Abstract
Individuals with Parkinson's disease (PD) have shown impaired performance on the verbal suppression component of the Haylings Sentence Completion Test (HSCT). The present study aimed to determine whether this performance related to (i) the inability to suppress a pre-potent response or (ii) difficulty in the generation of a strategy to facilitate task execution. The study adopted a novel variation of the HSCT that isolated each process and employed fMRI to examine the associated neural correlates in a comparison of individuals with PD and matched healthy controls. No significant behavioral differences were detected between these two groups. However, fMRI results revealed atypical underlying neural activity in the PD group. Controls exhibited increased activation in the left dorsolateral prefrontal cortex and striatum when generating a response independently, relative to generation when a supporting strategy was provided. The PD group demonstrated the opposite pattern of activation, in addition to greater recruitment of right hemisphere regions. This pattern of activation was postulated to be evidence of compensatory mechanisms, acting to bolster the output of frontostriatal circuits compromised by disease pathology.
Keywords: Lexical-semantics, Inhibition, Parkinson's disease, Prefrontal cortex, fMRI
Abbreviation: HSCT, Hayling Sentence Completion Task
Highlights
-
•
Verbal suppression and strategy generation was similar in PD and control groups.
-
•
Behavioral performance was subserved by atypical frontostriatal activity in PD.
-
•
Compensatory neural mechanisms may preserve behaviour in early disease stages.
1. Introduction
Language disturbances have been widely documented in Parkinson's Disease (PD; for reviews see Colman & Bastiaanse, 2011; Murray, 2008; Altmann and Troche, 2011), including disrupted verbal fluency (Auriacombe et al., 1993; Bayles et al., 1993; Flowers et al., 1995; Henry and Crawford, 2004; Herrera et al., 2012; Piatt et al., 1999; Raskin et al., 1992). Verbal fluency difficulties may reflect an underlying deficit in verbal selection and suppression, however verbal fluency tasks are complex in nature, and require several skills including lexical search, retrieval, selection, and suppression (Perret, 1974; Ruff et al., 1997). Determining the precise nature of these difficulties is of particular clinical significance, as decreased verbal fluency performance has been linked to increased risk of dementia development (Jacobs et al., 1995; Levy et al., 2002; Williams-Gray et al., 2007).
A task that has been employed to further isolate some of these component processes is the Hayling Sentence Completion Task (HSCT), intended to measure verbal selection and suppression (Burgess and Shallice, 1996). This task presents participants with contextually constrained sentences that have had their final word removed. In response, participants are required to either produce a word that is congruent with the sentence (Part A, posited to measure verbal selection) or a word that is incongruent (Part B, posited to measure verbal suppression). Burgess and Shallice further hypothesised that the ability to internally develop and apply a strategy would assist in generating an incongruent response in Part B (e.g. naming objects in the room) and so developed a scoring system that allowed for this to be subjectively measured.
Participants with PD have typically performed poorly on the HSCT. It has been observed that PD cohorts record significantly slower response times on Part B of the task relative to healthy controls (Bouquet et al., 2003; Obeso et al., 2011), and some authors have also reported increased comission of inhibition errors (Obeso et al., 2011; O'Callaghan et al., 2013a, O'Callaghan et al., 2013b). Such findings have been interpretted as evidence of an impairment in the ability to suppress a highly prepotent response in favor of a task appropriate response. This conclusion is further supported by studies of individuals with surgically managed PD. Castner et al. (2007) administered the HSCT to a group of PD patients receiving bilateral stimulation of the subthalamic nucleus (STN), and found that when off stimulation, the PD participants demonstrated significantly slower response times and made a larger number of errors on the HSCT Part B, relative to healthy controls. When stimulators were switched on, performance was improved to a level commensurate with controls. While providing further evidence of disrupted verbal suppression in PD, these results additionally suggest a role for the subthalamic nucleus in facilitating aspects of verbal suppression or related processes.
To date, assumptions regarding the neural mechanisms underlying verbal selection and suppression differences between healthy controls and the PD population have been primarily based on comparison of behavioral performance and known basal ganglia pathology in PD. In healthy controls, functional neuroimaging has consistently demonstrated recruitment of the frontal lobe during completion of both components of the task, though the precise location varies. Typically, responses in both conditions are associated with increased activity in the left inferior frontal gyrus (IFG), while mid-frontal and orbitofrontal activation is commonly observed during the Part B in parallel with slower response times relative to Part A (Allen et al., 2008; Collette et al., 2001; Nathaniel-James et al., 1997).
It is well-established that regions of the frontal cortex are functionally connected to nuclei of the basal ganglia, via a number of parallel, closed-circuit feedback loops known as basal-ganglia-thalamo-cortical (BGTC) circuits or frontostriatal networks, and that signalling along these pathways is altered in PD as a result of the nigrostriatal pathology (ref). In the motor realm these tracts allow the basal ganglia to provide top-down control over the selection and inhibition of competing motor plans in order to facilitate fluid movement (Alexander and Crutcher, 1990; Frank, 2006; Mink, 1996). Given that the subcortical circuitry believed to subserve cognitive functions is analogous to that of the motor realm, it has been hypothesised that their function may also be similar (Frank, 2006; Redgrave et al., 1999), and indeed, the role of frontostriatal circuitry in facilitating cognitive functions has now been widely documented (Dirnberger and Jahanshahi, 2013; Lewis et al., 2003; Owen, 2004). Furthermore, it has been speculated that the subtle impairments in language production associated with PD are the result of the interaction between cognitive processes (mediated by the frontostriatal networks) and linguistic processing in cerebral regions (for review see Altmann and Troche, 2011; Murray, 2008; Pell and Monetta, 2008). Taken together with the evidence provided by Castner et al. (2007) of a possible modulatory role for the STN, it therefore appears reasonable to hypothesise that the difficulties exhibited by individuals with PD completing the HSCT may have their origin in disrupted frontostriatal signalling, secondary to disease pathology within the basal ganglia. However this is yet to be directly confirmed through the utilisation neuroimaging in a PD population.
In addition to a paucity of neuroimaging evidence in PD cohorts, it can also be argued that the conclusions that can be drawn from such studies are limited by the task's design and scoring. The process for analysis of results, described by Burgess and Shallice (1996), dictates that the score on Part B minus the score on Part A indirectly indexes the demands of suppressing the prepotent response. This design does not account for the underlying component processes that are at play in each part of the HSCT, following the presentation of the sentence stem. In Part A, participants are presumed to activate a set of possible responses, enhance the activation of the most appropriate response, suppress competing alternatives, and verbally produce the selected word. In comparison, Part B will initiate activation of a set of prepotent responses, one of which will likely be automatically enhanced as the most contextually accurate. All of these responses must then be suppressed. At this point, the participant is required to generate an alternative response and produce the selected word. It can therefore be noted that in addition to the likely differences in suppression of a non-prepotent (Part A) vs. a strongly prepotent (Part B) response, Part B also has an additional step involving the generation of an alternative response. It may be assumed that this process would be best facilitated by the application of a strategy that streamlines the search, retrieval and selection of an alternative word from within a large pool of possibilities. Internal strategy formulation is closely related to cognitive control, and has been previously identified as being impaired in PD (Taylor et al., 1986). It could therefore be suggested that either verbal suppression, strategy generation, or both of these cognitive functions underlie the difficulties observed on the HSCT in this population. As previously discussed, Burgess and Shallice (1996) only accounted for the possible contribution of strategy generation by subjectively rating the participant's responses based on whether they appear to be strategic in nature.
These limitations also have implications for the interpretation of imaging findings, such as those described above, in that the potential to draw associations between structure and function (i.e. verbal suppression vs. verbal selection) may have been confounded by the interference of strategic processing. In an attempt to address this issue, de Zubicaray et al. (2000) developed a novel fMRI task, analogous to the HSCT, to allow for increased precision in the isolation of verbal selection, suppression, and strategy use. In young, healthy adult males they found 94% of responses in the suppression condition were generated based on use of strategy, and that relative to the initiation condition this correlated with increased activity in a network of frontal regions including the left dorsolateral prefrontal cortex (dlPFC) and the anterior cingulate cortex (ACC). Both of these regions have been identified as participating in frontostriatal feedback loops described above. Indeed, a current theory of cognitive control posits that the dlPFC provides top-down signals that bias activity in other cortical areas in order to favor a weak but task-relevant response, via these frontostriatal pathways (Miller and Cohen, 2001). In the context of the findings of de Zubicaray et al. (2000) this would appear to support the notion that the dlPFC was recruited during the component of the task requiring participants to implement a strategy and generate an alternative, incongruent response. It therefore appears likely that administering the HSCT in the PD population in a manner that allows for quantitative measurement of the influence of strategy may offer further insight into the mechanisms underlying their difficulty with the task.
For the present study, an fMRI paradigm was designed to examine brain activity associated with the component processes underlying the HSCT in individuals with PD relative to healthy controls. Specifically, the study aimed to test whether the altered performance of PD participants relative to controls on the HSCT was the result of deficits in verbal suppression, or strategy generation and implementation. This was achieved by comparing performance in the HSCT Part B (requiring strategy formation) with a novel condition in which individuals were provided with a strategy for producing an unrelated word. Based on the literature reviewed above, it was hypothesised that the ability to formulate a strategy would place significant demands upon the dlPFC frontostriatal loop, and thus it was expected that activity here would be decreased in the PD group as a result of disease-driven dysfunction in this circuitry.
2. Methods
2.1. Participants
Thirteen participants (6 males) with idiopathic PD were recruited. All participants in the PD group were required to meet the following inclusion criteria: (1) diagnosis of idiopathic PD prior to inclusion in the study (diagnosis confirmed using Calne et al., 1992); (2) right-handed, confirmed with the Annett Hand Preference Questionnaire (Annett, 1970); (3) English as a first language; (4) Hoehn and Yahr, 2001 rating of 1–3. Applicants were excluded if there was a history of substance abuse, head trauma, stereotaxic surgery and/or neurological disease other than PD. The Geriatric Depression Scale (GDS; Sheikh and Yesavage, 1986) was administered to screen for untreated clinical depression. A score greater than eight was considered indicative of major clinical depression in PD and any participants scoring in this range were excluded (Dissanayaka et al., 2011; Dissanayaka et al., 2007). The Montreal Cognitive Assessment (MoCA v7.1; Nasreddine et al., 2005) was administered in order to screen for significant cognitive impairment. Participants who achieved a score that was >1 SD below the expected range for their age group (Rossetti et al., 2011) were excluded from further involvement in the study. Potential participants were also excluded if they presented with moderate-severe dysarthria (in order to minimize variation in response transcription due to poor intelligibility of speech) or an uncorrected hearing or visual impairment that could affect the validity of task performance (self-reported). Years of education (YOE) was calculated for each participant and included years spent undertaking primary, secondary, bachelor, post-graduate, and diploma/certificate studies. Levodopa equivalent daily dosage (LEDD) was calculated for each PD participant based on the procedures outlined by Tomlinson et al. (2010). The demographic and neurological characteristics of the PD participants are presented inTable 1.
Table 1.
Characteristics of participants with PD.
| Participant | Agea | Sex | Disease durationa | YOE | HY | LEDD | MoCA | GDS |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | 1 | 18 | 1 | 100 | 29 | 1 |
| 2 | 62 | M | 14 | 9 | 2 | 298 | 20 | 4 |
| 3+ | 59 | F | 4 | 16 | 2 | 364 | 27 | 8 |
| 4 | 55 | F | 3 | 20 | 1 | 512.5 | 26 | 0b |
| 5 | 70 | F | 1 | 12 | 1 | 100 | 25 | 4 |
| 6 | 57 | M | 7 | 12 | 2 | 1787.5 | 23 | 5b |
| 7 | 62 | M | 7 | 9 | 2 | 1050 | 24 | 5b |
| 8 | 69 | M | 10 | 10 | 1 | 191 | 24 | 1b |
| 9 | 73 | M | 4 | 17 | 2 | 500 | 26 | 4 |
| 10 | 49 | F | 2 | 11 | 1 | 600 | 24 | 3 |
| 11+ | 61 | F | 7 | 12 | 2 | 348 | 27 | 6b |
| 12 | 58 | F | 4 | 14 | 1 | 450 | 26 | 0 |
| 13 | 69 | M | 6 | 17 | 1 | 349.5 | 24 | 0 |
| M | 62.23 | NA | 5.39 | 13.62 | 1.46 | 511.58 | 25 | 3.3 |
| SD | 6.83 | 3.8 | 3.64 | 0.52 | 455.67 | 2.24 | 2.6 |
Note. YOE = Years of Education; LEDD = Levodopa Equivalent Daily Dosage (mg/day); HY = Hoehn & Yahr rating. MoCA = Montreal Cognitive Assessment. GDS = Geriatric Depression Scale; M = male; F = female.
Participant subsequently excluded prior to data analysis.
Age and Disease duration are reported in years.
Participant was taking anti-depressant medication at time of testing.
Eighteen neurologically healthy participants were recruited as controls (6 males, mean age = 68.06 years [9.52], mean YOE = 16 [4]). There was no significant difference between the control and PD groups for age (p = .07), YOE (p = .1) or gender (x2 = 0.71). Controls were excluded if: (1) they were left handed (Annett, 1970); (2) they had a history of alcohol and/or substance abuse, neurological disease, surgery and/or trauma; (3) they had an uncorrected vision or hearing impairment that could affect validity of task performance; or (4) they achieved a score on the MoCA (Nasreddine et al., 2005) that was >1 SD below the expected range for their age group and level of education (Rossetti et al., 2011). The mean total MoCA score for the control group was 27 [1.8].
A battery of neurocognitive assessments was also administered to all participants in order to establish cognitive baselines. These assessments included the Boston Naming Test 2nd Edition (BNT; Kaplan et al., 2001), selected subtests of the Test of Everyday Attention (TEA; Robertson et al., 1994) including Elevator Counting and Elevator Counting with Distraction, the National Adult Reading Test (NART; Nelson and Willison, 1991), digits forwards and backwards, and verbal fluency (phonemic, semantic, and cued).
The study was approved by the Human Research Ethics Committee of the University of Queensland and was therefore in accordance with the 2007 NHMRC National Statement on Ethical Conduct in Human Research. Participants provided written informed consent prior to their inclusion in the study. All participants were financially compensated for their participation in the study.
2.2. Experimental design and stimuli
The study employed a novel variation on the HSCT originally described by (Burgess and Shallice, 1996). This modification was required in order to differentiate between the processes of response inhibition, response initiation and strategy formation. Stimuli consisted of 120 high cloze probability sentences, 6–8 words in length (Mlength = 7.2 [0.8]), with the final word removed. These were obtained from an expanded version of Bloom and Fischler (1980) sentence completion norms, compiled by Block and Baldwin (2010). This database comprises 400 high cloze probability sentences standardised against an undergraduate student population. N-watch software (Davis, 2005) was employed to determine the CELEX spoken word frequency of the final word (i.e. the most frequently provided ‘most probable’ response) in the high cloze probability sentences.
Three conditions were constructed termed complete, unrelated, and strategy. In the complete condition sentence stems were presented followed by the instruction “complete”. Participants were required to provide a single word that accurately completed the sentence conceptually and grammatically. Participants completed this condition twice (60 trials in total), using alternative sets of stimuli. In the unrelated condition sentence stems were presented followed by the instruction “unrelated”. Participants were required to provide a single word that was completely unrelated to the context of the sentence. Participants completed this condition once only (30 trials in total).
In the novel strategy condition sentence stems were presented followed by a semantic category cue (e.g., “fruit” or “transport”) that was unrelated to the context of the sentence. In order to minimize potential priming effects, categories were unrelated to the context of sentence stems included in this condition, and did not relate semantically to any responses predicted for the complete condition. Participants were required to generate a single word that is derived from the given category. Eight high frequency semantic categories (six experimental and two for practice trials) were selected from the Battig and Montague (1969) norms. These included color, transport, fruit, furniture, sport, and clothing for experimental trials, and tools and vegetables for practice trials. The Battig and Montague (1969) norms reported the mean total number of members generated for each of the experimental categories to be as follows: color (M = 9.73 [3]), transport (M = 7.02 [23]), fruit (M = 7.82 [14]), furniture (M = 7.25 [18]), sport (M = 7.93 [13]), and clothing (M = 9.54 [5]). The strategy condition contained 30 trials in total and each category appeared five times. The occurrence of each category cue was pseudo-randomized such that a minimum of five items separated repetitions of the same category. Sentence stems were pseudo-randomized across all conditions (complete, unrelated, and strategy) in order to minimize semantic associations existing between lexical items contained within each sentence stem, the most probable responses, and the selected semantic categories provided in the strategy condition (for example, exposure to the sentence stem “On Valentines day the women received a single red…” in the complete condition could potentially prime the response “red” in relation to the semantic category of color when encountered in the subsequent strategy condition). One-way ANOVA demonstrated no significant differences between conditions with respect to sentence stem length (F [3, 116] = 0.744, p = .528), cloze probability (F [3, 116] = 0.134, p = .940), or CELEX spoken word frequency of the most probable response (F [3, 116] = 0.734, p = .534).
Participants completed two runs of 60 trials successively within one scanning session, with a short break in between. The first run examined the complete and unrelated conditions, with the second involving the complete and strategy conditions. This sequence of events was designed to prevent participants from using the semantic categories provided in the cued condition to aid response generation in the unrelated condition, and thus remained constant for each participant. Conditions were presented sequentially (five trials per block) within each experimental run in order to minimize cognitive set-switching demands.
2.3. Procedure
Prior to commencing each experimental run, participants received five practice trials of each condition in order to familiarize themselves with the task requirements. Practice trials for complete and unrelated conditions were presented prior to run 1 (outside of scanner), with trials for the strategy condition only presented prior to Run 2 (in scanner). This arrangement was designed to prevent participants from utilising the semantic category approach of the strategy condition to support completion of unrelated trials (note however that if that if participants spontaneously utilised this strategy independently during the unrelated condition their responses were still considered valid). Practice stimuli were selected such that the occurrence of semantic associations between practice and test items was minimized. During practice trials, corrective feedback was provided by the examiner as per the original HSCT protocol (Burgess and Shallice, 1996). For the novel strategy condition, participants were corrected if they provided a word that was unrelated to the sentence stem but did not belong to the given category. Importantly, the categories used during practice trials were not included in the experimental trials. Participants were discouraged from providing the same response to multiple items (e.g., providing “banana” for every item).
Stimuli were presented on a computer monitor using Cogent 2000 software (Wellcome Department of Imaging Neuroscience, 2013) operating via a Matlab R2011b platform (MathWorks, 2011) with a screen resolution of 1024 × 768, Arial font in size 50. The screen projected onto a monitor visible to the participants within the bore of the magnet. Each trial began with a fixation cross for 250 ms. Sentence stems appeared one word at time with an interval of 500 ms between each individual word. Then, 500 ms after the offset of the last word, a prompt “_____” appeared, followed by a written instruction that informed participants of the response required (“complete”, “unrelated” or a semantic category cue e.g., “fruit”). This design was intended to discourage participants from ignoring or not processing the sentence and thus reducing suppression requirements. The sentence stem and instruction remained on screen for 5000 ms before automatically progressing to the beginning of the next trial, after which any responses were discounted. For each item, participants were asked to provide a response as quickly as possible following appearance of the response instruction. The total time to complete both runs was approximately 20 min.
2.4. Image acquisition
Imaging was acquired using a Siemens Trio (3 T; Siemens AG, Germany). Functional imaging was conducted using a gradient echo EPI sequence (echo time [TE] = 36 ms, repetition time [TR] = 2500 ms, field of view [FOV] = 210 × 210 mm, flip angle 80, in-plane resolution of 3.6 × 3.6 mm, and 36 slices × 3 mm, with a 0.6 mm gap). In each run, 242 image volumes were collected. Three-dimensional T1-weighted images were also acquired in the same session, using a magnetisation-prepared rapid acquisition with gradient echo sequence (TE = 2.99 ms, TR = 2200 ms, inversion time [TI] = 900 ms, FOV = 256 × 256 × 192 mm, 192 phase encodings in the slice direction, isotropic voxel size of 1 mm3). A fluid-attenuated inversion recovery (FLAIR) sequence was also included in order to remove signal from cerebrospinal fluid from resulting images (FLAIR TE/TR 93/7000 ms, TI = 2500 ms, resolution = 0.86 × 0.86 × 4 mm, FOV = 220 mm).
2.5. Imaging data processing
Raw imaging data was processed using Statistical Parametric Mapping Version 12 software (SPM12; Wellcome Trust Centre for Neuroimaging, 2014) operating through Matlab R2013b (MathWorks, 2013). Pre-processing steps included realigning and unwarping the fMRI time series, and applying slice time correction. Both sessions were then co-registered to a within-session, high-resolution T1 structural image. At this point, a motion finger-printing tool was employed in order to automatically assess and correct for the effects of motion within the fMRI time series. At this point, a motion finger-printing tool was employed in order to automatically assess and correct for the effects of motion within the fMRI time series. As described by Wilke (2012), this process pulls out the maximal motion of total displacement from scan to scan. This procedure detects direct motion in the brain, but also incorporates changes due to motion by B0 interaction (Wilke, 2012), and generates several timeseries to include as regressors of no interest. The multiple regressors were unique to each participant, and included three of the motion fingerprint timecourses that shared the least variance with each other (i.e. the most independent representations of motion), as per Wilke (2012). Following motion finger-printing, T1 images were segmented into grey matter, white matter and cerebrospinal fluid using a tissue classification method. The images were spatially normalized using DARTEL spatial normalisation (Ashburner, 2007). An 8 mm, full-width, half-maximum Gaussian kernel was then be used to smooth the resulting images. This kernel size was selected based on the findings of Hopfinger et al. (2000), which demonstrated that subcortical activations (of primary interest in the present investigation) were detected with greater sensitivity when larger smoothing kernels were utilised. The task design was convolved with the hemodynamic response function to create the general linear model, from which an ANOVA was constructed modeling condition (complete, unrelated, strategy) by group (PD, control). Included in the GLM were regressors to remove global signal and motion. Independent t-tests were also conducted where relevant to examine between group differences for the strategy and unrelated conditions separately.
A hypothesis-driven region of interest (ROI) analysis was also conducted. A spherical ROIs (of 8 mm radius) capturing the left dlPFC (−38 30 32) was developed within MNI atlas space using MarsBar ROI toolbox (Brett et al., 2002) for SPM12 (Wellcome Trust Centre for Neuroimaging, 2014). As discussed above, the left dlPFC has been implicated in previous studies of the HSCT and its analogues (de Zubicaray et al., 2000; Nathaniel-James et al., 1997) and is a critical component of the cognitive frontostriatal loop (Middleton and Strick, 2000). Two anatomically derived ROIs were also obtained using WFU Pickatlas software (Maldjian et al., 2003). These included the left dorsal striatum (caudate and putamen) and the left ACC, due to their participation in a cognitive frontostriatal circuits implicated in PD (Middleton and Strick, 2000).
2.6. Scoring of behavioral data
Audio files containing verbal responses were digitally filtered to reduce interference from scanner noise using Audacity software (v2.1.2) and response times were manually extracted. Response time was measured from the offset of the written instruction indicating required response (e.g. “unrelated”, “complete”, “color”) to the onset of the participant's verbal response (in order to avoid contamination from non-verbal artifacts such as coughing). The PD and control groups were compared in terms of both response latency and response accuracy. Responses were scored as either correct or incorrect. Responses were incorrect if they contained excessive interjections or false starts, or self-corrections. For the complete condition, a single word that completed the sentence in a way that made sense and was grammatical was considered to be a correct response. For the unrelated condition, each response was judged on how semantically related it was to the sentence, as outlined by Burgess and Shallice (1996), with a correct response being a single word that was unrelated to any component of the sentence. For the strategy condition, a correct response had to be a member of the cued semantic category. Repetitions in the unrelated and strategy conditions were not permitted. Response scoring was conducted by two markers. Cohen's kappa was run to determine inter-rater agreement and returned an acceptable level of agreement, κ = 0.781 (95% CI 0.768, 0.794), p < .001.
3. Results
Initial exploration of ROI data (see Section 3.2.1) identified three significant outliers (2 PD, 1 control). Outliers were identified based on interquartile range (IQR). A data point (representing the mean percentage blood‑oxygen-level dependent [BOLD] signal change) was considered to be an outlier if it met one of the following conditions: <25th percentile – 1.5*IQR, or >75th percentile +1.5*IQR. These participants were excluded from all further analysis including whole brain results. These exclusions did not result in significant differences between groups in terms of age (p = .152), YOE (p = .128), or gender (p = .441).
3.1. Behavioral results
3.1.1. Neurocognitive battery
A series of independent t-tests were conducted in order to identify any significant differences between groups across the battery of neurocognitive measures. Results are presented in Table 2. Note that excluded participants, as discussed above, were not included in statistical analysis of this assessment data. For selected items, sample size is also reduced due to some participants being unable to complete the task as a result of fatigue or time constraints. No significant differences were identified.
Table 2.
Baseline measurements of neurocognitive performance of PD and control groups.
| Measure | Group | n | MScore | SD | Significance |
|---|---|---|---|---|---|
| Semantic fluency | PD | 11 | 17.53 | 5.21 | .079 |
| Control | 17 | 20.84 | 3.25 | ||
| Phonemic fluency | PD | 11 | 13.88 | 4.48 | .086 |
| Control | 17 | 16.61 | 2.49 | ||
| BNT | PD | 11 | 55.45 | 2.38 | .988 |
| Control | 17 | 55.47 | 3.10 | ||
| TEA – EC | PD | 11 | 6.91 | 0.30 | .758 |
| Control | 17 | 6.94 | 0.24 | ||
| TEA – ECD | PD | 11 | 9.18 | 2.96 | .395 |
| Control | 16 | 10.06 | 2.32 | ||
| Digits forward | PD | 10 | 7.20 | 1.03 | .253 |
| Control | 17 | 7.76 | 1.30 | ||
| Digits backward | PD | 10 | 5.30 | 1.25 | .698 |
| Control | 17 | 5.12 | 1.11 | ||
| NART_FISQ | PD | 10 | 112.50 | 11.43 | .228 |
| Control | 17 | 117.53 | 6.80 | ||
| Cued fluency | PD | 11 | 22.64 | 3.96 | .161 |
| Control | 17 | 24.47 | 2.78 |
Note. BNT = Boston Naming Test 2nd Edition; NART_FISQ = National Adult Reading Test Full Scale IQ; TEA – EC = Test of Everyday Attention – Elevator Counting; TEA – ECD = Test of Everyday Attention – Elevator Counting with Distraction.
3.1.2. Response time
Only correct trials were included in the analysis of response time. Furthermore, only those trials in which a response was provided within a window 250 ms to 2500 ms were included. As a result, 26% of trials in the PD group and 20% of trials in the control group were discarded. A Shapiro-Wilks test indicated a departure from normality in the distribution of the response time data for both groups. A square-root transformation was performed to rectify this and the resulting distribution was satisfactory. This transformed data was submitted to a Linear Mixed Model (LMM) analysis with group and condition modelled as fixed effects and participant number as a random effect.
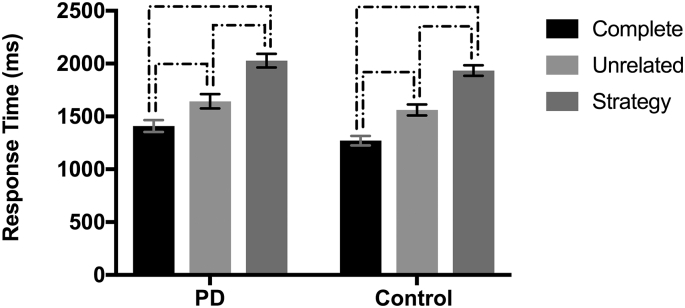
Results demonstrated a significant main effect of condition, F (2, 2138) = 313.71, p < .001. Pairwise comparisons further demonstrated that significant differences were present between all conditions, with the fastest responses in the complete condition relative to both unrelated (p < .001) and strategy (p < .001) conditions, and unrelated significantly faster than strategy (p < .001). Independent testing of PD and control groups separately revealed that this pattern of performance was present and significant in both groups, see Fig. 1. Results here are reported in raw form, for ease of interpretation. No main effect of group or group by condition interaction was detected.
Fig. 1.
Mean response time (ms) for complete, unrelated, and strategy conditions by group. Brackets indicate significant differences (p < .05). Error bars indicate standard error of the mean.
3.1.3. Accuracy
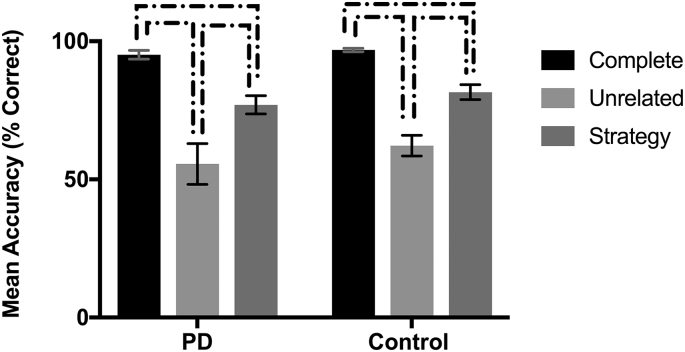
The mean percent correct responses per condition were generated for each participant and submitted to an LMM. Group and condition were modelled as fixed effects, and participant as a random effect. Results returned a main effect of condition, F (2, 56) = 82.64, p < .001. Pairwise comparisons confirmed significant differences between all three conditions, wherein complete was more accurate than unrelated (p < .001) and strategy (p < .001), and strategy was more accurate than unrelated (p < .001). This pattern of performance was present independently in both PD and control groups (see Fig. 2). No main effect of group or group by condition interaction was present.
Fig. 2.
Mean accuracy (percentage correct responses) for complete, unrelated, and strategy conditions by group. Brackets indicate significant differences (p < .05). Error bars indicate standard error of the mean.
3.2. Imaging results
3.2.1. Region of interest analysis
In order to reliably compare performance across groups in the unrelated and strategy conditions, it was necessary to control for differences in the relative baseline. The complete condition was assumed to provide a baseline measure to control for speech production. Mean BOLD signal in the unrelated condition and strategy condition were therefore subtracted from the complete condition for each ROI (i.e. complete minus strategy and complete minus unrelated) and these figures submitted to a generalised linear model (GLM), repeated measures ANOVA in order to observe the effects of group and condition. Results indicated group by condition interactions in the left dlPFC (F [1, 26] = 7.417, p = .011, partial eta squared = 0.222) and left striatum (F [1, 26] = 11.125, p = .003, partial eta squared = 0.3), and left ACC (F [1, 26] = 8.756, p = .006, partial ETA = 0.252). However, in order to interpret these findings accurately, it was necessary to ensure that the two groups did not differ significantly in their complete baseline measure. Independent t-tests demonstrated that the control and PD groups recorded equivalent baseline activations in the left dlPFC and left striatum (p > .05), however group differences were detected for the complete condition in the left ACC (p = .041). As inferences concerning strategy and suppression relative to completion could not be drawn in this case, left ACC results derived from the Complete-Unrelated and Complete-Strategy ANOVA were not further considered.
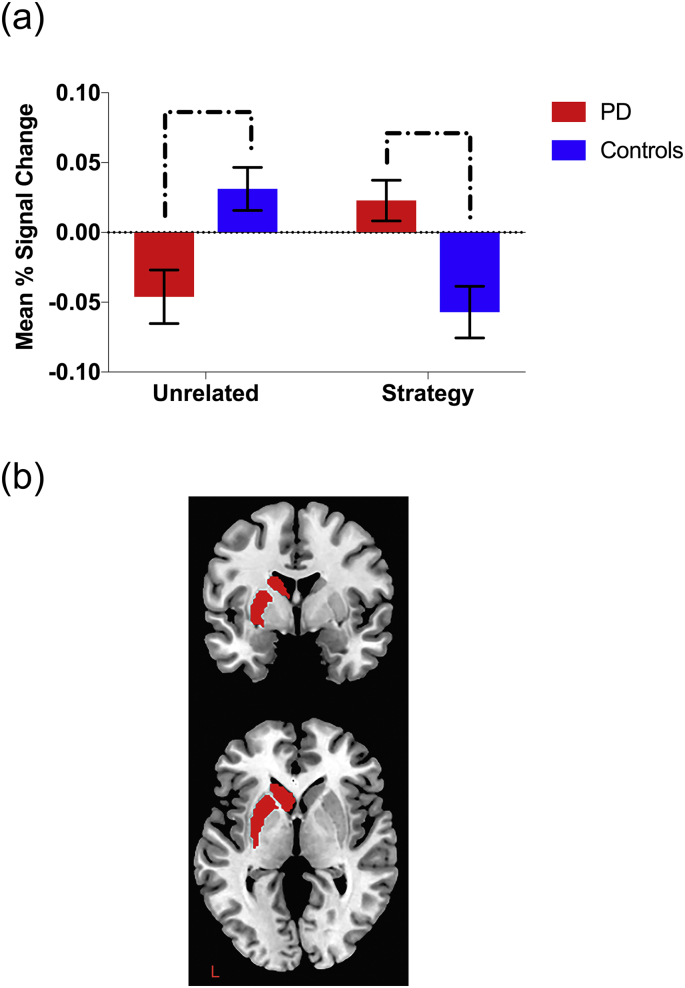
Significant group by condition interactions detected in the left dlPFC and the left striatum were further examined in order to define the nature of the interaction. An independent t-test revealed significant between-group differences in activation of the left dorsal striatum for both unrelated (t [26] = −3.14, p = .004) and strategy conditions (t [26] = 3.08, p = .005). This difference was characterised by increased activation during the unrelated condition and decreased activation during the strategy condition in the control participants, while the opposite pattern (decreased during unrelated and increased during strategy) was observed in the PD group.
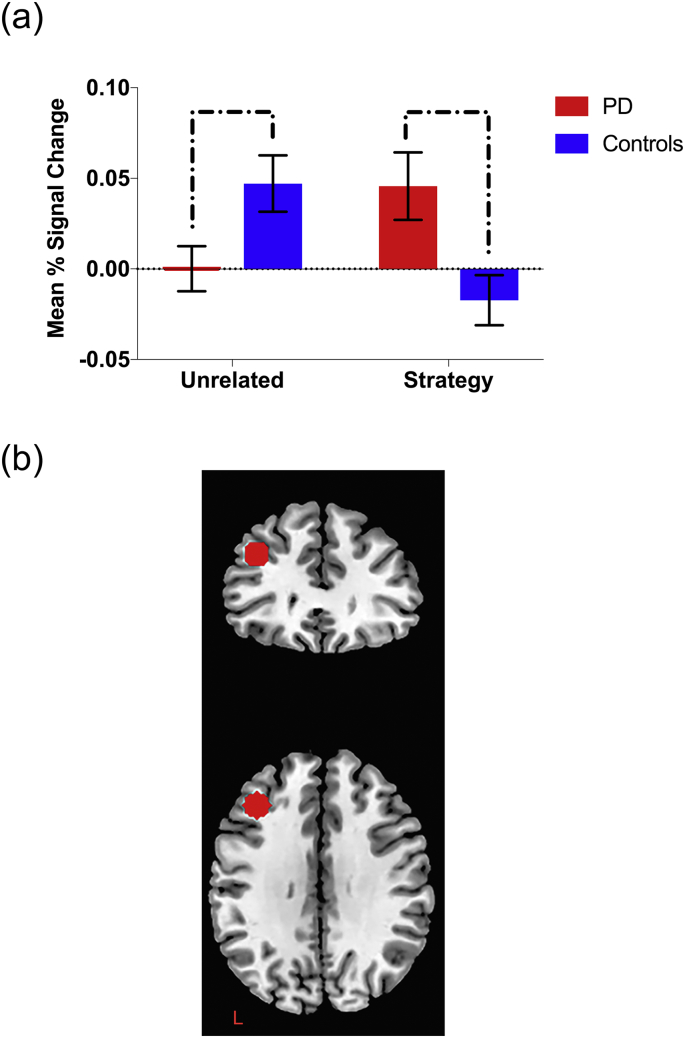
Independent t-tests also identified significant differences between groups in activation of the left dlPFC for both the unrelated (t [26] = −2.36, p = .026) and strategy conditions (t [26] = 2.76, p = .01). Paired t-tests examining the change in activation between unrelated and strategy conditions further revealed that the control group appeared to modulate recruitment of this region as a function of condition (t (16) =2.33, p = .033). This was characterised by a decrease in activity in the strategy condition relative to unrelated. The PD group did not record a significant change in left dlPFC activation across these conditions (t (10) = −1.74, p = .113). Significant findings in the left striatum and left dlPFC are plotted in Fig. 3 and Fig. 4 respectively.
Fig. 3.
Region of interest analysis for the left striatum. Bar graph (a) indicates relative mean percentage change in BOLD signal in left dorsal striatum as a function of condition (unrelated vs. strategy, each subtracted from the complete baseline). Brackets indicate significant between-group differences in activation (p < .05). Error bars indicate standard error of the mean. Figure (b) displays axial and coronal slices of a priori defined anatomical ROI.
Fig. 4.
Region of interest analysis for the left dlPFC. Bar graph (a) indicates relative mean percentage change in BOLD signal in left dlPFC as a function of condition (unrelated vs. strategy, each subtracted from the complete baseline). Brackets indicate significant between-group differences in activation (p < .05). Error bars indicate standard error of the mean. Figure (b) displays axial and coronal slices of a priori defined spherical ROI.
3.2.2. Whole brain analysis
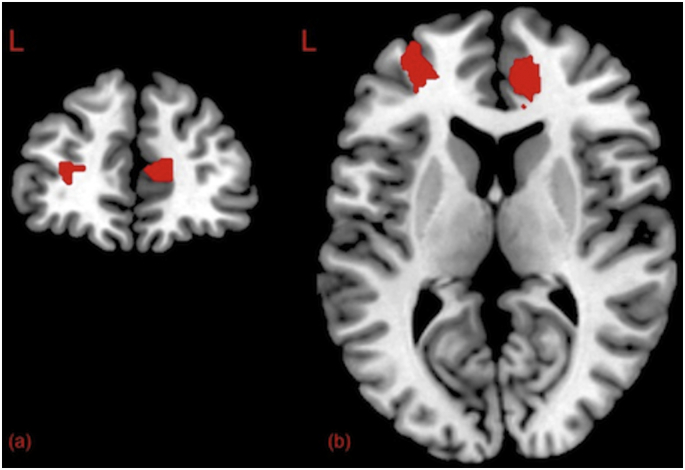
An exploratory whole brain analysis was conducted and results are reported for height threshold of p < .001 uncorrected and clusters family wise error (FWE) corrected (p < .05) according to SPM12 (Wellcome Trust Centre for Neuroimaging, 2014). Results were masked to the grey matter. Anatomical labels for peak coordinates were obtained using the Neuromorphemetrics atlas associated with SPM12. No main effects of group or condition were detected, nor was a group by condition interaction. An independent t-test identified a significant difference (p < .001 uncorrected and cluster FWE p < .05) between PD and control groups in those neural regions that were more strongly activated during the strategy condition relative to the unrelated condition. The coordinates of peak activations identified in this analysis are detailed in Table 3 and pictured in Fig. 5. This effect was characterised by greater activation in the right middle superior frontal gyrus, medial segment (SMFGm), and left MFG in the PD group during the strategy condition relative to the unrelated condition, in comparison to the control group. The region defined here as the left MFG shares some anatomical overlap with the dlPFC. The dlPFC label has therefore been used throughout the discussion below for consistency. It must, however, be noted that the spatial configuration of these findings indicate that the maximum sensitivity for the whole-brain analyses was outside of the a priori ROIs.
Table 3.
Whole-Brain Analysis: Peak Maxima of Clusters Demonstrating Significant Differences in Activity between PD and Control Groups as a Function of Condition.
| Contrast and anatomical label of activation peak | z-score | k | Voxel level |
MNI coordinates |
||
|---|---|---|---|---|---|---|
| pFWE | x | y | z | |||
| Strategy > Unrelated (PD > Control) | ||||||
| Right MSFGm | 3.99 | 417 | <.043 | 10 | 48 | 10 |
| Left MFG | 4.02 | 435 | <.035 | −28 | 44 | 8 |
Note. MNI coordinates of peak activation from whole brain analysis for clusters corrected at the voxel level (p < .05). FWE = family wise error. k = cluster size (voxels). MFG = middle frontal gyrus. MSFGm = middle superior frontal gyrus medial segment.
Fig. 5.
Coronal (a) and axial (b) slices displaying clusters in left middle frontal gyrus and right middle superior frontal gyrus (medial segment). These regions demonstrated significant differences in activity between PD and control groups as a function of condition. Clusters corrected at the voxel level (p < .05).
4. Discussion
The present study aimed to determine whether the deficits observed in the HSCT, when administered to a PD population, result from disrupted verbal suppression or from difficulty in generating and implementing a strategy that can facilitate execution of the task. We further sought to identify the neural substrates recruited for these processes in PD participants relative to healthy controls. We addressed these aims using a variation on the traditional HSCT that incorporated a novel strategy condition in combination with fMRI. While behaviorally this PD cohort was able to suppress a prepotent verbal response and generate a task-relevant unrelated alternative (presumably through the implementation of an internally generated strategy) with a degree of proficiency equal to controls, the process is seemingly subserved by an atypical neural network.
The control group recruited the left striatum and the left dlPFC to support execution of the unrelated condition. This finding is in line with our hypothesis and previous studies of the HSCT and its analogues in healthy younger adults (Collette et al., 2001; de Zubicaray et al., 2000; Nathaniel-James et al., 1997). Both the dlPFC and the striatum participate in the frontostriatal cognitive loop known to subserve cognitive control processes such as inhibition, working memory, strategy, and attention (for reviews see Hanganu et al., 2015; de la Fuente-Fernandez, 2012; Zgaljardic et al., 2006), all of which are presumably at play during the unrelated component of the HSCT. However, relative to the control group, the PD group demonstrated significantly reduced recruitment of left dlPFC and striatum during this condition. The whole brain analyses conducted did not reveal any additional neural recruitment in the PD group during the unrelated condition, and the a priori ROIs also did not appear to participate in this alternative network. It is possible that increased functional connectivity may have compensated for decreased activity in critical frontostriatal structures, as has been observed in previous studies of PD populations (Gorges et al., 2015; Yang et al., 2016), however testing of this hypothesis was beyond the scope of the present study. Thus, further investigation is required to determine patterns of connectivity that helped the PD group maintain their behavioral performance during the unrelated condition, in the face of decreased activity in frontostriatal network nodes.
In contrast to the unrelated condition, whole brain and ROI analysis indicated that the PD group were more reliant on the increased recruitment of left striatum, left dlPFC, and regions of the right frontal cortex, to maintain performance in the strategy condition. This pattern of activity is in contrast to that observed in the control group, who showed significantly decreased activity within these regions, and demonstrated significantly less recruitment of the right hemisphere. The additional neural activity observed in the PD group to maintain behavioral performance in the strategy condition may be explained by closer examination of the cognitive demands associated with the strategy task. Given that similar activity was not observed during the unrelated task, and verbal suppression was expected to be critical to both conditions, it may be assumed that this function was not responsible for the observed increase in activity. In the strategy condition, participants were required to generate members of a given semantic category under strict time constraints, and were asked not to repeat any of their responses. As each category appeared five times throughout the task, it can be assumed that this placed significant demands upon working memory resources, lexical access, and retrieval. In this way, the strategy condition bears resemblance to a semantic fluency task. Patients with PD consistently demonstrate difficulty in performing such tasks (for meta-analysis see Henry and Crawford, 2004). The PD group in the present study performed at a level commensurate with controls on a measure of semantic fluency. However, given the similarity of this task to the strategy task, this was not unexpected and may strengthen the hypothesis that both are subserved by the same atypical neural network.
Verbal fluency tasks are traditionally complex to analyse, due to the large number of cognitive skills at play during their execution. However, in their study of healthy adults, Shao et al. (2014) demonstrated that mean score in a semantic fluency task was better predicted by the ability to store and update relevant information in working memory, than by lexical access speed or vocabulary size. Interestingly, the PD group in the present study performed at a level commensurate with controls in the Boston Naming Test (a picture-naming assessment; Kaplan et al., 2001). This may suggest that vocabulary and lexical access were relatively intact in this cohort, lending support to the possibility of underlying problems with working memory.
The possibility of increased activation during the strategy condition to support working memory is corroborated by the assumed functions of the neural regions in question. In the PD group these were the left striatum (putamen and caudate), left dlPFC, and right MSFGm. The dlPFC and the dorsal caudate nucleus (a component of the striatum) are known to participate in the frontostriatal cognitive loops subserving executive functions (Cole and Schneider, 2007; D'Esposito, 2007; Grahn et al., 2009; MacDonald et al., 2000; for meta-analysis see Niendam et al., 2012). More specifically, there exists a growing body of evidence demonstrating a relationship between striatal dopamine uptake, PFC activation, and working memory performance (Gazzaley et al., 2004; Landau et al., 2009; Lewis et al., 2004). Indeed, Frank et al. (2001) have developed a computational neural network model of working memory, in which the selective firing of neurons in the striatum is posited to operate as a dynamic gating mechanism, enabling memory representations maintained in the frontal cortex to be rapidly updated according to task-relevant goals. In the PD population, several studies have linked disruptions to the maintenance or manipulation of information in working memory to reduced striatal uptake of dopamine (Holthoff-Detto et al., 1997; Rinne et al., 2000; van Beilen et al., 2008), and reduced activity in larger-scale frontostriatal networks (Gabrieli et al., 1996; Lewis et al., 2003; Owen, 2004). It may therefore be hypothesised that in the present study, the increased recruitment of the striatum observed in the PD group is evidence of a compensatory neural mechanism, driving the frequent updating of task-relevant working memory representations.
It must be noted that this postulation is highly speculative; the labelling of atypical activity in clinical populations as evidence of compensation rather than inefficient processing can be difficult to justify. However, while the present study appears to be the first to tentatively identify such compensation during a verbal selection/suppression paradigm, support for the notion may be gained from previous studies that have identified similar patterns of compensation during other cognitively demanding tasks. For example, both Gerrits et al. (2015) and Poston et al. (2016) have reported preserved cognitive performance in the face of altered neural recruitment in PD cohorts during a set-switching task and a modified Sternberg task, respectively. Tinaz et al. (2008) used fMRI to examine the functional integrity of frontostriatal circuits in PD participants during a semantic sequencing task (previously demonstrated to recruit cognitive control regions in young healthy controls). Similarly to the present study, the PD and control groups did not differ significantly in terms of behavioral performance (response time and accuracy). However, the PD group demonstrated increased activity in the left MFG relative to the control group. This region is considered relevant to the task, and is thought to be involved in the maintenance of information held in working memory. The increased activity was therefore considered to reflect compensatory activity that allowed these participants to maintain their behavioral performance. Tinaz et al. additionally found greater recruitment of a right hemisphere network in the PD group, relative to both age-matched and younger healthy controls, and this too was attributed to compensatory mechanisms. Such an interpretation is line with previous findings of greater recruitment of the contralateral hemisphere both in PD participants during tasks that are hemispherically lateralised in control groups (Carbon and Marié, 2003) and more generally as a mechanism of compensation in healthy ageing (Berlingeri et al., 2013; Cabeza, 2002; Reuter-Lorenz and Cappell, 2008).
Similar conclusion may be drawn regarding the present study. Presumably the strategy condition places additional demands upon working memory due to the need to recall which category members have already been provided as a response. In the control group, these working memory demands were manageable, however perhaps due to the decreased availability of attentional resources thought to be associated with PD (Dirnberger and Jahanshahi, 2013), this group were required to increase activity in relevant left-hemisphere regions, as well as in the right frontal hemisphere, in order to maintain performance. With regard to the latter finding, it should be noted that participant recruitment controlled for handedness and all other experimental conditions revealed activity that was largely focused in left hemisphere networks, further qualifying the suggestion that the right-hemisphere activity in this PD group was compensatory in nature.
Taken together, the findings of Gerrits et al. (2015), Poston et al. (2016), Tinaz et al. (2008), and the present study suggest that for those processes that necessarily recruit frontostriatal networks, cognitively intact PD participants are able to maintain behavioral performance through the increased recruitment of regions both intrinsic to the task at-hand, and in some cases, in novel regions beyond this network. The regions demonstrating this enhanced activity appear to be dictated by the demands of the given task, however components of the frontostriatal network appear to be frequently involved in this compensatory network. In the studies analysed here, this may due to the common factor of increased working memory demands, particularly the maintenance of items in working memory, and the dependence of this activity upon nigrostriatal pathways known to be compromised by the pathology of PD. It may be that as nigrostriatal loss progresses, the system is no longer able to compensate sufficiently, and this coincides with the onset of cognitive impairment.
The small sample size employed in the present study places considerable limitations upon the generalisation of its findings, and the authors particularly note the negative impact of small sample size upon the reproducibility of ROI analyses in task-based fMRI (Paul et al., 2017). However, it can be said that as concerns the left frontal cortex, the findings of the ROI analysis were mirrored in that of the whole-brain analysis, providing some support for their validity. A further, potential limitation of the present study's design concerns the processing demands associated with each condition of the task. Though the experimental paradigm was designed with the intent of minimizing variations in task demands across conditions (other than response requirements), it must be acknowledged that some inconsistency may be introduced in terms of how response generation was impacted by the preceding sentence stem. It is possible that in the strategy condition, the preceding sentence stem was more readily disregarded during response formulation due to the availability of the category cue. However, as this category cue was only provided after presentation of the high cloze probability sentence stem, it may be assumed that the influence of this confound was considerably limited.
A number of inconsistencies were noted between the present results and the results of previous studies of the HSCT in PD and healthy populations. The finding of no overall behavioral difference in the PD group relative to the control group is not consistent with previous reports (see Section 1), where PD participants have recorded slower response times and/or greater number of errors on the suppression component of this task (Bouquet et al., 2003; Castner et al., 2007; Obeso et al., 2011). However, it must be noted that several of the studies that found impaired performance in PD recruited participants with greater disease severity and lengthier disease duration relative to our cohort of mild-moderately affected participants. These differences in clinical characteristics may explain the discrepancies present in performance, given the well-established heterogeneity of PD, particularly in terms of the rate of dopaminergic depletion and degree of cognitive impairment (for reviews see de la Fuente-Fernandez, 2012; Monchi et al., 2016; Owen, 2004).
It is also noted that the present investigation was unable to account for the possibility of altered neural recruitment in both the PD and control groups as a result of typical age-related compensatory mechanisms (Cabeza, 2002; Reuter-Lorenz and Cappell, 2008). Though the regions recruited by the control group do reflect those reported in studies of healthy younger adults (as discussed above), the inclusion of such a comparison group in future investigations could allow for greater rigour in labelling activity patterns as typical or atypical.
Importantly, the novel adaptations to the HSCT have revealed that provision of a strategy improves the accuracy of performance on the traditional verbal suppression component of the task, in both the control and PD groups. Assuming that completion of the strategy condition required the suppression of the prepotent response, it may be inferred that it is therefore the process of strategy generation and implementation that accounts for the increased error rate observed in both groups in the unrelated condition. This represents a novel finding, as performance on this task was previously attributed solely to difficulty suppressing a prepotent verbal response (Belleville et al., 2006; Burgess and Shallice, 1996; Chan et al., 2008).
5. Conclusions
In conclusion, the present study demonstrated that this cohort of mild-moderate PD participants were able to maintain behavioral performance that was commensurate with controls in our novel variation of the HSCT. However, this performance was achieved in the PD group through the recruitment of compensatory mechanisms that were assumed to bolster working memory function in task-relevant, left hemisphere frontostriatal circuits, and regions of the right frontal cortex. In addition, the novel variation on the HSCT employed here determined that the capacity to develop and implement a strategy that supports task execution is a critical component of the suppression task in the HSCT and should therefore be considered when this paradigm in utilised in future investigations.
Acknowledgments
Acknowledgements
The authors thank K. O'Brien, M. West, and A. Rawlings for their assistance with data collection and/or scoring, and J. Yang for her assistance with the pre-processing of fMRI data.
Declarations of interest
None.
Funding
This work is supported by the Australian Research Council [grant number DP120104420]. M. Isaacs was supported by an Australian Government Research Training Program Scholarship and the Asia-Pacific Centre for Neuromodulation. D. Copland was supported by the University of Queensland Vice Chancellor's Fellowship and ARC Future Fellowship.
References
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Allen P., Mechelli A., Stephan K.E., Day F., Dalton J., Williams S., McGuire P.K. Fronto-temporal interactions during overt verbal initiation and suppression. J. Cogn. Neurosci. 2008;20(9):1656–1669. doi: 10.1162/jocn.2008.20107. [DOI] [PubMed] [Google Scholar]
- Altmann L.J., Troche M.S. High-level language production in Parkinson's disease: a review. Parkinson's Dis. 2011;2011 doi: 10.4061/2011/238956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br. J. Psychol. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Auriacombe S., Grossman M., Carvell S., Gollomp S., Stern M.B., Hurtig H.I. Verbal fluency deficits in Parkinson's disease. Neuropsychology. 1993;7(2):182–192. [Google Scholar]
- Battig W.F., Montague W.E. Category norms of verbal items in 56 categories. A replication and extension of the Connecticut category norms. J. Exp. Psychol. 1969;80(3, Pt.2):1–46. [Google Scholar]
- Bayles K.A., Trosset M.W., Tomoeda C.K., Montgomery E.B., Jr., Wilson J. Generative naming in Parkinson disease patients. J. Clin. Exp. Neuropsychol. 1993;15(4):547–562. doi: 10.1080/01688639308402578. [DOI] [PubMed] [Google Scholar]
- Belleville S., Rouleau N., Van der Linden M. Use of the Hayling task to measure inhibition of prepotent responses in normal aging and Alzheimer's disease. Brain Cogn. 2006;62(2):113–119. doi: 10.1016/j.bandc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Berlingeri M., Danelli L., Bottini G., Sberna M., Paulesu E. Reassessing the HAROLD model: is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp. Brain Res. 2013;224(3):393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- Block C.K., Baldwin C.L. Cloze probability and completion norms for 498 sentences: behavioral and neural validation using event-related potentials. Behav. Res. Methods. 2010;42(3):665–670. doi: 10.3758/BRM.42.3.665. [DOI] [PubMed] [Google Scholar]
- Bloom P.A., Fischler I. Completion norms for 329 sentence contexts. Mem. Cogn. 1980;8(6):631–642. doi: 10.3758/bf03213783. [DOI] [PubMed] [Google Scholar]
- Bouquet C.A., Bonnaud V., Gil R. Investigation of supervisory attentional system functions in patients with Parkinson's disease using the Hayling task. J. Clin. Exp. Neuropsychol. 2003;25(6):751–760. doi: 10.1076/jcen.25.6.751.16478. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. Paper Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan: 2002. Region of Interest Analysis Using an SPM Toolbox [Abstract] [Google Scholar]
- Burgess P.W., Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34(4):263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD Model. Psychol. Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Calne D.B., Snow B.J., Lee C. Criteria for diagnosing Parkinson's disease. Ann. Neurol. 1992;32(S1):S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Carbon M., Marié R.-M. Functional imaging of cognition in Parkinson's disease. Curr. Opin. Neurol. 2003;16(4) doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Castner J.E., Copland D.A., Silburn P.A., Coyne T.J., Sinclair F., Chenery H.J. Lexical-semantic inhibitory mechanisms in Parkinson's disease as a function of subthalamic stimulation. Neuropsychologia. 2007;45(14):3167–3177. doi: 10.1016/j.neuropsychologia.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Shum D., Toulopoulou T., Chen E.Y.H. Assessment of executive functions: review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008;23(2):201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Collette F., Van der Linden M., Delfiore G., Degueldre C., Luxen A., Salmon E. The functional anatomy of inhibition processes investigated with the Hayling task. NeuroImage. 2001;14(2):258–267. doi: 10.1006/nimg.2001.0846. [DOI] [PubMed] [Google Scholar]
- Colman K., Bastiaanse R. 2011. Language processing in Parkinson's disease patients without dementia. In Diagnostics and rehabilitation of Parkinson's disease: InTech. [Google Scholar]
- Davis C. N-Watch: a program for deriving neighborhood size and other psycholinguistic statistics. Behav. Res. Methods. 2005;37(1):65–70. doi: 10.3758/bf03206399. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R. Frontostriatal cognitive staging in Parkinson's disease. Parkinson's Dis. 2012;2012:561046. doi: 10.1155/2012/561046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G.I., Zelaya F.O., Andrew C., Williams S.C.R., Bullmore E.T. Cerebral regions associated with verbal response initiation, suppression and strategy use. Neuropsychologia. 2000;38(9):1292–1304. doi: 10.1016/s0028-3932(00)00026-9. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2007;362(1481):761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G., Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J. Neuropsychol. 2013;7(2):193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Dissanayaka N.N., Sellbach A., Matheson S., Marsh R., Silburn P.A., O'Sullivan J.D., Mellick G.D. Validity of Hamilton depression inventory in Parkinson's disease. Mov. Disord. 2007;22(3):399–403. doi: 10.1002/mds.21309. [DOI] [PubMed] [Google Scholar]
- Dissanayaka N.N., O'Sullivan J.D., Silburn P.A., Mellick G.D. Assessment methods and factors associated with depression in Parkinson's disease. J. Neurol. Sci. 2011;310(1–2):208–210. doi: 10.1016/j.jns.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Flowers K.A., Robertson C., Sheridan M.R. Some characteristics of word fluency in Parkinson's disease. J. Neurolinguistics. 1995;9(1):33–46. [Google Scholar]
- Frank M.J. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19(8):1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frank M., Loughry B., O'Reilly R. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Singh J., Stebbins G.T., Goetz C.G. Reduced working memory span in Parkinson's disease: evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology. 1996;10(3):322–332. [Google Scholar]
- Gazzaley A., Rissman J., D'Esposito M. Functional connectivity during working memory maintenance. Cogn. Affect. Behav. Neurosci. 2004;4(4):580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Gerrits N.J., van der Werf Y.D., Verhoef K.M., Veltman D.J., Groenewegen H.J., Berendse H.W., van den Heuvel O.A. Compensatory fronto-parietal hyperactivation during set-shifting in unmedicated patients with Parkinson's disease. Neuropsychologia. 2015;68:107–116. doi: 10.1016/j.neuropsychologia.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Gorges M., Muller H.P., Lule D., Pinkhardt E.H., Ludolph A.C., Kassubek J. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol. Aging. 2015;36(4):1727–1735. doi: 10.1016/j.neurobiolaging.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav. Brain Res. 2009;199(1):53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Hanganu A., Provost J.-S., Monchi O. Neuroimaging studies of striatum in cognition part II: Parkinson's disease. (Disease/disorder overview) Front. Syst. Neurosci. 2015 doi: 10.3389/fnsys.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J.D., Crawford J.R. Verbal fluency deficits in Parkinsons disease: a meta-analysis. J. Int. Neuropsychol. Soc. 2004;10(4):608–622. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- Herrera E., Cuetos F., Ribacoba R. Verbal fluency in Parkinson's disease patients on/off dopamine medication. Neuropsychologia. 2012;50(14):3636–3640. doi: 10.1016/j.neuropsychologia.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57(10 Suppl 3):S11. [PubMed] [Google Scholar]
- Holthoff-Detto V.A., Kessler J., Herholz K. Functional effects of striatal dysfunction in Parkinson disease. Arch. Neurol. 1997;54(2):145–150. doi: 10.1001/archneur.1997.00550140025008. [DOI] [PubMed] [Google Scholar]
- Hopfinger J.B., Buchel C., Holmes A.P., Friston K.J. A study of analysis parameters that influence the sensitivity of event-related fMRI analyses. NeuroImage. 2000;11(4):326–333. doi: 10.1006/nimg.2000.0549. [DOI] [PubMed] [Google Scholar]
- Jacobs D.M., Marder K., Cote L., Sano M., Stern Y., Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson's disease. Neurology. 1995;45(9):1691–1696. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. 2nd ed. Lippincott, Williams, & Wilkins; Philadelphia: 2001. Boston Naming Test. [Google Scholar]
- Landau S.M., Lal R., O'Neil J.P., Baker S., Jagust W.J. Striatal dopamine and working memory. Cereb. Cortex. 2009;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G., Jacobs D.M., Tang M.X., Cote L.J., Louis E.D., Alfaro B., Marder K. Memory and executive function impairment predict dementia in Parkinson's disease. Mov. Disord. 2002;17(6):1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- Lewis S., Dove A., Robbins T., Barker R., Owen A. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003;23(15):6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.J.G., Dove A., Robbins T.W., Barker R.A., Owen A.M. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur. J. Neurosci. 2004;19(3):755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarhcitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- MathWorks MatLab (R2011b) [Software] 2011. http://www.mathworks.com.au/products/new_products/release2011b.html Retreived from.
- MathWorks MatLab (R2013b) [Software] 2013. https://au.mathworks.com/products/new_products/release2013b.html Retrieved from.
- Middleton F.A., Strick P.L. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Monchi O., Hanganu A., Bellec P. Markers of cognitive decline in PD: the case for heterogeneity. Parkinsonism Relat. Disord. 2016;24:8–14. doi: 10.1016/j.parkreldis.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Murray L.L. Language and Parkinson's Disease. Ann. Rev. Appl. Linguist. 2008;28:113. [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James D.A., Fletcher P., Frith C.D. The functional anatomy of verbal initiation and suppression using the Hayling test. Neuropsychologia. 1997;35(4):559–566. doi: 10.1016/s0028-3932(96)00104-2. [DOI] [PubMed] [Google Scholar]
- Nelson H.E., Willison J. NFER-Nelson; Windsor, UK: 1991. The Revised National Adult Reading Test – Test Manual. [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I., Wilkinson L., Casabona E., Bringas M.L., Alvarez M., Alvarez L., Jahanshahi M. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp. Brain Res. 2011;212(3):371–384. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C., Naismith S.L., Hodges J.R., Lewis S.J., Hornberger M. Fronto-striatal atrophy correlates of inhibitory dysfunction in Parkinson's disease versus behavioural variant frontotemporal dementia. Cortex. 2013;49(7):1833–1843. doi: 10.1016/j.cortex.2012.12.003. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C., Naismith S.L., Shine J.M., Bertoux M., Lewis S.J., Hornberger M. A novel bedside task to tap inhibitory dysfunction and fronto-striatal atrophy in Parkinson's disease. Parkinsonism Relat. Disord. 2013;19(9):827–830. doi: 10.1016/j.parkreldis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Owen A.M. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Paul E.J., Turner B., Miller M.B., Barbey A.K. How sample size influences the reproducibility of task-based fMRI. bioRxiv. 2017 [Google Scholar]
- Pell M.D., Monetta L. How Parkinson's disease affects non-verbal communication and language processing. Lang Ling Compass. 2008;2(5):739–759. [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behavior. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Piatt A.L., Fields J.A., Paolo A.M., Koller W.C., Tröster A.I. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J. Clin. Exp. Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Poston K.L., York-Williams S., Zhang K., Cai W., Everling D., Tayim F.M., Menon V. Compensatory neural mechanisms in cognitively unimpaired Parkinson disease. Ann. Neurol. 2016;79(3):448–463. doi: 10.1002/ana.24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin S.A., Sliwinski M., Borod J.C. Clustering strategies on tasks of verbal fluency in Parkinson's disease. Neuropsychologia. 1992;30(1):95–99. doi: 10.1016/0028-3932(92)90018-h. [DOI] [PubMed] [Google Scholar]
- Redgrave P., Prescott T.J., Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89(4):1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Cappell K.A. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17(3):177–182. [Google Scholar]
- Rinne J.O., Portin R., Ruottinen H., Nurmi E., Bergman J., Haaparanta M., Solin O. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]Fluorodopa positron emission tomographic study. Arch. Neurol. 2000;57(4):470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Robertson I.H., Ward T., Ridgeway V., Nimmo-Smith I. Thames Valley Test Company; Bury St. Edmunds, UK: 1994. The Test of Everyday Attention. [Google Scholar]
- Rossetti H.C., Lacritz L.H., Cullum C.M., Weiner M.F. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77(13):1272. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- Ruff R.M., Light R.H., Parker S.B., Levin H.S. The psychological construct of word fluency. Brain Lang. 1997;57(3):394–405. doi: 10.1006/brln.1997.1755. [DOI] [PubMed] [Google Scholar]
- Shao Z., Janse E., Visser K., Meyer A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin. Gerontol. 1986;5(1/2):165–173. [Google Scholar]
- Taylor A.E., Saint-cyr J.A., Lang A.E. Frontal lobe dysfunction in Parkinson's disease: the cortical focus of neostriatal outflow. Brain. 1986;109(5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Tinaz S., Schendan H.E., Stern C.E. Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiol. Aging. 2008;29(3):397–407. doi: 10.1016/j.neurobiolaging.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25(15):2649. doi: 10.1002/mds.23429. Official Journal of the Movement Disorder Society. [DOI] [PubMed] [Google Scholar]
- van Beilen M., Portman A.T., Kiers H.A., Maguire R.P., Kaasinen V., Koning M., Leenders K.L. Striatal FDOPA uptake and cognition in advanced non-demented Parkinson's disease: a clinical and FDOPA-PET study. Parkinsonism Relat. Disord. 2008;14(3):224–228. doi: 10.1016/j.parkreldis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Wellcome Department of Imaging Neuroscience Cogent (2000) [Software] 2013. http://www.vislab.ucl.ac.uk/cogent.php Retrieved from.
- Wellcome Trust Centre for Neuroimaging Statistical Parametric Mapping (Version 12) [Software] 2014. http://www.fil.ion.ucl.ac.uk/spm/ Retrieved from.
- Wilke M. An alternative approach towards assessing and accounting for individual motion in fMRI timeseries. NeuroImage. 2012;59(3):2062–2072. doi: 10.1016/j.neuroimage.2011.10.043. [DOI] [PubMed] [Google Scholar]
- Williams-Gray C.H., Foltynie T., Brayne C.E., Robbins T.W., Barker R.A. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Yang W., Liu B., Huang B., Huang R., Wang L., Zhang Y., Wu K. Altered resting-state functional connectivity of the striatum in Parkinson's disease after levodopa administration. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic D.J., Borod J.C., Foldi N.S., Mattis P.J., Gordon M.F., Feigin A., Eidelberg D. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. J. Clin. Exp. Neuropsychol. 2006;28(7):1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]