Chemistry. 2011;401(2):635–646. doi: 10.1007/s00216-011-5098-9. [DOI] [PubMed] [Google Scholar]
- 26.Sun H.-Z., Wang D.-M., Wang B., et al. Metabolomics of four biofluids from dairy cows: Potential biomarkers for milk production and quality. Journal of Proteome Research. 2015;14(2):1287–1298. doi: 10.1021/pr501305g. [DOI] [PubMed] [Google Scholar]
- 27.Langmead L., Rampton D. S. Review article: complementary and alternative therapies for inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2006;23(3):341–349. doi: 10.1111/j.1365-2036.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- 28.Jackson L. N., Zhou Y., Qiu S., Wang Q., Mark Evers B. Alternative Medicine Products as a Novel Treatment Strategy for Inflammatory Bowel Disease. American Journal of Chinese Medicine. 2008;36(05):953–965. doi: 10.1142/S0192415X08006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y.-T., Wen C.-C., Chen Y.-H., et al. Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064152.e64152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R., Alex P., Ye M., Zhang T., Liu L., Li X. An old herbal medicine with a potentially new therapeutic application in inflammatory bowel disease. International Journal of Clinical and Experimental Medicine. 2011;4(4):309–319. [PMC free article] [PubMed] [Google Scholar]
- 31.Lee W., Yang E.-J., Ku S.-K., Song K.-S., Bae J.-S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation. 2013;36(1):94–102. doi: 10.1007/s10753-012-9523-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen P., Zhou X., Zhang L., et al. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. Journal of Ethnopharmacology. 2015;162:207–214. doi: 10.1016/j.jep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Onizawa M., Nagaishi T., Kanai T., et al. Signaling pathway via TNF-α/NF-κB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(4):G850–G859. doi: 10.1152/ajpgi.00071.2008. [DOI] [PubMed] [Google Scholar]
- 34.Song W.-B., Lv Y.-H., Zhang Z.-S., et al. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World Journal of Gastroenterology. 2009;15(31):3916–3919. doi: 10.3748/wjg.15.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernia P., Caprilli R., Latella G., Barbetti F., Magliocca F. M., Cittadini M. Fecal Lactate and Ulcerative Colitis. Gastroenterology. 1998;95(6):1564–1568. doi: 10.1016/S0016-5085(88)80078-7. [DOI] [PubMed] [Google Scholar]
- 36.van der Wiel Korstanje J. A. A., Winkler K. C. The faecal flora in ulcerative colitis. Journal of Medical Microbiology. 1975;8(4):491–501. doi: 10.1099/00222615-8-4-491. [DOI] [PubMed] [Google Scholar]
- 37.Scharff J. R., Longo W. E., Vartanian S. M., Jacobs D. L., Bahadursingh A. N., Kaminski D. L. Ischemic colitis: spectrum of disease and outcome. Surgery. 2003;134(4):624–630. doi: 10.1016/S0039-6060(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 38.Gilshtein H., Hallon K., Kluger Y. Ischemic colitis caused increased early and delayed mortality. World Journal of Emergency Surgery. 2018;13(1, article no 31) doi: 10.1186/s13017-018-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoda R., Matsueda K., Yamato S., Umeda N. Epidemiologic analysis of Crohn disease in Japan: Increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. American Journal of Clinical Nutrition. 1996;63(5):741–745. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 40.Calder P. C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition. 2006;83(6):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 41.Milanski M., Degasperi G., Coope A., et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. The Journal of Neuroscience. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devillard E., McIntosh F. M., Duncan S. H., Wallace R. J. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. Journal of Bacteriology. 2007;189(6):2566–2570. doi: 10.1128/jb.01359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntosh F. M., Shingfield K. J., Devillard E., Russell W. R., Wallace R. J. Mechanism of conjugated linoleic acid and vaccenic acid formation in human faecal suspensions and pure cultures of intestinal bacteria. Microbiology. 2009;155(1):285–294. doi: 10.1099/mic.0.022921-0. [DOI] [PubMed] [Google Scholar]
- 44.Gorissen L., Raes K., Weckx S., et al. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Applied Microbiology and Biotechnology. 2010;87(6):2257–2266. doi: 10.1007/s00253-010-2713-1. [DOI] [PubMed] [Google Scholar]
- 45.Hontecillas R., Wannemeulher M. J., Zimmerman D. R., et al. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. Journal of Nutrition. 2002;132(7):2019–2027. doi: 10.1093/jn/132.7.2019. [DOI] [PubMed] [Google Scholar]
- 46.Bassaganya-Riera J., Reynolds K., Martino-Catt S., et al. Activation of PPARγ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127(3):777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 47.Clarke J. M., Topping D. L., Christophersen C. T., et al. Butyrate esterified to starch is released in the human gastrointestinal tract. American Journal of Clinical Nutrition. 2011;94(5):1276–1283. doi: 10.3945/ajcn.111.017228. [DOI] [PubMed] [Google Scholar]
- 48.Hofmanová J., Ciganek M., Slavík J., et al. Lipid alterations in human colon epithelial cells induced to differentiation and/or apoptosis by butyrate and polyunsaturated fatty acids. The Journal of Nutritional Biochemistry. 2012;23(6):539–548. doi: 10.1016/j.jnutbio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Marchesi J. R., Holmes E., Khan F., et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. Journal of Proteome Research. 2007;6(2):546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed I., Greenwood R., de Costello B. L., Ratcliffe N. M., Probert C. S. An Investigation of Fecal Volatile Organic Metabolites in Irritable Bowel Syndrome. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058204.e58204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirnov K. S., Maier T. V., Walker A., et al. Challenges of metabolomics in human gut microbiota research. International Journal of Medical Microbiology. 2016;306:266–279. doi: 10.1016/j.ijmm.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Weir T. L., Manter D. K., Sheflin A. M., Barnett B. A., Heuberger A. L., Ryan E. P. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070803.e70803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassaganya-Riera J., Hontecillas R., Beitz D. C. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clinical Nutrition. 2002;21(6):451–459. doi: 10.1054/clnu.2002.0594. [DOI] [PubMed] [Google Scholar]
- 54.O'Shea M., Bassaganya-Riera J., Mohede I. C. Immunomodulatory properties of conjugated linoleic acid. American Journal of Clinical Nutrition. 2004;79(6):1199S–1206S. doi: 10.1093/ajcn/79.6.1199S. [DOI] [PubMed] [Google Scholar]
- 55.Serini S., Piccioni E., Merendino N., Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14(2):135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 56.Cheng L., Jin H., Qiang Y., et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. International Immunopharmacology. 2016;40:1–10. doi: 10.1016/j.intimp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 58.Smith P. M., Howitt M. R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bassaganya-Riera J., Pogranichniy R. M., Jobgen S. C., et al. Conjugated linoleic acid ameliorates viral infectivity in a pig model of virally induced immunosuppression. Journal of Nutrition. 2003;133(10):3204–3214. doi: 10.1093/jn/133.10.3204. [DOI] [PubMed] [Google Scholar]
- 60.McHardy I. H., Goudarzi M., Tong M., et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1(1, article no 17) doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan S. H., Louis P., Flint H. J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Applied and Environmental Microbiology. 2004;70(10):5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjerrum J. T., Wang Y., Hao F., et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics. 2015;11(1):122–133. doi: 10.1007/s11306-014-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Chemical structures of the ten marker compounds in Jackyakgamcho-tang. Supplementary Figure 2. Schematic diagram of acute colitis induction and treatment. Supplementary Figure 3. Representative HPLC chromatograms of the (A) standard mixture and (B) Jackyakgamcho-tang sample at UV wavelengths 230 (I), 250 (II), 255 (III), 270 (IV), 275 (V), and 360 (VI) nm. Supplementary Figure 4. RT-PCR analysis of mucosal cytokine expression in acute colitis. Supplementary Figure 5. Representative GC-MS total ion current (TIC) chromatograms of (A) serum and (B) feces samples. Supplementary Figure 6. Microbial community analysis results. Supplementary Table 1. Quality control data of Jackyakgamcho-tang. Supplementary Table 2. Amounts of the ten marker compounds in Jackyakgamcho-tang by HPLC (n=3). Supplementary Table 3. Identified metabolites in serum and feces samples. Supplementary Table 4. Metabolic changes by acetic acid-induced colitis and Jackyakgamcho-tang administration in serum and feces. Supplementary Table 5. Microbial richness and diversity of 16S rRNA libraries based on 97% identity OTUs from airborne bacteria collected with groups, respectively.
Data Availability Statement
The metabolomics data used to support the findings of this study are available from the corresponding author upon request.