Abstract
Adrenal insufficiency is defined as impaired adrenocortical hormone synthesis. According to its source, the deficit is classified as primary (adrenal steroidogenesis impairment), secondary (pituitary adrenocorticotropic hormone deficit) or tertiary (hypothalamic corticotropin-releasing hormone deficit). The management of adrenal insufficiency resides primarily in physiological replacement of glucocorticoid secretion. Standard glucocorticoid therapy is shrouded in several controversies. Along the difficulties arising from the inability to accurately replicate the pulsatile circadian cortisol rhythm, come the uncertainties of dose adjustment and treatment monitoring (absence of reliable biomarkers). Furthermore, side effects of inadequate replacement significantly hinder the quality of life of patients. Therefore, transition to circadian hydrocortisone therapy gains prominence. Recent therapeutic advancements consist of oral hydrocortisone modified-release compounds (immediate, delayed and sustained absorption formulations) or continuous subcutaneous hydrocortisone infusion. In addition to illustrating the current knowledge on conventional glucocorticoid regimens, this review outlines the latest research outcomes. We also describe the management of pediatric patients and suggest a novel strategy for glucocorticoid replacement therapy in adults.
Keywords: adrenal insufficiency, cortisol, glucocorticoids, glucocorticoid replacement therapy, modified-release compounds
Introduction
Adrenal insufficiency (AI) was first described by Thomas Addison in 1849, during a session of the South London Medical Society, as a form of anemia in adults suffering from fatigue, muscle weakness, loss of appetite, and eventually heart failure.1 Three patients were autopsied and in all of which Addison described ‘a diseased condition of the suprarenal capsules. Life expectancy of those patients was greatly improved after the discovery of cortisol by Hench, Kendall and Reichtein in the late 1930s, and the first-in-man use of synthetic deoxycorticosterone acetate to treat patients suffering from AI.1,2
AI corresponds to an impaired adrenocortical hormone secretion due either to an adrenal (primary insufficiency) or a hypothalamo-pituitary pathology (respectively secondary and tertiary insufficiency). While primary forms are associated with both glucocorticoid and mineralocorticoid deficiencies, the secondary and the tertiary forms only exhibit cortisol deficiency.3,4 AI is a rare disease, with a prevalence of the primary form estimated at 93–144 cases per million, whereas cases of secondary AI (SAI) are believed to be twice as numerous.4,5 Auto-immune aggression of the adrenal gland accounts for the vast majority (90%) of primary AI (PAI), the remaining being infectious diseases (e.g. tuberculosis), cancers, surgery, and congenital forms.6,7 Genetic defects of steroidogenesis include various forms of congenital adrenal hyperplasia (CYP21A2, CYP11B1, HSD3B2, CYP17A1, POR mutations), and syndromic (e.g. adrenoleukodystrophy) or familial (e.g. familial glucocorticoid deficiency) forms.8 Glucocorticoid therapy or a tumor affecting the hypothalamo-pituitary region are the major causes of secondary and tertiary forms of AI.
The typical clinical picture of AI displays the symptoms described by Addison himself, and may include low blood pressure, hyponatremia and hyperkalemia or hypoglycemia. However, the diagnosis of AI is challenged by the numerous cases of a- or pauci-symptomatic AI. The diagnosis of AI may thus be evoked on the basis of low serum total cortisol levels (measured before 09:00) and low DHEA-S levels for age and sex, as this sulfated form of androgen is specific to the adrenals. The level of adrenocorticotropic hormone (ACTH) is suggestive of PAI when doubled and associated to low morning cortisol (<140 nmol/l).9 Diagnosis is confirmed by short 250 µg ACTH test with cortisol failing to rise above 500 nmol/l or 18 µg/dl.3 Plasma renin level, associated to the measurement of aldosterone, is a reliable diagnostic criterion for mineralocorticoid deficiency.
The treatment of AI consists of physiological replacement of glucocorticoid secretion accompanied or not by mineralocorticoid substitution. However, endogenous cortisol production is difficult to accurately reproduce, because of inter- and intra-individual variability of glucocorticoid secretion and the lack of reliable biomarkers of treatment monitoring.6–8 These difficulties are responsible for important discordance in substitution protocols among practitioners, and the lack of precise guidelines.
Defining an appropriate glucocorticoid regimen poses numerous challenges, the most important being the implementation of an individualized approach to physiological cortisol secretion and preventing under- and overtreatment. Recent data showed that suboptimal replacement therapy generates a twofold increased risk of mortality from cardiovascular disease, infectious disease and cancer.10,11 Underdosed regimens generate metabolic disturbances,12–15 whereas overdosed ones induce hypercortisolism-related anomalies such as impaired glucose metabolism, insulin resistance, obesity, and hypertension.16–19 In addition, a recent review20 has outlined that both temporary over- and underdosed regimens greatly increase the risk for cerebrovascular diseases. Our review aims to describe glucocorticoid replacement therapy (GRT) in both pediatric and adult chronic AI, and to propose a new prescription regimen of GRT in adults.
Physiological endogenous cortisol production
Hypothalamic–pituitary–adrenal axis
The central pacemaker located in the hypothalamic suprachiasmatic nuclei (SCN) generates circadian and ultradian rhythmic signals which regulate the activity of the hypothalamic–pituitary–adrenal axis (HPA).18 While the circadian–nyctohemeral rhythm controls daily variation, the ultracircadian one controls short-term level variations with pulsations every 15 min.21 The SCN pulsatility initiates corticotropic-releasing hormone (CRH) production in the neuroendocrine neurons of the hypothalamic paraventricular nuclei. As a result, CRH induces the pituitary synthesis of ACTH, which determines glucocorticoid hormones production through steroidogenesis in the zona fasciculata of the adrenal cortex.18,21 Finally, via a negative feedback loop, steroid hormones will inhibit the HPA axis.16
Circadian clock system
The most intriguing aspect of the adrenal glucocorticoid synthesis is by far its pulsatile secretion. Apart from the well-known role of the SCN, the adrenal gland itself integrates a circadian clock that regulates the sensitivity of adrenocortical cells to ACTH and which sets specific time intervals for maximal response.8,22 The adrenal molecular clockwork exhibits autonomous activity and can be modulated by the activation of the autonomic SCN–adrenal pathway (adrenal gland innervation through splanchnic nerve).18 Furthermore, rhythmic expression of clock (i.e. CLOCK or BMAL1) and steroidogenic pathway proteins (i.e. steroidogenic acute regulatory protein) in the zona fasciculata accompanies the rhythmicity of cortisol secretion in plasma and brain.22,23
Interactions between central and peripheral circadian clocks result in releasing zenith concentrations early in the morning (plasma surge between 04:00 and 08:00) and nadir concentrations late at night (less than 50% of the morning value).23,24 Four distinct secretory phases have been formally described: a minimal secretion occurring 4 h prior and 2 h after sleep onset, followed by nocturnal production between the third and the fifth hours of sleep, followed by a main secretory phase consisting of three to five episodes between the sixth and the eighth hours of sleep which prolongs during the first hour of wakefulness, and an intermittent secretory phase consisting of up to nine episodes during 2–12 h in the waking period.22
Cortisol production rate
The cortisol production rate demonstrates a high rate of variability both intra- and interpersonally.24,25 Established confounding factors are weight, age, sex, cortisol-binding proteins, and cortisol metabolism.26 Studies (Table 1) evaluating the total amount of cortisol produced daily in adults in physiological conditions, by using isotope dilution and measurement by gas-chromatography mass spectrometry, have identified oscillating values between 5 and 11 mg/m2/day with a mean of 7 mg/m2/day.27 Although pubertal status was not always notified in cohort studies, children and women are described to have lower cortisol production rates (5–6 mg/m2/day), whereas elders have lower peak and higher nadir values compared with youngsters.22,26,27
Table 1.
Characteristics of clinical evaluations of endogenous cortisol production in healthy subjects.
| Study | Patients, n | Age, sex | Measurement technique | Cortisol production rate |
|---|---|---|---|---|
| Purnell et al.28 | 54 | Adults, M + F | Stable isotope dilution (LC/MS) | 7 mg/m2/ day |
| Linder et al.29 | 33 | Children, teenagers, M + F | Stable isotope dilution (LC/MS) | 4.9–8.7 mg/m2/day |
| Esteban et al.27 | 12 | Adults, M + F | Stable isotope dilution (LC/MS) | 5.7 mg/m2/day |
| Kerrigan et al.26 | 18 | Puberty, M | Deconvolution analysis | 5–6.5 mg/m2/day |
| Kraan et al.30 | 4 | Adults, M | Stable isotope dilution (LC/MS) | 9–11 mg/m2/day |
| Brandon et al.31 | 22 | Children and adults, M + F | Stable isotope dilution (LC/MS) | Children: 4.6 mg/m2/day Adults ♂: 8.7 mg/m2/ day; Adults ♀: 6.3 mg/m2/ day |
F, female; LC, liquid chromatography; M, male; MS, mass spectrometry.
Physiological roles of glucocorticoids
Glucocorticoid receptors
Glucocorticoids elicit ubiquitous roles and govern resting and stress-related homeostasis through their genomic actions.8 Pleiotropic effects are mediated by the glucocorticoid (GRs) and mineralocorticoid receptors (MRs), members of the nuclear receptor superfamily that regulates gene transcription in a ligand-dependent manner.32 GR gene NR3C1 (nuclear receptor subfamily 3, group C member 1) is located on chromosome 5q31-32. Alternative splicing of exon 9 produces two distinct receptor isoforms: the α GR that is ubiquitously expressed in every cell (except for SCN) and which binds glucocorticoids, and the β GR that does not bind glucocorticoids, and which represses the transcriptional activity of the α isoform.8,32
Glucocorticoid binding induces a conformational change in the receptor with dissociation from the chaperon heat proteins and immunophilins. After translocation into the nucleus, the activated α GR binds to the specific glucocorticoid response elements within the promoter sequences of the target genes, and regulates their transcription.32 Global gene expression analysis demonstrated that glucocorticoids influence up to 20% of the human leukocyte transcriptome, with two thirds of them being induced and one third being suppressed.33 Besides DNA direct binding, gene expression modulation can be performed by physical interactions with other transcription factors such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and signal transducers and activators of transcription (STATs).8 At the end of secretory pulses, the α GR–cortisol complex is destroyed by the proteasome.34
Role on the immune system
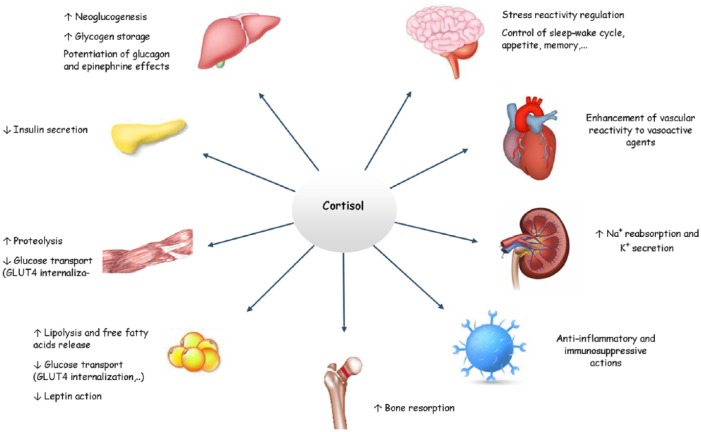
Repression of the activities of AP-1 and NF-κB, considered the central mediators of inflammatory and immune response through induction of the expression of various proinflammatory cytokines [e.g. interleukins 2, 3, and 6; tumor necrosis factor-α (TNF-α)], sets the pathway for the major anti-inflammatory and immunosuppressive actions.32,35 Furthermore, by synergistically enhancing the activity of STAT3 and STAT5, the α GR–cortisol complex protects cells at sites of injury or inflammation from succumbing to inflammation-induced apoptosis.32 Another mechanism involved in immune-response regulation is the initiation of programmed cell death in immune cells (i.e. T lymphocytes, eosinophils, mast cells, dendritic cells). Glucocorticoids inhibit T- and B-cell proliferation, natural killer cell (NK) activity, and differentiation of macrophages, and limit TNF-α toxicity and lymphocyte response to interleukin 2 (IL-2).33
Metabolic effects
Glucocorticoids mainly exert catabolic actions in muscle and adipose tissue, and anabolic effects in liver.8 Cortisol acts as a counter-regulatory hormone for glucose metabolism, antagonizing insulin roles, and creating the premises for diabetogenic effects of excessive glucocorticoids. The hyperglycemic effect is attained through stimulation of hepatic gluconeogenesis and glycogenesis, mobilization of peripheral gluconeogenic substrates, potentiation of glucagon and epinephrine effects on glycogenolysis and gluconeogenesis, and inhibition of peripheral glucose utilization.32 In adipocytes, glucocorticoids stimulate free fatty acid release and inhibit glucose transport and leptin action, thus favoring central obesity when in excess.33
On the cardiovascular system, the main role of glucocorticoids is the maintenance of normal blood pressure. This results from the enhancement of vascular reactivity to vasoactive agents (angiotensin II, norepinephrine) through expression of α1B and β2 receptors, the induction of Ca2+ voltage-dependent channels in vascular smooth muscle cells, the induction of Na/K-adenosine triphosphatase (ATPase) in cardiomyocytes, and the inhibition of cyclooxygenase and inducible nitric oxide synthase.33 As for the hydro-electrolytic balance, glucocorticoids directly increase epithelial Na+ absorption and K+ secretion; they also exhibit a natriuretic and diuretic effect through stimulation of atrial natriuretic factor secretion.32,33
Influences on central nervous system
Endogenous glucocorticoids play a fundamental role in various central nervous system functions such as behavior, mood, sleep–wake cycle, learning, and memory.36,37 Excitability and electrical activity of neurons is modulated through the GRs in neurons and glial cells, the MRs expressed in the dentate gyrus, pyramidal cells of the hippocampus and other areas of the limbic system. Basal cortisol levels maintain neuronal excitability by MRs and stress-induced levels suppress stimulated neuronal activity via GRs. Both deficit and excess of corticosteroids can cause loss of hippocampal neurons.33
From a behavioral standpoint, cortisol regulates early-life programming of stress reactivity and controls appetite (peak coincides with hormone circadian zenith), feeding and food-seeking behavior; it also influences the sleep–wake cycle (evening cortisolemia induces insomnia). Cognitive effects reside in memory consolidation and retrieval through enhancing hippocampal excitability and synaptic plasticity. At basal levels, glucocorticoids interact with specific receptors located in the prefrontal cortex, hippocampus and basolateral amygdala, promoting a neuroprotective effect. Acute cortisolemia temporarily impairs long-term memory functions and inhibits traumatic memory retrieval.37 Harbeck and colleagues have shown that elevated levels of cortisol can exert a negative impact even on short-term memory performance.25
The influence on hippocampal function is also conveyed through the roles played in sleep physiology. Cortisol is able to initiate and maintain both REM (rapid eye movement) and non-REM sleep cycles. Nocturnal concentration variations influence the transition from one cycle to another with higher levels that arrive late at night corresponding to REM cycle followed by the zenith value early in the morning preceding the non-REM stage.38 As a result of pulsatile secretion loss, patients with Addison disease experience disrupted sleep architecture consisting of multiple night awakenings and difficulties in falling asleep.39
Stress-related actions
All the baseline physiological roles culminate with the regulation of stress-related homeostasis.33 Acute stressors increase glucocorticoid production through activation of the HPA axis that will further interact with the other components of the stress system (i.e. locus caeruleus).40 Glucocorticoids control the organism’s adaptive response through permissive actions (maintenance of primary homeostatic defense mechanisms) and suppressive effects (limitation of overactivated defense mechanisms).33 Under stressful conditions, they also influence the circadian clock system. They temporarily disconnect the peripheral clocks from the central one which will restore connections a few days after the end of the stress event.40
An overview of the relevant effects of cortisol is available in Figure 1.
Figure 1.
Physiological effects of cortisol.
Standard glucocorticoid therapy
Short-acting glucocorticoids
In both pediatric and adult AI, pharmacological treatment relies on oral replacement therapy, mainly with hydrocortisone (HC), a short-acting glucocorticoid. Another option is cortisone acetate which is less preferred due to the necessity of hepatic activation by the 11β-hydroxysteroid dehydrogenase type I and the lack of absorption when administered intramuscularly.41 A mean cortisol production rate of 5–11 mg/m2/day is equivalent to 7.5–15 mg/m2/day of HC and to 20 mg/m2/day of cortisone acetate.9 The Endocrine Society guidelines recommend a total daily dose between 15 and 25 mg of HC divided into two or three digressive doses; the highest dose given in the morning, the second one either 2 h after lunch (two-dose regimen), either at noon followed by a third dose in the evening, 4–6 h before bedtime. As for cortisone acetate, the recommended dose is between 20 and 35 mg per day.42
Pharmacokinetics and pharmacodynamics
HC, a 11β-hydroxyl compound corresponding chemically to 11β,17α,21-trihydroxypregn-4-ene-3,20-dione (formula C21H30O5; molecular weight of 362.5 g/mol/l), is highly permeable with a gastrointestinal rate of absorption, without hepatic bioactivation, of about 93–96% for an oral dose of 20 mg.43–45 After intravenous administration, the distribution phase of HC has an 8-min half-life, whereas the half-life of HC is 90 min after oral administration.46 The absorption of HC is equivalent regardless of the route of administration (intravenous, intramuscular, gastric, sublingual).47 Jung and colleagues48 have compared peak serum cortisol levels after a physiological HC dose of 20 mg and a stress dose of 50 mg in a group of eight healthy adults (endogenous production previously suppressed by dexamethasone). While important peaks of serum cortisol were noticed within 30 min after intravenous administration, similar levels were identified 2 h after both oral and intravenous administration. An interesting finding was the supraphysiological serum cortisol levels observed after an oral dose of 20 mg.
Once in the bloodstream, 95% of HC binds to plasmatic proteins (67–87% to cortisol binding protein, which has a high affinity and a low capacity, and 7–19% to albumin, which exhibits a low affinity and a high capacity), only the free cortisol fraction being biologically active. Supraphysiological doses of HC overpass binding capacities when serum cortisol levels exceed 200 ng/ml, the albumin-bound fraction rises and the free cortisol fraction increases up to 25%.49 The increase of serum free cortisol levels leads to an increased hepatic and renal clearance,50 explaining the nonlinear relationship between oral HC dose and serum cortisol levels. Toothaker and colleagues have shown that HC’s in vivo dissolution is reduced at high doses: oral administration of HC doses from 5 to 40 mg generates disproportionate plasmatic values.49,51 In a clinical study, three healthy adults (with endogenous production previously suppressed by dexamethasone) attained Cmax serum cortisol levels of 199, 393 and 419 ng/ml, 1.0, 1.0 and 1.7 h after receiving 10, 30 and 50 mg of oral HC, respectively.52
Free cortisol binds GRs and MRs through mediation of 11β-hydroxysteroid dehydrogenase enzymes (11βHSD) type 1 (with a low affinity and high capacity) expressed mainly in the liver and of 11βHSD type 2 (with a high affinity and low capacity) expressed mainly in the kidney but also in other mineralocorticoid target tissues such as salivary glands, colon and placenta.53,54 11βHSD type 1 reduces cortisone (inactive form) to cortisol thus regenerating cortisol and maximizing glucocorticoid effects.54,55 The intense regenerative activity makes it difficult to evaluate the actual intracellular impact of a peak of serum cortisol which is usually attained 1 h after oral administration with a distribution volume of 34 l.45 11βHSD type 2 oxidizes cortisol to cortisone-protecting cells from mineralocorticoid effects of cortisol excess.54 High normal physiological serum cortisol can saturate the enzyme leading to MR activation with renal Na+ retention and consecutive hypertension.55
Furthermore, cortisol and cortisone are metabolized to tetrahydrocortisol, 5α-tetrahydrocortisol and tetrahydrocortisone which are excreted in the urine (50% of the secreted cortisol). A decreased activity of 11βHSD type 2 can be detected through measurement of urinary cortisol metabolites (increased ratio between tetrahydrocortisol,5α-tetrahydrocortisol and tetrahydrocortisone).54 Another urinary metabolite is 6-β-hydrocortisol, produced after 6β-hydroxylase (CYP3A4) metabolization.56 CYP3A4, an enzyme of the cytochrome P450 superfamily, is a minor pathway (1%), but it can be induced by certain drugs and in case of hypercortisolism.57 Excretion of HC is bimodal, 75% biliary and 25% urinary, independently of the route of administration.45
Clinical relevance
In the pediatric population, the main cause for PAI is congenital adrenal hyperplasia (CAH) in preschool children, followed by autoimmune insufficiency in adolescents.58 HC is the treatment of choice for physiological cortisol replacement, the main advantages being its low potency and short duration of action, which facilitate dose titration.42,59–61 HC is also associated with fewer side effects having a lower impact on linear growth compared with long-acting corticosteroids.59–62 A study performed by Bonfig and colleagues63 on 125 patients with CAH treated with prednisone until final height was reached showed a significantly reduced adult height compared with estimated target height. Moreover, the HC dose levels at the beginning of puberty were negatively correlated with final height. Whenever the dose exceeded 20 mg/m2/day in infants and 15–17 mg/m2/day in adolescents, shorter adult height occurred.62,63
Up to 95% of CAH stem from mutations in CYP21A2 with a subsequent 21-hydroxylase deficiency, resulting in cortisol secretion impairment with increased pituitary ACTH release and excessive adrenal androgen synthesis.58 Therefore, besides replacing cortisol secretion, the aim of HC therapy is to suppress androgen overproduction to prevent virilization and to ensure optimal growth and fertility.58,61 CAH treatment protocols require high HC doses: up to 25 mg/m2/day in early infancy and between 10 and 15 mg/m2/day after the first year of life.61,63 The main therapeutic challenge is to strike a balance between hypercortisolism and hyperandrogenism, the recommendation being to reduce HC doses immediately after reaching target levels of androgens. Yet this balance may be perturbed by the increase in cortisol clearance during puberty.62
For other types of pediatric PAI, the total HC dose ranges from 7 to 12 mg/m2/day, with a starting dose of 8 mg/m2/day.62 The recommended regimen is usually a thrice-daily administration. The daily dose of HC tends to be higher than the mean physiological cortisol production rate because of the short half-life and the partial destruction by gastric acidity.59,62 In central AI (secondary or tertiary), treatment aims towards a lower daily HC dose, between 7 and 9 mg/m2/day, divided in three doses.59 Because central insufficiency most frequently results from a partial ACTH deficit, overtreatment can occur at lower-range HC doses and treatment must be cautiously titrated.59,64–66 On the other hand, suboptimal dosing generates nocturnal hypocortisolemia with subsequent hypoglycemia that can hinder neurological development in small children.67
Long-acting glucocorticoids
Long-acting glucocorticoids such as prednisone, prednisolone and dexamethasone are an alternative to HC in adolescents who have reached final height, or in adults. Standard regimens consist of a twice-daily administration.46 Main indications are lack of compliance to a multidaily regimen and severe impairment of quality of life (QoL) as a result of circadian cortisol rhythm mismatches.45,46 Prednisolone is preferred over prednisone because it does not necessitate hepatic activation through 11βHSD type 1. The recommended dose of prednisone ranges from 3 to 5 mg per day in PAI, administered orally once or twice.42 In CAH, the usual prednisone dose is between 2 and 4 mg/m2/day and should represent one fifth of the HC dose.62 Dexamethasone is administered once a day at a dose range between 0.25 and 0.375 mg/m2/day. As emphasized by the Endocrine Society,42 dexamethasone is not recommended for children because of its long-acting properties and is usually disregarded in pregnant women because it does not undergo placental inactivation. Furthermore, dexamethasone may trigger adrenal crisis if no concurrent fludrocortisone is given, as it does not exert mineralocorticoid activity. Also, due to difficulties for dose titration, patients under dexamethasone substitution are at increased risk of Cushing-like side effects. For these reasons, dexamethasone is the least preferred glucocorticoid for substitution, according to the Endocrine Guidelines.42
Pharmacokinetics and pharmacodynamics
Structural changes in the basic steroid nucleus and its side groups determine the pharmacological differences between long-acting glucocorticoids, in terms of bioavailability, GR and MR interaction, and genomic modulation. A decisive structural characteristic is the polarity of the substituent in position 6 or 16. Potency is stimulated by 6-α methyl, 16-methylene hydrophobic substitution, by the Δ1-dehydro-configuration (prednisolone), by 6α and 9α-fluorination (dexamethasone). The first two mechanisms also attenuate mineralocorticoid potency.68,69 Hence, prednisone and prednisolone exert minimal mineralocorticoid activity and dexamethasone has none.70
Once in the bloodstream, 60% of the prednisolone binds to CBG and 99% of dexamethasone binds to albumin. Therefore dexamethasone has protein-bound fractions (60–70%) which are concentration-independent, whereas prednisolone is concentration dependent with an affinity ranging from 90% in lower concentrations to 60% in higher ones.69–72 Protein binding patterns create a non-linear pharmacokinetics, higher doses generating more rapid clearance rates.69 An increase in an intravenous dose administration from 5 mg of prednisolone to 40 mg corresponds to a 75% increase in clearance. The moment of administration also influences prednisolone clearance, which is lower by up to 25% in the morning.71
Inactivation occurs through hepatic reduction of Δ4 double bond and of the 3-keto group followed by glucuronidation and sulfation.72 The elimination half-life is 1 h for prednisone, 3 h for prednisolone and 4 h for dexamethasone.71 On the other hand, plasma half-life inaccurately estimates the duration of action.70 Biological half-lives are longer than the plasmatic ones and are dependent on the tissue glucocorticoid levels.72 Actually, evaluation of biologic activity relies on the ability of a single dose to suppress ACTH during a certain period of time. In this regard, prednisone and prednisolone exhibit a biologic half-life between 8 and 12 h, and dexamethasone reaches a half-life range between 36 and 54 h.69
Clinical relevance
Prednisolone is the therapy of choice, especially in patients who are unable to tolerate multiple-dose regimens (e.g. lack of compliance, daily fatigue, sleep disorders). Moreover, its longer half-life allows a once-daily administration in cases where lower replacement doses are required (primary and central insufficiency).73,74 Williams and colleagues73 have recently examined the prednisolone pharmacokinetic profile through ultraperformance liquid chromatography in healthy subjects and patients with AI. A single mean dose of 3.86 mg did not only attain adequate replacement but it also mimicked physiological cortisol profile. Dose fractioning extended the total glucocorticoid exposure through increasing the total (0–24 h) AUC.
In CAH, evening prednisolone administration more easily achieves androgen target levels with a better suppression of the morning 17-hydroxyprogesterone surge. However, Ajish and colleagues75 did not demonstrate significant improvements when compared with the equivalent HC dose. Cadalto and colleagues76 compared the benefits of a once-daily morning dose of prednisolone in CAH with conventional HC replacement in patients aged from 1 to 21 years. At 1 year of follow up better improvements were shown in suppression of steroid levels and in control of skeletal maturation advancement. On the other hand, a similar study77 performed on 15 pediatric patients showed no difference between prednisolone and HC; the efficacy between the two regimens being comparable.
When drawing a comparison between prednisolone and HC, deleterious-effect assessment becomes a priority. In children, prednisolone is well known for inflicting growth suppression through inhibition of growth hormone production and tissue sensitivity and limiting IGF-1 bioactivity.76 In adults, the metabolic side effects are in the spot light. Authors of one retrospective study78 carried out on behalf of the European Adrenal Insufficiency Registry concluded that prednisolone and HC are equivalent steroid replacement therapies that exhibit the same cardiovascular risk. Another 3-year observational study performed by the same group showed that patients treated with prednisolone developed higher plasma low-density lipoprotein (LDL) lipid profiles.79
The use of dexamethasone is restricted to CAH treatment in adults, as per international guidelines.61,62 Regarding adrenal androgen suppression, dexamethasone’s potency is evaluated as 80–100 times greater than HC. Indications for CAH treatment in pediatric patients stir numerous controversies. Rivkees and colleagues80 have treated eight children with once-daily low dose of dexamethasone (0.25 mg/m2/day) since neonatal period. A mean observation period of the 6.5 years revealed normal linear growth, skeletal maturation and body mass index evolution. The same group also obtained favorable growth results during a 7-year follow up on 26 children treated with a single-morning low dexamethasone dose (0.27 ± 0.01 mg/m2/day).81 Nocturnal administration efficacy was investigated by Dauber and colleagues in five prepubertal children.82 Dexamethasone given at 1/50 total HC daily dose proved to suppress more efficiently both morning ACTH and adrenal steroids than conventional HC replacement.
Acute stress management
Adrenal crisis
The most feared complication of AI is adrenal crisis. In spite of all the efforts invested in early diagnosis and management, the incidence fails to decrease. It is responsible for the death of 1 in 200 patients per year, having an incidence between 4.4 and 17/100 patient-years.83 A retrospective study performed on 444 patients revealed an incidence of 6.6/100 patient-years, the main precipitating factor being gastrointestinal disease (33%) and various infections (24%).84 Another survey conducted on 1000 patients with PAI from four countries has identified 8% cases of adrenal crisis happening every year.85 During a 2-year prospective follow up of 423 patients with primary or secondary AI, 64 adrenal crises occurred, four of which were fatal.86
No consensual definition of adrenal crisis has been internationally accepted. A recent review proposed a revised definition: ‘…an acute health impairment accompanied by features such as hypotension (systolic blood pressure < 100 mmHg, or 20 mmHg lower than the usual value), acute abdominal symptomatology, altered sensorium, acute metabolic disturbances (hyponatremia, hyperkalemia, hypoglycemia), pyrexia, which resolve after parenteral glucocorticoid administration.’2 The clinical features are a consequence of the incapacity of adrenocortical hormones levels to meet requirements during physical and physiological stress.2,83
The first-line management relies on fluid resuscitation and stress-dosing glucocorticoid replacement. While hypovolemia and hyponatremia need rapid intravenous fluid resuscitation with isotonic sodium chloride 0.9%, hypoglycemia is corrected with intravenous 5% dextrose. As for glucocorticoid replacement, parenteral HC should be administered immediately, either intramuscularly or intravenously, to prevent further clinical deterioration. An important advantage of HC is its mineralocorticoid effect when it exceeds 50 mg/day.87 If HC is not available, prednisolone is the second-line preferred treatment and dexamethasone is the least preferred.2,57
For adults, Endocrine Society guidelines recommend an intravenous bolus of 100 mg HC, followed by 200 mg/24 h as a continuous infusion or 6-hourly injection and reduced to 100 mg/day the next day. Glucocorticoids should be tapered to maintenance dosage in 1–3 days in accordance with the clinical evolution.42,83 In children, the stress dose varies according to age as follows: 25 mg/m2 in infants, 50 mg/m2 in preschool children and 100 mg/m2 in children above the age of 12 years.42 The bolus is followed by the same dose divided 6 hourly for 24–48 h.63 As for fluid resuscitation, a rapid bolus of 20 ml/kg is recommended. In case of shock, it can be increased up to 60 ml/kg within 1 h. Hypoglycemia correction, which is much more common in children necessitates 0.5–1 g/kg of intravenous (IV) dextrose.42
Prevention of adrenal crisis
Prevention of adrenal crisis is based on identifying both the precipitating and the risk factors. The most common precipitating factor remains infection (gastrointestinal illness 23–56%, other infections 17–25%).2,88 Its potency stems from the inadequate level of cortisol that is unable to modulate the immune response, thus favoring the inflammatory cytokines excess, and to enhance vascular reactivity to catecholamines.2,89 Other precipitators are surgical procedures, physical or psychological stress, or abrupt interruption of corticosteroid replacement.2,88 As for risk factors, patients with PAI are much more prone because of the deficit of mineralocorticoid hormones.57 As for SAI, of the 202 patients included in the prospective study by Hahner et al., 47% reported adrenal crisis at baseline and 17 adrenal crises were identified during the 2-year follow up.86
In practice, adrenal crisis is prevented by increasing the glucocorticoid doses and dividing them into three to four intakes during an acute illness or adequately adapting them before surgical procedures and by avoiding abrupt cessation of glucocorticoid therapy.88,90 International guidelines recommend doubled (fever > 38°C) or tripled (fever > 39°C) HC doses for 2–3 days in febrile illnesses. In case of gastric intolerance, adults receive a parenteral dose of 100 mg and children one between 25 and 100 mg/m2.42 Intramuscular administration provides a coverage of 6–8 h before reaching professional medical care.64 Subcutaneous HC administration may represent a more acceptable alternative for patients that still needs further scientific evaluation before implementation.88
In healthy subjects, minor surgeries need a mean cortisol production rate between 10 mg/day and 50 mg/day, whereas more important ones need up to 75–200 mg/day.2 Therefore, minor to moderate surgeries require 25–75 mg/24 h HC in adults, and in children, either intramuscularly (IM) 50 mg/m2 or doubled/tripled HC replacement.42 A preoperative stress dose can be given 30–60 min before general anesthesia induction, followed by a second one given as a constant infusion or as a 6 h divided intravenous bolus for 24 h.64 For major surgeries, 100 mg IM HC or 50–100 mg IV HC is administered before anesthesia, followed by continuous infusion of 200 mg for 24 h or 100 mg IM or IV every 6 h until oral tolerance is regained or for 24–48 h. Afterwards, oral dose can be doubled for minimum 48 h in long recoveries and for 24–48 h in short recoveries.2,42
The one measure playing a pivotal role is patient education, which needs special priority assignment. Physicians must instruct patients to correctly adapt their oral dose during stressful events and recognize the situations when IM or subcutaneous HC administration is needed.42,57 Guidelines stipulate that patients need to be equipped not only with an emergency card but also with an emergency kit for parenteral glucocorticoid self-administration.83 A recent survey89 performed in Germany on 33 patients showed that less than 70% carried an emergency kit, almost 80% were unable to correctly adjust the glucocorticoid dose during common illnesses and only 20% could identify the symptoms of over- and undertreatment.
Conventional glucocorticoid replacement regimens
Current glucocorticoid replacement regimens are variable and heterogenous. In view of AI prevalence, oral glucocorticoid regimens have not been the subject of randomized and controlled studies. A multicenter prospective study91 performed on behalf of European Adrenal Insufficiency Registry that included more than 1000 patients revealed an impressive diversity not only among the dose and the type of corticosteroid, but also on the regimen design. Almost 90% of patients were receiving HC. Among them, 5.5% were receiving a unique dose, 48.7% received two doses, 43.6% received three doses, and 2.1% received four doses per day. A thrice-daily regimen was common in patients with PAI and the twice daily for SAI. The total daily doses varied between 20 and 25 mg, which is equivalent to an approximate dose of 11–14 mg/m2/day for an adult with a mean weight of 70 kg, confirming a tendency for supraphysiological replacements. The general conclusion was that treatment adaptation relies on clinical assessment (symptoms, patient lifestyle), instead of being based on reliable biological parameters. Unfortunately, this study did not correlate the results with the clinical traits of patients, nor with the occurrence of adverse effects (metabolic syndrome, weight gain, etc.). What is more, it only reflects the clinical experience in a tertiary center and not in a primary care context.
Treatment monitoring
The lack of significant biomarkers stems directly from the particular patterns of HC pharmacokinetics. Volume distribution, plasmatic protein binding, metabolism and excretion of active principle, cytochrome-P450-mediated drug interactions, all influence the interindividual variability. Current biomarkers such as cortisolemia, ACTH levels and 24 h urine profiles prove to lack reliability.92 Howlett and colleagues93 have retrospectively analyzed the urinary profiles of 130 patients (174 profiles) under a twice-daily or thrice-daily HC regimen. The following criteria evaluated the efficacy of each type of regimen: 09:00 urinary free cortisol (age reference interval), 12:30 and 17:30 plasmatic cortisol > 50 nmol/l (ideally >100 nmol/l). A sum of 60% patients receiving three doses met the criteria. For the twice-daily treatment, only 15% reached correct levels, the tendency being of extremely high morning cortisolemia and very low 17:30 values. An initial posology of 10 mg in the morning, 5 mg at noon and another 5 mg in the evening was proposed. Haas and colleagues94 also proposed using urinary cortisol profiles (from 00:00 to 08:00) as a monitoring tool for 14 AI patients. While patients’ urinary cortisol excretion rates correlated with cortisolemia, healthy subjects exhibited a wide interindividual variability (14.0 ± 7.8 μg/8 h, range: 0.24–35.4 µg). The recommendation was to use it for diagnostic purposes, rather than monitoring ones.
Cortisol plasmatic profiles (blood samples every 2 h from 06:00 to 00:00) were prospectively investigated by Simon and colleagues95 in 50 adult patients who were under 13 different HC replacement regimens (total daily dose from 15 to 50 mg). Results were compared with physiological cortisol concentrations at key moments throughout the day. Totals of 79%, 55% and 45% patients proved to be outside the physiological ranges at 08:00, 16:00 and 00:00, respectively. The regimen that had the most appropriate cortisol levels (between 32% and 54%) was divided into three doses of 10 mg at 07:30, 5 mg at 12:00 and 5 mg at 16:30. Attainment of physiological cortisol profiles was also used by Swords and colleagues96 to compare low-dose HC regimens with cortisone acetate ones in adult patients with hypopituitarism. Behan and colleagues97 have chosen the same criteria to compare three twice-daily regimens consisting of: (a) 20 mg/08:00, 10 mg/16:00; (b) 10 mg/08:00/16:00; or (c) 10 mg/08:00, 5 mg/16:00 during a 6-week prospective randomized crossover study. The profiles of patients under the lowest-dose regimen (10/5 mg) proved to replicate most accurately physiological cortisol production.
Dose adjustment
The role of HC pharmacokinetics in tailoring personalized replacement therapy incited Mah and colleagues98 to investigate the pharmacokinetic parameters of a single morning-dose regimen. An oral fixed HC dose of 10 mg was compared either with a body-surface-area-adapted one of 5.5 mg/m2, or to a weight-based one of 0.12 mg/kg. The use of a fixed dose led to a greater interpatient variability of plasmatic levels and to a treatment overexposure of 6.3%. Weight adjustment reduced the overexposure to 5% and the intervariability from 31% to 7%. Plasmatic cortisol interindividual variability was also the scope of a study performed by Connell and colleagues,99 who evaluated cortisol profiles after an HC morning dose. Oral doses of 10, 15 or 20 mg gave a Cmax with a range from 422 to 1.554 nmol/l, which was attained after 15 min in some patients and after more than 2 h in others. The same group hypothesized salivary cortisol as a monitoring alternative, but it failed to correlate with plasma correlations and, in fact, showed even greater intervariability with this method.
Based on their kinetic analysis findings, Mah and colleagues98 also proposed a weight-based thrice-daily HC regimen. The total daily dose varied from 6.5 to 10.1 mg/m2/day (mean value of 8.3 mg/m2/day for a weight below 94 kg), given by 50% in the morning before breakfast, followed by 25% in the afternoon and the remaining 25% in the evening (21:00). This regimen permits homogenous doses administration (approximately 9 mg/m2/day) for patients weighing between 75 and 84 kg. However, for patients outside this weight range, the HC dose variability increases, making them more prone to overdosing (Table 2). Cortisol normograms showed that 23.1% patients reached 4 h cortisol levels above the 90th percentile.98 Plasma cortisol kinetic analysis also served as a tool for Rousseau and colleagues to adjust a thrice-daily regimen in PAI.100 For more than 80% of the patients (n = 27) under a total daily dose of 6 mg/m2/day HC, mean cortisol area under the curve (AUC) mimicked physiological profiles. Doses of 10 and 14 mg/m2/day proved to induce overtreatment. Contrary to previous studies, cortisol concentrations measured at 10:00 and 14:00 strongly correlated with the daytime AUC (from 08:00 to 19:00), suggesting the 2 h postmorning-dose cortisolemia as a valid adjustment parameter.
Table 2.
Interindividual HC dose variability according to the regimen proposed by Mah et al.98
| Weight (kg) | Dose expressed in mg/m2/day (body surface, m2) | Total daily dose (mg) |
|---|---|---|
| 50–54 | 6.8–6.5 (1.47–1.55) | 10 |
| 55–74 | 9.6–8.1 (1.56–1.84) | 15 |
| 75–84 | 9.4–8.9 (1.86–1.97) | 17.5 |
| 85–94 | 10.1–9.1 (1.98–2.2) | 20 |
HC, hydrocortisone.
The same study investigated the potential role of ACTH as a biomarker. Cortisol normograms did not correlate neither with the ACTH decrease rate nor with the ACTH-specific time values.100 On the other hand, plasma ACTH suppression was an indicator of overtreatment for the 10 and 14 mg/m2/day regimens. Feek and colleagues101 described limitations of ACTH through a randomized study on 12 Addisonian patients. Morning plasma ACTH showed a great variability after oral administration of HC or cortisone acetate, and no correlations with ACTH levels could be identified. This could be related to an altered pituitary sensitivity to cortisol suppression. Laureti and colleagues102 recommended against using plasma ACTH concentrations as a single monitoring parameter. Instead, these authors proposed a combined use of plasma ACTH and cortisol levels, plus urinary free cortisol measurement to compare twice daily and thrice-daily cortisone acetate regimens in PAI. With this method, they observed, after a 6-month prospective follow up, the superiority of a three-dose administration, with higher levels of urinary free cortisol and lower ACTH levels, compared with the twice-daily regimen, whereas with three daily doses, patients had almost physiological cortisolemia throughout the day. The Endocrine Society recommends against dosing ACTH for monitoring purposes, to prevent overtreatment.42
Besides the twice-daily and thrice-daily posology previously described, another proposal is a fourth-daily one. Ekman and colleagues103 have compared a four-dose HC regimen (10 + 10 + 5 + 5 mg) with a two-dose one [20 + 0 (placebo) + 10 + 0 (placebo) mg] during a randomized, prospective, double-blind, crossover (two periods of 4 weeks) study on 15 patients. Even though the total HC daily dose was the same, a subdivision into four administrations led to more physiological cortisol profiles and median ACTH levels. Contrary to Howeltt and colleagues’ findings,93 the two-dose regimen produced lower cortisol levels and higher ACTH levels than the four-dose administration. An explanation could be the earlier administration of HC doses compared with a classic twice-daily regimen. At the end of the study,103 QoL questionnaires revealed that patients preferred receiving four administrations. They have reported improvement in their sleep quality and less daytime fatigue. Lower cortisol peaks after the morning dose could explain the impact on the QoL.
In conclusion, based on the physiological repartition of cortisolemia, clinical studies recommend dividing the HC posology into three times per day and to perform adjustments according to either weight or to body surface area. A total daily dose should be limited between 6 and 10 mg/m2/day in order to prevent overtreatment. It is noteworthy to mention the fact that few studies convey the occurrence of AI-related symptoms during low-dose replacement therapy.
Limitations of standard glucocorticoid therapy
Mortality
At present, regardless of diagnostic advances and worldwide availability of replacement therapy, AI still doubles the mortality risk from cardiovascular diseases, infections and cancer.5,6 This risk only adds to the mortality risk from adrenal crises estimated at 0.5/100 patient-years.10 A Norwegian study104 found that adrenal crises accounted for 15% of deaths among a population study of 800 patients with Addison disease. It also identified higher mortality rates in men before the age of 40, infections accounting for 10% and sudden deaths for 9.2%. The inaccuracies in GRT could partially explain these tendencies, with under- or overtreatment rendering the organism incapable of properly fighting back infection and illness.5 Corticosteroid therapy mismatches can increase mortality by 1.5–2-fold, reducing life expectancy by 3 years in females and by 11 years in males.103 In a retrospective study performed on patients with secondary AI, overdosed replacement regimens, either with weight-adjusted or absolute HC doses, significantly increased mortality.105 Concomitant presence of other endocrine disorders such as diabetes mellitus or thyroid disease only worsens the survival rate in AI patients.104
Side effects
Cardiovascular risk
Apart from the lack of reliable biomarkers previously mentioned, the greatest limitations arise from glucocorticoid side effects. Inadequate steroid replacement results in risk exacerbation of both cardiovascular disease and metabolic syndrome.19,25 A recent randomized double-blind, crossover Dutch study106 compared the cardiovascular impact of two HC regimens (0.2–0.3 mg/kg/day and 0.4–0.6 mg/kg/day). The higher regimen was associated with a higher systolic blood pressure (+5 mmHg, p = 0.011). The same study found that HC doses regarded as physiological (between 14 and 22 mg/m2/day) were able to suppress aldosterone, renin and noradrenalin, thus altering the cardiovascular homeostasis. Similar results were conveyed by a study90 of the European Adrenal Insufficiency Registry where among 141 HC substituted patients (total daily dose of 15–30 mg/kg/day), 35.5% developed arterial hypertension (mean value of 131/79 mmHg) and 26.9% were under statin treatment.
Peterson and colleagues107 have studied the cardiovascular consequences of a short-term increase in HC equivalent total dose from 20 mg/day to 30 mg/day for 17 patients with SAI. After 1 week, patients experienced reduced reactive hyperemia index compatible with endothelial dysfunction. There was no impact on the glucose metabolism. The cardiovascular benefits of an HC dose reduction were investigated by Behan and colleagues108 in a recent prospective crossover (two periods of 6 weeks) randomized study. Ten pituitary patients were randomized to receive three types of regimens: 20 mg/08:00, 10 mg/16:00; 10 mg/08:00/16:00; 10 mg/08:00, 5 mg/16:00. Lowest-dose regimen led to lower Ambulatory Arterial Stiffness Index and to attainment of more physiologically normal nocturnal blood pressure profiles (absence of nocturnal systolic dip < 10%).
Metabolic syndrome
The presence of metabolic syndrome was evaluated by Giordano and colleagues through comparing 38 patients with Addison disease who were under either cortisone acetate (37.5 mg/day), or HC (30 mg/day) with age- and body mass index (BMI)-matched controls. Patients had higher rates of central adiposity (83% versus 44%), hypertriglyceridemia (18% versus 8%), hypercholesterolemia (10% versus 3%) and glucose intolerance (8%).109 For patients with SAI, Filipsson and colleagues110 have shown that a total daily HC equivalent dose greater than 20 mg/day led to metabolic perturbations. A total of 2424 patients with hypopituitarism were divided into four groups: the first three according to the type of corticosteroid substitution and the fourth one included ACTH-sufficient (AS) patients. An important finding was that patients on HC and prednisolone had higher glycated hemoglobin (HbA1C) levels and central obesity prevalence than those on cortisone acetate. Also, in the setting of hypopituitarism, Danilowicz and colleagues111 showed that a reduction of HC dose from 20–30 mg/day to 10–15 mg/day improved body composition and lipid profiles of patients within 6–12 months.
Similar to anti-inflammatory corticotherapy, replacement therapy exerts a negative impact on BMI. The risk of overweight was estimated by Biermasz et al.112 by comparing hair cortisol concentrations in 132 patients with primary or secondary AI, in 42 patients with ACTH-sufficient hypopituitarism and in healthy controls. HC-substituted patients presented higher cortisol concentrations, which in males were correlated with increased BMIs (average value 27.57 kg/m2), despite a low-dose regimen (average HC dose of 21 mg/day). Table 3 lists BMI values (range between 24 and 28.8 kg/m2, with an average of 27.6 kg/m2) from various clinical studies in patients with AI. Except for Benson and colleagues’ study,113 results were not systematically matched with healthy controls.
Table 3.
Studies evaluating BMI in patients with PAI or SAI.
| Study | PAI/SAI | BMI (kg/m2) ± DS | Patients, n |
|---|---|---|---|
| Benson et al.113 | SAI | 27.0 ± 7.4 (versus 26.0 ± 3.2 controls, p = 0.58) |
18 |
| Werumeus Buning et al.114 | SAI | 26.6 | 47 |
| Ekman et al.103 | PAI | 24.0 ± 3.4 | 15 |
| Harbeck et al.115 | PAI or SAI | 25.3 ± 4.4 (long-term R/) 27.5 ± 5.2 (short-term R/) |
14 |
| Murray et al.91 | PAI or SAI | 26.2 ± 4.8 (PAI) 28.8 ± 5.1 (SAI) |
321(PAI); 695 (SAI) |
| Nilsson et al.116 | PAI | 25.5 ± 4.1 | 71 |
| Rousseau et al.100 | PAI | 26.1 ± 6.2 | 27 |
| Schulz et al.117 | PAI (and CAH) | 26.4 ± 4.6 | 57 with PAI |
BMI, body mass index; CAH, congenital adrenal hyperplasia; DS, ; PAI, primary adrenal insufficiency; SAI, secondary adrenal insufficiency.
Neurocognitive impairment
From a cognitive standpoint, a physiological elevated level of cortisol can impair memory and executive cognitive functions.114 The physiopathological explanation relies on the overexpression of GRs in the hippocampus (memory) and prefrontal cortex (executive functioning skills, attention, and social cognition). Studies evaluating cognitive performances in patients with AI are rare and often reach contradictory conclusions (Table 4). Results’ incongruence is a consequence of the high variability of study methodology, under-represented cohorts, heterogeneity of study groups (treatment duration, dose, age, sex, environmental factors, etc.). The team of Tiemensma118 performed multiple neuropsychological studies on patients diagnosed with AI for 10 ± 8 years. Patients had lower performances in hearing tests, visual memory and executive functioning tasks than their matched controls. However, the external validity of this particular study is difficult to evaluate given the patients’ better results in concentration and attention evaluations (features identified as potentially altered by corticotherapy; p < 0.05).
Table 4.
Studies evaluating cognitive performances in patients with primary adrenal insufficiency (PAI) and secondary insufficiency (SAI).
| Study | PAI/SAI | Variable | Cognitive performances | Patients, n (cohorts) |
|---|---|---|---|---|
| Werumeus Buning et al.114 | SAI | Dose | No effect‡ | 47 |
| Harbeck et al.115 | PAI or SAI | Substitution duration | No effect¥ | 14 (long versus short term) |
| Henry et al.119 | PAI | PAI | No effect (but effect on sleep)* | 60 (versus healthy controls) |
| Klement et al.120 | PAI | PAI | ↓* | 8 (versus healthy controls) |
| Schultebraucks et al.121 | PAI | PAI | No effect (except for verbal expression)Ѡ | 30 (versus healthy controls) |
| Tytherleigh et al.122 | PAI | GC ± MC | ↓Ѧ | 9 (GC versus GC + MC) |
| Tiemensma et al.118 | PAI or SAI | PAI | ↓₡ | 31 (versus healthy controls) |
Memory, attention, executive functioning and social cognition.
Memory attention, vigilance, intellectual functioning.
Selective attention, short-term memory;
Executive functioning, concentration, verbal memory, visual memory, work memory, autobiographic memory.
Tests for numbers, letters, names, items recognition, HVLT, comprehension speed, categories;
Verbal intelligence, work memory, short-term memory, mental flexibility, verbal debit, psychometrics rapidity, processing speed, executive functioning, long span attention.
CAH, congenital adrenal hyperplasia; GC, glucocorticoids; HVLT, ; MC, mineralocorticoids; PAI: primary adrenal insufficiency; SAI: secondary adrenal insufficiency.
Bone mineral density
Even at physiological doses, corticosteroids can inflict deleterious effects on bone mineral density (BMD). These effects were prospectively investigated by Schulz and colleagues117 on patients with PAI (n = 57) and with CAH (n = 33). According to their HC dose, patients were divided into three groups: unchanged (25.2 mg/day, n = 50), increased (18.7–25.9 mg/day, n = 13) and decreased (30.8–21.4 mg/day, n = 27). Dual-energy X-ray absorptiometry at baseline and after 2 years showed decreased BMD in the increased-dose group and an improvement in the decreased-dose group (p < 0.05). In addition, BMD negatively correlated with HC dose. The BMD for the decreased-dose group (reduction from 14.3 mg/m2/day to 13.6 mg/m2/day) was close to the 50th percentile.
Other prospective studies in HC substituted patients (Table 5) found near-normal BMD, whereas almost 50% of these patients had Z scores lower than −2 or −2.5. Regarding patients with PAI treated with prednisolone, a recent study123 found that 46% of them (men and women) met the criteria for osteoporosis (Z score < −2.5) after the age of 50 years. The BMD Z score of these patients were lower in the different sites studied [lumbar spine (−0.42), femoral neck (−0.50) and hip (−0.58)]. Surprisingly, this study did not find any correlation between BMD Z scores and prednisolone dose, or treatment duration, whereas it negatively correlated with BMI.
Table 5.
Studies evaluating BMD (DXA scan) in patients with primary (PAI) or secondary (SAI) adrenal insufficiency.
| Study | PAI/SAI | BMD evaluation | Patients, n (cohort) |
|---|---|---|---|
| Jodar124 | PAI | Normal (on average but 56% < −2.5DS; 30 mg/day or prednisolone)¥ | 25 |
| Schulz117 | PAI (et HCS) | Normal (25 mg/day) or ↓ (31 mg/day) | 90 |
| Valero125 | PAI | Normal (30 mg/day or prednisolone)₡ | 30 (versus healthy controls) |
| Zelissen126 | PAI | ↓ (32% men and 7% women)* | 91 |
Same team as Valero. Determinations of bone turnover markers (calcium, alkaline phosphatase, osteocalcin, PTH and 25(OH) vitamin D.
Bone turnover markers (calcium, alkaline phosphatase, osteocalcin, PTH and 25(OH) vitamin D, type I procollagen.
BMD reduction defined as at least one measure (lumbar spine, right femoral neck, left femoral neck) < −2DS; patients under substitution for 10.6 years (average).
BMD, bone mineral density; CAH, congenital adrenal hyperplasia; DXA, ; GC, glucocorticoids; MC, mineralocorticoids; OH, hydroxy; PAI, primary adrenal insufficiency; PTH, parathyroid hormone; SAI, secondary adrenal insufficiency.
Quality of life
The inability to accurately replicate the pulsatile cortisol secretion by conventional corticosteroid replacement therapy is the main factor that hinders patients’ QoL. Some clinical features of AI such as sleep disturbances and important daytime fatigue resemble circadian disorders.26,31 Patients’ QoL seems to be inversely proportional to diagnosis delay, emphasizing the importance of a precocious therapy instauration.42,127 Other altering factors are advanced age, female sex and presence of comorbidities (i.e. celiac disease, ovarian insufficiency, atrophic gastritis).127 Recent clinical studies estimating the QoL (Table 6) conveyed a deleterious effect of replacement glucocorticoid therapy in comparison with healthy subjects or patients under a low corticosteroid dose.
Table 6.
Studies evaluating quality of life in patients with primary (PAI) or secondary (SAI) adrenal insufficiency.
| Studies | PAI/SAI | QoL | Patients, n (cohort) |
|---|---|---|---|
| Andela et al.128 | PAI or SAI | ↓§ (p < 0.001 in comparison with controls, no connection with cortisol hair concentrations) | 120 |
| Benson et al.113 | SAI | ↓# | 18 |
| Bleicken et al.129 | PAI or SAI | ↓Ø (high versus low dose, thrice versus twice/day) | 334 |
| Werumeus Buning et al.114 | SAI | ↑ with higher HC dose‡ | 47 |
| Hahner et al.130 | PAI or SAI | ↓# | 256 |
| Harbeck et al.115 | PAI or SAI | No effect (↑ nervosity) |
14 (long versus short term) |
| Henry et al.119 | PAI | ↓* | 60 (versus healthy controls) |
| Løvås et al.131 | PAI | ↓# | 79 |
| Meyer et al.127 | PAI | ↓# | 200 |
| Tiemensma et al.118 | PAI or SAI | ↓₡ | 54 (versus healthy controls) |
Short-Form-36.
Short-Form-36, EQ-5D, HADS, MFI-20.
Short-Form-36, Giessen Complaint List (GBB-24), HADS.
Health questionnaire (PHQ-15, PHQ-9), anxiety (GAD-7), well-being (RAND-36), mood (HADS), fatigue (MFI-20).
Short-Form 36, mood (Beck Depression Inventory-II), sleep quality (Pittsburgh Sleep Quality Index).
Scale for apathy; scale for irritability; questionnaire for mood and anxiety; scale for hospitalization anxiety; scale for depression.
EQ-5D, ; GAD-7, ; PAI, primary adrenal insufficiency; HADS, Hospital Anxiety and Depression Scale; MFI-20, Multidimensional Fatigue Inventory-20; QoL, quality of life; RAND-36, RAND 36-Item Health Survey; SAI, secondary adrenal insufficiency.
Few studies have shown interest toward the occurrence of AI-related symptomatology during low-dose HC regimens. A recent Dutch double-blind, randomized, crossover study evaluated the influence of a low dose (15–20 mg/day or 8–12 mg/m2/day) and a high dose (30–40 mg/day or 16–23 mg/m2 /day) HC regimen on developing the symptoms. Health-related questionnaires revealed that patients experienced less depression, anxiety and fatigue while on a higher dose. An important bias in the study was that statistically significant differences were found only after the switch from high to low dose, raising suspicion for corticosteroid-withdrawal-related symptoms.114 Another study128 contradicted the results, proving correlations between HC dosage increase and negative consequences on QoL. Patients experiencing substantial physical fatigue, pain, reduced vitality, and poorly perceived health status were not only on higher doses but also presented with higher hair cortisol concentrations.
Compound formulations
Other significant limitations of conventional corticosteroids arise from the type of compound formulation used. Pediatric patients have to face the shortcomings of unlicensed compounded adult medications. A recent survey132 performed by the European Society of Pediatric Endocrinology including physicians from 16 countries revealed that 60% used off-label divided adult HC tablets and 55% used unlicensed individualized capsules. The doses varied between 0.5 and 5 mg with a preferred thrice-daily (94%) administration. The lack of licensed pediatric HC formulations leads to the usage of adult 10 mg HC tablets compounded by pharmacists. These tablets fail to meet the criteria of European Pharmacopeia in terms of mass and content uniformity, resulting in high dosing variability.133
In accordance with European Pharmacopeia criteria, a recent German study134 assessed 56 batches of HC capsules (1125) collected from CAH patients aged 0–14 years. A total of 21.4% of batched capsules did not meet the standards of the uniformity analysis (net mass and drug content). Surprisingly, 3.6% contained a different steroid than the one labeled, making a total of 25% unsuitable batches for treatment. In cases of good compliance, drug content inaccuracies can explain poor outcomes in CAH patients. A UK study135 outlined the difficulties that arise with the manipulation of 10 mg HC tablets. Physical properties such as hardness can alter dose accuracy depending on the device used for crushing. Another UK study60 demonstrated that tablet splitting is not consistent either; 10 mg HC were quartered in order to obtain a 2.5 mg dose. Dose uniformity analysis revealed that 50% were outside the content criteria required by US Pharmacopeia, whereas more than 40% failed to respect the European Pharmacopeia weight variation.
Recent therapeutic advancements
The numerous limitations deprive conventional immediate-release HC therapy from the possibility to improve the QoL of patients and to provide a better outcome. Therefore, in recent years, replicating the circadian rhythm of endogenous glucocorticoids has become pivotal in the design of GRT. Circadian HC therapy aims to replicate physiological cortisol rhythm, thus preventing the negative consequences of mismatches. The two main research directions evolve around delayed and sustained-release oral HC formulations and circadian IV or subcutaneous infusions of HC.136,137
Modified-release compounds
Plenadren®
Pharmacokinetics and pharmacodynamics
Plenadren® represents a modified-release HC that has an immediate-release outer-layer coating and an extended-release core (i.e. dual-release HC).57,58 Compared with immediate-release HC, it presents a similar Cmax and a 20% reduced bioavailability; supplementary dose adjustment therefore being necessary. It produces higher morning cortisol concentrations and lower ones in the evening. The dual-release character offers a more extended serum profile, creating the possibility of a once-daily morning regimen with no necessity for a second afternoon dose. However, it is not able to reproduce the overnight cortisol rise.136,138
A randomized pharmacokinetic study performed by Johannsson and colleagues139 on betamethasone-suppressed healthy adults showed that a 20 mg dose of dual-release HC can reach a serum cortisol concentration > 200 nmol/l within less than 30 min and maintain it for minimum 6 h. Whereas the bioavailability of dual-release HC is dose- and food-intake dependent, its terminal half-life is dose independent and is almost 1.5 h longer than immediate-release HC. The same group140 compared the pharmacokinetic properties of once-daily dual-release HC regimen with those of conventional thrice-daily HC during a randomized prospective crossover study performed in 64 adults with PAI. Dual-release HC exhibited a 19.4% lower 24 h AUC and induced only one peak in cortisol concentration in 84% of patients, and as such, corresponded more to the physiological profile than immediate-release HC.
Clinical relevance
In the same study,140 after 3–6 months of treatment, patients on the dual-release HC had lower systolic blood pressures, HbA1C levels (p = 0.0006), and total cholesterol levels, as compared with those on immediate-release HC. QoL questionnaires favored dual-release HC for improvement of cognitive functioning, well-being and fatigue decrease. Favorable metabolic outcome was also demonstrated by Giordano and colleagues141 in an open-label study. Nineteen patients with Addison disease were switched from a thrice-daily HC administration to a single morning 20 mg Plenadren® administration. After 1 year, Plenadren® treatment reduced waist circumferences (p = 0.007), total and LDL cholesterol levels (p < 0.05), and HbA1C levels (p = 0.002) and improved 30-AddiQol total scores in these patients, whereas it decreased insulin requirements in type 1 diabetes patients.
A recent Italian single-blind randomized study142 went beyond the metabolic outcomes and evaluated the immune impact. In the 89 patients with PAI and SAI included, 46 were switched from a standard HC therapy to a once-daily morning modified-release HC regimen. After 6 months, treatment decreased the counts of pro-inflammatory CD14+CD16− monocytes and increased the counts of CD16+CD14− and CD16+ NKs, thus eliminating the low-grade inflammation status and restoring NKs’ cytotoxicity. Patients also experienced reduced recurrent infections. An essential conclusion is that the deleterious effect of conventional HC therapy on the immune system derives not only from inappropriate dosing, but also from the mismatch of the circadian physiological cortisol rhythm. Moreover, it was proposed that for patients with AI, the immunomodulator effect of glucocorticoids is stronger than the immunosuppressive one. The same team further demonstrated altered circadian expression of 19/68 genes in peripheral blood mononuclear cells in the same patients.143 After 3 months, modified-release HC treatment was able to restore the expression levels for 18 genes, as compared with the ones of healthy controls.
Long-term safety parameters were evaluated by Nilsson et al. in 71 adult patients with PAI who received a maintenance dose of Plenadren® during an open-label 5-year prospective study.144 Overall, the treatment was well tolerated with no safety concerns observed. Of the 1060 adverse effects reported, 73% were cortisol-deficiency related and they decreased to 43% at 1 year, and to 23% at 5 years of treatment. Contrary to the results of the Johannsson139 and Giordano141 teams, no significant improvements in metabolic profiles were observed. Parameters such as BMI, blood pressure, glucose metabolism, and lipid profile had a steady evolution. As for QoL evaluation, while physical functioning score on Fatigue Impact Scale worsened after the first 3 years, the Psychological General Well-Being Scale showed improvement tendencies toward the end.144
Chronocort®
Pharmacokinetics and pharmacodynamics
Chronocort® is another modified-release HC that, unlike Plenadren®, can be taken late at night, since it exhibits a delayed and sustained absorption profile. Hence, Chronocort® can reproduce the physiological overnight cortisol rise with an early peak in the morning.58,136 Debono and colleagues145 have compared, in a randomized crossover study, the pharmacokinetics of the pilot Chronocort® tablet (5, 15 mg) with the conventional 10 mg HC tablet in 28 dexamethasone-suppressed healthy subjects. Before randomization into four single-dose regimens, patients underwent cortisol profiling to obtain a physiological profile. Late evening (22:00) administration of 30 mg modified-release HC created a near-to-physiological cortisol daily AUC. However, Chronocort® induced an earlier cortisol peak (06:00 instead of 08:30) and a physiological cortisolemia only for 12 h after intake. The bioavailability was less than 75%. Administration of 15–20 mg Chronocort® at 23:00, followed by 10 mg at 07:00, produced cortisol profiles closer to normal.
Scalable technology reformulations led to the development of a multiparticulate Chronocort® tablet which contains an inert core coated with an HC layer, further coated with polymeric layers that ensure a more uniform delayed release, and an outer enteric layer. A phase II open-label study146 performed on 16 dexamethasone-suppressed healthy subjects showed an 89% bioavailability of multiparticulate Chronocort®. A twice-daily regimen (20 mg 23:00, 10 mg 07:00) of multiparticulate Chronocort® reached a Cmax of 665 nmol/l (physiological peak 549 nmol/l) in an average 8.5 h after the first dose. The first cortisol peak was noticed at 07:30 and the second peak later, between 14:00 and 16:00. It was concluded that a twice-daily administration of the compound resulted in a sustained and delayed absorption of HC followed by attainment of an almost normal physiological pharmacokinetic profile.
Clinical relevance
Given the favorable results coming from pharmacokinetic studies, several clinical studies have compared modified-release HC with conventional HC therapy. In a study by Quinckler et al.,147 patients with PAI and SAI treated with modified-release HC (n = 30) showed reduced BMIs and HbA1C levels, whereas patients under conventional HC experienced no improvement. The ability to replicate the overnight cortisol rise extended the indications of Chronocort® for CAH treatment for androgen suppression. Verma and colleagues148 evaluated the hormonal profiles of 14 CAH patients after 1 month of a single Chronocort® dose (given at 22:00) regimen, previously followed by 1 week of standard thrice-daily HC therapy. Even though the cortisol peak appeared earlier, at 06:00, morning steroid androgen levels were lower. However, their concentrations rose later, between 12:00 and 20:00. Mallapa and colleagues149 included 16 CAH adults who followed a 6-month treatment of a twice-daily modified-release HC regimen (20 mg 23:00; 10 mg 07:00). The 24 h, morning and afternoon androstenedione AUCs, along with 24 h, morning 17-hydroxyprogesterone AUCs significantly lowered in these patients. While body composition showed an increase in lean body mass, BMD slightly decreased.
Pediatric formulations
Neither Plenadren®, nor Chronocort® (Table 7 draws a comparison between the two compounds) is licensed for pediatric use.57 The TAIN (Treatment of Adrenal Insufficiency in Neonates) has developed an immediate-release granulated HC formulation called Infacort®. The formulation contains a microcrystalline core coated by an HC layer, further coated by binding layers and an outer ‘taste-making’ one. Doses of 0.5, 1, 2 and 5 mg are available. It can be administered with food, liquids or as dry granules. A phase I crossover pharmacokinetic study on 16 dexamethasone-suppressed healthy participants demonstrated the same bioequivalence with a 10 mg conventional HC tablet, and a linear relationship between cortisol Cmax and dose. No adverse reactions were reported.132
Table 7.
Comparison of pharmacological characteristics of Plenadren® and Chronocort®.
| Plenadren® | Multiparticulate Chronocort® | |
|---|---|---|
| Composition | Immediate-release hydrocortisone outer-layer coating Extended-release core |
Inert core coated with a hydrocortisone layer, coated with polymeric layers Outer enteric layer |
| • Dual release | • Delayed and sustained release | |
| Dose regimen | • Once daily, morning | • Twice daily (evening and morning) |
| Distribution profile | Higher morning cortisol concentrations Lower evening cortisol concentrations No overnight cortisol rise |
Early peak of cortisol in the morning Early peak of cortisol in the afternoon Overnight cortisol rise |
A further open-label phase III trial150 has studied Infacort® absorption and safety in three consecutive children cohorts (2–6 years, 28 days to 2 years, 1–28 days) diagnosed with CAH or hypopituitarism. The standard HC morning dose (median dose 2 mg) was replaced with Infacort®, followed by their usual treatment 8 h later. Cortisol concentrations measured at 0, 60, and 240 min revealed that Cmax (mean 626 nmol/l) was attained at 60 min, showing a similar absorption in young children and adults. At 4 h post administration, cortisol concentrations were lower than 50 nmol/l for 41.7% of the patients. No serious adverse effects were reported and more than 90% of the parents questioned were in favor of the granulated HC.
Continuous subcutaneous hydrocortisone infusion
The quest for obtaining an accurate replication of endogenous cortisol rhythm has led to the development of continuous subcutaneous HC infusion (CSHI)-using insulin pumps. Beside the basal infusion, boluses are given in case of acute stress. The main goal is to recreate the ultradian secretion.52,53 For seven adult patients with Addison disease, CSHI reduced the total daily HC dose, restored circadian cortisol variation, normalized both ACTH and salivary cortisol levels and improved Short Form 36 scores.151 For another 10 Addison patients included in a randomized double-blind placebo-controlled study, CSHI induced similar hormonal profiles with conventional oral HC therapy with the exception of higher morning salivary cortisol levels. However, no improvement in subjective health status was identified.152
Øksnes and colleagues153 have compared CSHI treatment with standard HC therapy on 33 patients with Addison disease. While the HC infusion (initial dose 10.5 mg/m2/day) was adapted according to salivary (6.00–8.00; 23.00–00.00) and morning serum cortisol levels, oral doses were weight adjusted and adapted according to serum cortisol normogram 4 h after the morning administration. After 3 months, CSHI treatment normalized ACTH and morning cortisol levels, restored night-time cortisol levels, lowered enzyme indices of 5α-reductase, 5β-reductases, and CYP3A4. Metabolic outcomes (HOMA index, lipid profile, BMI) were similar in both treatments. The more physiological cortisol profile attained by CSHI was also demonstrated by the same team in another crossover randomized study.154 In addition, they have evaluated the implications on insulin sensitivity, identifying the absence of the overnight glycemic decrease usually found with standard therapy.
Proposal for glucocorticoid substitution dose regimen
Because of the difficulty in evaluating the requirements of HC in adult patients with AI, and due to the lack of specific recommendations regarding adjustments for body composition, we aimed to propose, in this paragraph, a HC substitution dose protocol (both for PAI and SAI), based on the following elements:
the HC substitution dose regimen should take account of the patient’s anthropometry (i.e. weight and height) which is best estimated by the calculation of body surface based on normograms98 (mean BMI value of 22.5 kg/m2 was used for our calculations);
the mean daily HC substitution dose was evaluated at 8 mg/m2/day, by referring to data from the literature;
for values of height superior to the mean (for a defined body weight), body surface values were adapted when these led to a dose modification;
for convenience, the unitary doses were proposed as fractions of 20 mg.
A standard HC substitution protocol is proposed in Table 8. It is based on the actual knowledge in dose distribution (regression of doses from morning to bedtime) and it is proposed for patients without comorbidities or associated pathologies that may require an under- or overevaluation of daily HC dose. Some patients with SAI require other hormonal replacement therapy (e.g. growth hormone, estrogens) that may affect circulating cortisol levels. For these patients, or for others requiring higher doses of HC (because of metabolic characteristics or due to pharmacological interactions), an alternative HC substitution dose protocol should be evaluated. In Supplemental Table 1, we propose a HC substitution dose protocol for a total daily dose of 10 mg/m2/day.
Table 8.
Theoretical HC substitution regimen for adult patients, representing a total daily HC dose of 8 mg/m2/day.
| Body weight (kg) | Body surface (m2) | Total daily theoretical HC dose (mg/day) | Morning dose (mg) | Dose at lunch (mg) | Dose at supper (mg) | Total daily prescribed HC dose (mg/day) |
|---|---|---|---|---|---|---|
| 45–49 | 1.39 | 11.1 | 5 | 2.5 | 2.5 | 10 |
| If >155 cm: 1.5 | Cfr 12 | |||||
| 50–54 | 1.52 | 12.2 | 7.5 | 2.5 | 2.5 | 12.5 |
| If >170 cm: 1.7 | Cfr 14 | |||||
| 55–59 | 1.63 | 13 | 7.5 | 2.5 | 2.5 | 12.5 |
| If >175 cm: 1.8 | Cfr 14 | |||||
| 60–64 | 1.74 | 14 | 7.5 | 5 | 2.5 | 15 |
| If >175 cm: >1.85 | Cfr 14 | |||||
| 65–69 | 1.85 | 14.8 | 7.5 | 5 | 2.5 | 15 |
| If >185 cm: >2 | Cfr 16.5 | |||||
| 70–74 | 1.96 | 15.7 | 7.5 | 5 | 2.5 | 15 |
| If >185 cm: >2 | Cfr 16.5 | |||||
| 75–79 | 2.06 | 16.5 | 10 | 5 | 2.5 | 17.5 |
| If >185 cm: >2.1 | Cfr 16.5 | |||||
| 80–84 | 2.16 | 17.3 | 10 | 5 | 2.5 | 17.5 |
| If >200 cm: >2.3 | Cfr 18.4 | |||||
| 85–89 | 2.3 | 18.4 | 10 | 7.5 | 2.5 | 20 |
| 90–94 | 2.38 | 19 | 10 | 7.5 | 2.5 | 20 |
| 95–99 | 2.48 | 19.8 | 10 | 7.5 | 2.5 | 20 |
| 100–104 | 2.55 | 20.4 | 12.5 | 7.5 | 2.5 | 22.5 |
| 105–109 | 2.7 | 21.6 | 12.5 | 7.5 | 2.5 | 22.5 |
Cfr, ; HC, hydrocortisone.
Conclusion
In conclusion, despite being a lifesaving therapy, conventional GRT remains subjected to numerous limitations. Focus should be on side-effect acknowledgment in order to raise awareness among clinicians. An attainment of guidelines for worldwide uniformity is a high priority. In addition, reliable treatment biomarkers are crucial for developing individualized treatment regimens and thus improving compliance. Chronotherapy is a promising field and may be able to mimick physiological cortisol production in the future. Finally, achieving better pharmacokinetic profiles is important for minimizing adverse outcomes of chronic corticoid therapy.
Supplemental Material
Supplemental material, Supplemental_PDF for Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency by Alina Oprea, Nicolas C. G. Bonnet, Olivier Pollé and Philippe A. Lysy in Therapeutic Advances in Endocrinology and Metabolism
Acknowledgments
PL received grants from Fonds National de la Recherche Scientifique and from the BElgian Society for PEdiadtric Endocrinology and Diabetes (BESPEED). Ethics approval was not required for this review. AO: conception and design, manuscript writing; NB: manuscript writing; OP: manuscript writing; PL: conception and design; manuscript writing; administrative support; financial support; study supervision.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Philippe A. Lysy  https://orcid.org/0000-0003-1598-7998
https://orcid.org/0000-0003-1598-7998
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alina Oprea, Pediatric Endocrinology Unit, Cliniques Universitaires Saint Luc, Brussels, Belgium.
Nicolas C. G. Bonnet, Pediatric Endocrinology Unit, Cliniques Universitaires Saint Luc, Brussels, Belgium
Olivier Pollé, Pediatric Endocrinology Unit, Cliniques Universitaires Saint Luc, Brussels, Belgium.
Philippe A. Lysy, Pediatric Endocrinology Unit, Cliniques Universitaires Saint Luc, Pôle PEDI, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Av. Hippocrate 10, B-1200 Brussels, Belgium.
References
- 1. Bishop PM. The history of the discovery of Addison’s disease. Proc R Soc Med 1950; 43: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med 2016; 129: 339.e1–339.e9. [DOI] [PubMed] [Google Scholar]
- 3. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014; 383: 2152–2167. [DOI] [PubMed] [Google Scholar]
- 4. Guran T, Buonocore F, Saka N, et al. Rare causes of primary adrenal insufficiency: genetic and clinical characterization of a large nationwide cohort. J Clin Endocrinol Metab 2016; 101: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chabre O. The difficulties of pseudo-Cushing’s syndrome (or “non-neoplastic hypercortisolism”). Ann Endocrinol (Paris) 2018; 79: 138–145. [DOI] [PubMed] [Google Scholar]
- 6. Murray RD Ekman B Uddin S et al.;. The EU-AIR Investigators. Management of glucocorticoid replacement in adrenal insufficiency shows notable heterogeneity - data from the EU-AIR. Clin Endocrinol (Oxf) 2017; 86: 340–346. [DOI] [PubMed] [Google Scholar]
- 7. Charmandari E, Kino T, Chrousos GP. Glucocorticoids. In: Yaffe SJ, Aranda JV. (eds) Neonatal and pediatric pharmacology. 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2010, pp. 760–772. [Google Scholar]
- 8. Nicolaides NC, Charmandari E, Chrousos GP, et al. Circadian endocrine rhythms: the hypothalamic–pituitary–adrenal axis and its actions. Ann N Y Acad Sci 2014; 1318: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003; 361: 1881–1893. [DOI] [PubMed] [Google Scholar]
- 10. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, et al. Premature. Mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab 2006; 91: 4849–4853. [DOI] [PubMed] [Google Scholar]
- 11. Bensing S, Brandt L, Tabaroj F, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 2008; 69: 697–704. [DOI] [PubMed] [Google Scholar]
- 12. Schulz J, Frey KR, Cooper MS, et al. Reduction in daily hydrocortisone dose improves bone health in primary adrenal insufficiency. Eur J Endocrinol 2016; 174: 531–538. [DOI] [PubMed] [Google Scholar]
- 13. Werumeus Buning J, Van Faassen M, Brummelman P, et al. Effects of hydrocortisone on the regulation of blood pressure: results from a randomized controlled trial. J Clin Endocrinol Metab 2016; 101: 3691–3699. [DOI] [PubMed] [Google Scholar]
- 14. Reisch N, Arlt W. Fine tuning for quality of life: 21st century approach to treatment of Addison’s disease. Endocrinol Metab Clin North Am 2009; 38: 407–418, ix–x. [DOI] [PubMed] [Google Scholar]
- 15. Plat L, Byrne MM, Sturis J, et al. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol 1996; 270: E36–E42. [DOI] [PubMed] [Google Scholar]
- 16. Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18: 716–738. [DOI] [PubMed] [Google Scholar]
- 17. Plat L, Leproult R, L’Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 1999; 84: 3082–3092. [DOI] [PubMed] [Google Scholar]
- 18. Heshmati HM, Riggs BL, Burritt MF, et al. Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J Clin Endocrinol Metab 1998; 83: 751–756. [DOI] [PubMed] [Google Scholar]
- 19. Kalsbeek A, Van der Spek R, Lei J, et al. Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Mol Cell Endocrinol 2012; 349: 20–29. [DOI] [PubMed] [Google Scholar]
- 20. Rahvar AH, Haas CS, Danneberg S, et al. Increased cardiovascular risk in patients with adrenal insufficiency: a short review. Biomed Res Int 2017; 2017: 3691913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knutsson U, Dahlgren J, Marcus C, et al. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab 1997; 82: 536–540. [DOI] [PubMed] [Google Scholar]
- 22. Lightman SL, Wiles CC, Atkinson HC, et al. The significance of glucocorticoid pulsatility. Euro J Pharmacol 2008; 583: 255–262. [DOI] [PubMed] [Google Scholar]
- 23. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta 2011; 1812: 581–591. [DOI] [PubMed] [Google Scholar]
- 24. Oster H, Damerow S, Kiessling S, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 2006; 4: 163–173. [DOI] [PubMed] [Google Scholar]
- 25. Harbeck B, Kropp P, Mönig H. Effects of short-term nocturnal cortisol replacement on cognitive function and quality of life in patients with primary or secondary adrenal insufficiency: a pilot study. Appl Psychophysiol Biofeedback 2009; 34: 113–119. [DOI] [PubMed] [Google Scholar]
- 26. Kerrigan JR, Veldhuis JD, Leyo SA, et al. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993; 76: 1505–1510. [DOI] [PubMed] [Google Scholar]
- 27. Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72: 39–45. [DOI] [PubMed] [Google Scholar]
- 28. Purnell JQ, Brandon DD, Isabelle LM, et al. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab 2004; 89: 281–287. [DOI] [PubMed] [Google Scholar]
- 29. Linder BL, Esteban NV, Yergey AL, et al. Cortisol production rate in childhood and adolescence. J Pediatr 1990; 117: 892–896. [DOI] [PubMed] [Google Scholar]
- 30. Kraan GP, Dullaart RP, Pratt JJ, et al. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J Clin Endocrinol Metab 1998; 83: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 31. Brandon DD, Isabelle LM, Samuels MH, et al. Cortisol production rate measurement by stable isotope dilution using gas chromatography-negative ion chemical ionization mass spectrometry. Steroids 1999; 64: 372–378. [DOI] [PubMed] [Google Scholar]
- 32. Cidlowski JA, Maclchoff CD, Malchoff DM. Glucocorticoid receptors, their mechanisms of action, and glucocorticoid resistance physiology. In: Jameson JL, De Groot LJ, de Kretser DM, et al. (eds) Endocrinology adult and pediatric. 7th ed. Saunders, 2015, Chapter 98, pp. 1717–1726. [Google Scholar]
- 33. Chrousos GP. Glucocorticoid action: physiology In: Jameson JL, De Groot LJ, de Kretser DM, et al. (eds) Endocrinology adult and pediatric. 7th ed. Saunders, 2015, Chapter 99, pp. 1727–1740. [Google Scholar]
- 34. Conway-Campbell BL, McKenna MA, Wiles CC, et al. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 2007; 148: 5470–5477. [DOI] [PubMed] [Google Scholar]
- 35. Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax 2000; 55: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciriaco M, Ventrice P, Russo G, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother 2013; 4(Suppl. 1): S94–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci 2005; 63: 613–622. [DOI] [PubMed] [Google Scholar]
- 38. Payne JD, Nadel L. Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn Mem 2004; 11: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henry M, Ross IL, Wolf PSA, et al. Impaired quality and efficiency of sleep impairs cognitive functioning in Addison’s disease. Psychoneuroendocrinology 2017; 78: 237–245. [DOI] [PubMed] [Google Scholar]
- 40. Nicolaides NC, Charmandari E, Kino T, et al. Stress-related and circadian secretion and target tissue actions of glucocorticoids: impact on health. Front Endocrinol 2017; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nieman LK, Lacroix A, Martin KA. Treatment of adrenal insufficiency in adults In: Lacroix A, Martin K, eds Waltham, Mass. : UpToDate, 2016. Available at: https://www.uptodate.com/contents/treatment-of-adrenal-insufficiencyinadults?search=adrenal%20insufficiency&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3 (accessed 24 April 2018).
- 42. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dowty ME, Dietsch CR. Improved prediction of in vivo peroral absorption from in vitro intestinal permeability using an internal standard to control for intra- and inter-rat variability. Pharm Res 1997; 14: 1792–1797. [DOI] [PubMed] [Google Scholar]
- 44. Heimbach T, Oh DM, Li LY, et al. Absorption rate limit considerations for oral phosphate prodrugs. Pharm Res 2003; 20: 848–856. [DOI] [PubMed] [Google Scholar]
- 45. Derendorf H, Möllmann H, Barth J, et al. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol 1991; 31: 473–476. [DOI] [PubMed] [Google Scholar]
- 46. Hyde PM, Williams RH. Absorption and metabolism of hydrocortisone-4-C14. J Biol Chem 1957; 227: 1063–1081. [PubMed] [Google Scholar]
- 47. Mager DE, Lin SX, Blum RA, et al. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 2003; 43: 1216–1227. [DOI] [PubMed] [Google Scholar]
- 48. Jung C, Greco S, Nguyen HH, et al. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr Disord 2014; 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toothaker RD, Craig WA, Welling PG. Effect of dose size on the pharmacokinetics of oral hydrocortisone suspension. J Pharm Sci 1982; 71: 1182–1185. [DOI] [PubMed] [Google Scholar]
- 50. Beisel WR, Horton R, Yu Chao P, et al. Physiology of urinary cortisol excretion. J Clin Endocrinol Metab 1964; 24: 887–893. [DOI] [PubMed] [Google Scholar]
- 51. Toothaker RD, Welling PG. Effect of dose size on the pharmacokinetics of intravenous hydrocortisone during endogenous hydrocortisone suppression. J Pharmacokinet Biopharm 1982; 10: 147–156. [DOI] [PubMed] [Google Scholar]
- 52. Toothaker RD, Sundaresan GM, Hunt JP, et al. Oral hydrocortisone pharmacokinetics: a comparison of fluorescence and ultraviolet high-pressure liquid chromatographic assays for hydrocortisone in plasma. J Pharm Sci 1982; 71: 573–576. [DOI] [PubMed] [Google Scholar]
- 53. Diederich S, Eigendorff E, Burkhardt P, et al. 11beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab 2002; 87: 5695–5701. [DOI] [PubMed] [Google Scholar]
- 54. N’Gankam V, Uehlinger D, Dick B, et al. Increased cortisol and prednisone in metabolites and reduced activity of 11 beta-hydroxysteroid dehydrogenase in patients on hemodialysis. Kidney Int 2002; 61: 1859–1866. [DOI] [PubMed] [Google Scholar]
- 55. Ferrari P. The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim Biophys Acta 2010; 1802: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 56. Czock D, Keller F, Rasche FM, et al. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005; 44: 61–98. [DOI] [PubMed] [Google Scholar]
- 57. Øksnes M, Ross R, Løvås K. Optimal glucocorticoid replacement in adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2015; 29: 3–15. [DOI] [PubMed] [Google Scholar]
- 58. Porter J, Blair J, Ross RJ. Is physiological glucocorticoid replacement important in children? Arch Dis Child 2017; 102: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Donohoue PA. Treatment of adrenal insufficiency in children. In: Geffner ME, Hoppin AG, eds Waltham, Mass. : UpTodate 2017. Available at: https://www.uptodate.com/contents/treatment-of-adrenal-insufficiencyinchildren?search=adrenal%20insufficiency&source=search_result&selectedTitle=8~150&usage_type=default&display_rank=8 (accessed 24 April 2018).
- 60. Madathilethu J, Roberts M, Peak M, et al. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatrics Open 2018; 2: e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clayton PE, Miller WL, Oberfield SE, et al. Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm Res 2002; 58: 188–195. [DOI] [PubMed] [Google Scholar]
- 62. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95: 4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bonfig W, Bechtold S, Schmidt H, et al. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab 2007; 92: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 64. Shulman D, Palmert M, Kemp S. Adrenal insufficiency: still a cause of morbidity and death in childhood. Pediatrics 2007; 119: e484–e494. [DOI] [PubMed] [Google Scholar]
- 65. Nieman LK. Causes of secondary and tertiary adrenal insufficiency in adults. In: Lacroix A, Martin K, (eds) Waltham, Mass. : UpToDate, 2016. Available at: https://www.uptodate.com/contents/causes-of-secondary-and-tertiary-adrenal-insufficiency-in-adults (accessed 24 April 2018). [Google Scholar]
- 66. Nieman LK, Neary N. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2010; 17: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cambiaso P, Schiaffini R, Pontrelli G, et al. Nocturnal hypoglycaemia in ACTH and GH deficient children: role of continuous glucose monitoring. Clin Endocrinol (Oxf) 2013; 79: 232–237. [DOI] [PubMed] [Google Scholar]
- 68. Nicolaides NC, Chrousos GP, Charmandari E. Adrenal insufficiency [Updated 14 October 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al., (eds). Endotext. South Dartmouth, MA: MDText.com, Inc, 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279083/ [Google Scholar]
- 69. Nicolaides NC, Pavlaki AN, Maria Alexandra MA, et al. Glucocorticoid Therapy and Adrenal Suppression. 2018 Oct 19. In: De Groot LJ, Chrousos G, Dungan K, et al., (eds). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279156/ [Google Scholar]
- 70. Schadt C, Jackson SM. Glucocorticoids. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier, 2018, pp. 2186–2199. [Google Scholar]
- 71. Saag K, Buttgereit F. Systemic glucocorticoids in rheumatology. In: Hochberg M. (ed.), Rheumatology. 7th ed. Elsevier, 2019, pp. 488–498. [Google Scholar]
- 72. Kirou K, Boumpas D. Systemic glucocorticoid therapy in systemic lupus erythematosus. In: Dubois’ Lupus erythematosus and related syndromes. Elsevier, 2013, pp. 591–600. [Google Scholar]
- 73. Williams EL, Choudhury S, Tan T, et al. Prednisolone replacement therapy mimics the circadian rhythm more closely than other glucocorticoids. J Appl Lab Med 2016; 1: 152–161. [DOI] [PubMed] [Google Scholar]
- 74. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Int Med 2014; 275: 104–115. [DOI] [PubMed] [Google Scholar]
- 75. Ajish TP, Praveen VP, Nisha B, et al. Comparison of different glucocorticoid regimens in the management of classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Indian J Endocrinol Metabol 2014; 18: 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Caldato MC, Fernandes VT, Kater CE. One year clinical evaluation of single morning dose prednisolone therapy for 21-hydroxylase deficiency. Arq Bras Endocrinol Metabol 2004; 48: 705–712. [DOI] [PubMed] [Google Scholar]
- 77. Leite FM, Longui CA, Kochi C, et al. Comparative study of prednisolone versus hydrocortisone acetate for treatment of patients with the classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Arq Bras Endocrinol Metabol 2008; 52: 101–108. [DOI] [PubMed] [Google Scholar]
- 78. Smith DJF, Prabhudev H, Choudhury S, et al. Prednisolone has the same cardiovascular risk profile as hydrocortisone in glucocorticoid replacement. Endocrine Connections 2017; 6: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Quinkler M, Ekman B, Marelli C, et al. Prednisolone is associated with a worse lipid profile than hydrocortisone in patients with adrenal insufficiency. Endocrine Connections 2017; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rivkees SA, Stephenson K. Low-dose dexamethasone therapy from infancy of virilizing congenital adrenal hyperplasia. Int J Pediatric Endocrinol 2009; 2009: 274682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics 2000; 106: 767–773. [DOI] [PubMed] [Google Scholar]
- 82. Dauber A, Feldman HA, Majzoub JA. Nocturnal dexamethasone versus hydrocortisone for the treatment of children with congenital adrenal hyperplasia. Int J Pediatric Endocrinol 2010; 2010: 347636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hahner S. Acute adrenal crisis and mortality in adrenal insufficiency: still a concern in 2018! Annal Endocrinol 2018; 79: 164–166. [DOI] [PubMed] [Google Scholar]
- 84. Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol 2010; 162: 597–602. [DOI] [PubMed] [Google Scholar]
- 85. White K, Arlt W. Adrenal crisis in treated Addison’s disease: a predictable but under-managed event. Eur J Endocrinol 2010; 162: 115–120. [DOI] [PubMed] [Google Scholar]
- 86. Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 2015; 100: 407–416. [DOI] [PubMed] [Google Scholar]
- 87. Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab 2009; 94: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 88. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises: perspectives and research directions. Endocrine 2017; 55: 336. [DOI] [PubMed] [Google Scholar]
- 89. Kampmeyer D, Haas C, Stefan Moenig H, et al. Self-management in adrenal insufficiency — towards a better understanding. Endocrine J 2017; 64: 379–385. [DOI] [PubMed] [Google Scholar]
- 90. Allolio B. Extensive experience in endocrinology: adrenal crisis. Eur J Endocrinol 2015; 172: R115–R124. [DOI] [PubMed] [Google Scholar]
- 91. Murray RD Ekman B Uddin S et al.;. The EU-AIR Investigators. Management of glucocorticoid replacement in adrenal insufficiency shows notable heterogeneity – data from the EU-AIR. Clin Endocrinol 2017; 86: 340–346. [DOI] [PubMed] [Google Scholar]
- 92. Mallappa A, Debono M. Recent advances in hydrocortisone replacement treatment. Endocr Dev 2016; 30: 42–53. [DOI] [PubMed] [Google Scholar]
- 93. Howlett TA. An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf). 1997; 46: 263–268. [DOI] [PubMed] [Google Scholar]
- 94. Haas CS, Rahvar AH, Danneberg S, et al. Low impact of urinary cortisol in the assessment of hydrocortisone replacement therapy. Horm Metab Res 2016; 48: 571–574. [DOI] [PubMed] [Google Scholar]
- 95. Simon N, Castinetti F, Ouliac F, et al. Pharmacokinetic evidence for suboptimal treatment of adrenal insufficiency with currently available hydrocortisone tablets. Clin Pharmacokinet 2010; 49: 455–463. [DOI] [PubMed] [Google Scholar]
- 96. Swords FM, Carroll PV, Kisalu J, et al. The effects of growth hormone deficiency and replacement on glucocorticoid exposure in hypopituitary patients on cortisone acetate and hydrocortisone replacement. Clin Endocrinol (Oxf) 2003; 59: 613–620. [DOI] [PubMed] [Google Scholar]
- 97. Behan LA, Rogers B, Hannon MJ, et al. Optimizing glucocorticoid replacement therapy in severely adrenocorticotropin-deficient hypopituitary male patients. Clin Endocrinol (Oxf) 2011; 75: 505–513. [DOI] [PubMed] [Google Scholar]
- 98. Mah PM, Jenkins RC, Rostami-Hodjegan A, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2004; 61: 367–375. [DOI] [PubMed] [Google Scholar]
- 99. Thomson AH, Devers MC, Wallace AM, et al. Variability in hydrocortisone plasma and saliva pharmacokinetics following intravenous and oral administration to patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2007; 66: 789–796. [DOI] [PubMed] [Google Scholar]
- 100. Rousseau E, Joubert M, Trzepla G, et al. Usefulness of time-point serum cortisol and ACTH measurements for the adjustment of glucocorticoid replacement in adrenal insufficiency. PLOS One 2015; 10: e0135975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Feek CM, Ratcliffe JG, Seth J, et al. Patterns of plasma cortisol and ACTH concentrations in patients with Addison’s disease treated with conventional corticosteroid replacement. Clin Endocrinol (Oxf) 1981; 14: 451–458. [DOI] [PubMed] [Google Scholar]
- 102. Laureti S, Falorni A, Santeusanio F. Improvement of treatment of primary adrenal insufficiency by administration of cortisone acetate in three daily doses. J Endocrinol Invest 2003; 26: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 103. Ekman B, Bachrach-Lindström M, Lindström T, et al. A randomized, double-blind, crossover study comparing two- and four-dose hydrocortisone regimen with regard to quality of life, cortisol and ACTH profiles in patients with primary adrenal insufficiency. Clin Endocrinol (Oxf) 2012; 77: 18–25. [DOI] [PubMed] [Google Scholar]
- 104. Erichsen MM, Lovas K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160: 233–237. [DOI] [PubMed] [Google Scholar]
- 105. Zueger T, Kirchner P, Herren C, et al. Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab 2012; 97: E1938–E1942. [DOI] [PubMed] [Google Scholar]
- 106. Werumeus Buning J, Van Faassen M, Brummelman P, et al. Effects of hydrocortisone on the regulation of blood pressure: results from a randomized controlled trial. J Clin Endocrinol Metab 2016; 101: 3691–3699. [DOI] [PubMed] [Google Scholar]
- 107. Carolyn JP, Brenda LM, Campbell HT, et al. Acute effect of increasing glucocorticoid replacement dose on cardiovascular risk and insulin sensitivity in patients with adrenocorticotrophin deficiency. J Clin Endocrinol Metab 2014; 99: 2269–2276. [DOI] [PubMed] [Google Scholar]
- 108. Behan LA, Carmody D, Rogers B, et al. Low-dose hydrocortisone replacement is associated with improved arterial stiffness index and blood pressure dynamics in severely adrenocorticotrophin-deficient hypopituitary male patients. Eur J Endocrinol 2016; 174: 791–799. [DOI] [PubMed] [Google Scholar]
- 109. Giordano R, Marzotti S, Balbo M, et al. Metabolic and cardiovascular profile in patients with Addison’s disease under conventional glucocorticoid replacement. J Endocrinol Invest 2009; 32: 917–923. [DOI] [PubMed] [Google Scholar]
- 110. Filipsson H, Monson JP, Koltowska-Haggstrom M, et al. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab 2006; 91: 3954–3961. [DOI] [PubMed] [Google Scholar]
- 111. Danilowicz K, Bruno OD, Manavela M, et al. Correction of cortisol overreplacement ameliorates morbidities in patients with hypopituitarism: a pilot study. Pituitary 2008; 11: 279–285. [DOI] [PubMed] [Google Scholar]
- 112. Staufenbiel SM, Andela CD, Manenschijn L, et al. Increased hair cortisol concentrations and BMI in patients with pituitary-adrenal disease on hydrocortisone replacement. J Clin Endocrinol Metab 2015; 100: 2456–2462. [DOI] [PubMed] [Google Scholar]
- 113. Benson S, Neumann P, Unger N, et al. Effects of standard glucocorticoid replacement therapies on subjective well-being: a randomized, double-blind, crossover study in patients with secondary adrenal insufficiency. Eur J Endocrinol 2012; 167: 679–685. [DOI] [PubMed] [Google Scholar]
- 114. Werumeus Buning J, Brummelman P, Koerts J, et al. Hydrocortisone dose influences pain, depressive symptoms and perceived health in adrenal insufficiency: a randomized controlled trial. Neuroendocrinology 2016; 103: 771–778. [DOI] [PubMed] [Google Scholar]
- 115. Harbeck B, Danneberg S, Rahvar AH, et al. Exploring the impact of short- and long-term hydrocortisone replacement on cognitive function, quality of life and catecholamine secretion: a pilot study. Appl Psychophysiol Biofeedback 2016; 41: 341–347. [DOI] [PubMed] [Google Scholar]
- 116. Nilsson AG, Bergthorsdottir R, Burman P, et al. Long-term safety of once-daily, dual-release hydrocortisone in patients with adrenal insufficiency: a phase 3b, open-label, extension study. Eur J Endocrinol 2017; 176: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schulz J, Frey KR, Cooper MS, et al. Reduction in daily hydrocortisone dose improves bone health in primary adrenal insufficiency. Eur J Endocrinol 2016; 174: 531–538. [DOI] [PubMed] [Google Scholar]
- 118. Tiemensma J, Andela CD, Biermasz NR, et al. Mild cognitive deficits in patients with primary adrenal insufficiency. Psychoneuroendocrinology 2016; 63: 170–177. [DOI] [PubMed] [Google Scholar]
- 119. Henry M, Wolf PS, Ross IL, et al. Poor quality of life, depressed mood, and memory impairment may be mediated by sleep disruption in patients with Addison’s disease. Physiol Behav 2015; 151: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Klement J, Hubold C, Hallschmid M, et al. Effects of glucose infusion on neuroendocrine and cognitive parameters in Addison disease. Metabolism 2009; 58: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 121. Schultebraucks K, Wingenfeld K, Heimes J, et al. Cognitive function in patients with primary adrenal insufficiency (Addison’sdisease). Psychoneuroendocrinology 2015; 55: 1–7. [DOI] [PubMed] [Google Scholar]
- 122. Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison’s disease. Psychoneuroendocrinology 2004; 29: 712–723. [DOI] [PubMed] [Google Scholar]
- 123. Chandy DD, Bhatia E. Bone mineral density in patients with addison disease on replacement therapy with prednisolone. Endocr Pract 2016; 22: 434–439. [DOI] [PubMed] [Google Scholar]
- 124. Jodar E, Valdepenas MP, Martinez G, et al. Long-term follow-up of bone mineral density in Addison’s disease. Clin Endocrinol (Oxf) 2003; 58: 617–620. [DOI] [PubMed] [Google Scholar]
- 125. Valero MA, Leon M, Ruiz Valdepenas MP, et al. Bone density and turnover in Addison’s disease: effect of glucocorticoid treatment. Bone Miner 1994; 26: 9–17. [DOI] [PubMed] [Google Scholar]
- 126. Zelissen PM, Croughs RJ, Van Rijk PP, et al. Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann Intern Med 1994; 120: 207–210. [DOI] [PubMed] [Google Scholar]
- 127. Meyer G, Hackemann A, Penna-Martinez M, et al. What affects the quality of life in autoimmune Addison’s disease? Horm Metab Res 2013; 45: 92–95. [DOI] [PubMed] [Google Scholar]
- 128. Andela CD, Staufenbiel SM, Joustra SD, et al. Quality of life in patients with adrenal insufficiency correlates stronger with hydrocortisone dosage, than with long-term systemic cortisol levels. Psychoneuroendocrinology 2016; 72: 80–86. [DOI] [PubMed] [Google Scholar]
- 129. Bleicken B, Hahner S, Loeffler M, et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2010; 72: 297–304. [DOI] [PubMed] [Google Scholar]
- 130. Hahner S, Loeffler M, Fassnacht M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab 2007; 92: 3912–3922. [DOI] [PubMed] [Google Scholar]
- 131. Løvås K, Curran S, Oksnes M, et al. Development of a disease-specific quality of life questionnaire in Addison’s disease. J Clin Endocrinol Metab 2010; 95: 545–545. [DOI] [PubMed] [Google Scholar]
- 132. Whitaker MJ, Spielmann S, Digweed D, et al. Development and testing in healthy adults of oral hydrocortisone granules with taste masking for the treatment of neonates and infants with adrenal insufficiency. J Clin Endocrinol Metab 2015; 100: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 133. Kauzor D, Spielmann S, Ross R, et al. Medication safety study investigating hydrocortisone individually and extemporaneously compounded capsules for paediatric use in congenital adrenal hyperplasia. In: Proceedings from the 16th European Conference of Endocrinology, Wroclaw, Poland, 3–7 May 2014, abstract P825. [Google Scholar]
- 134. Neumann U, Burau D, Spielmann S, et al. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur J Endocrinol 2017; 177: 239–242. [DOI] [PubMed] [Google Scholar]
- 135. Saimbi S, Madden V, Stirling H, et al. Comparison of hydrocortisone 10 mg tablets: tablet hardness optimised for adult use has negative consequences for paediatric use. Arch Dis Child 2016; 101: e2. [DOI] [PubMed] [Google Scholar]
- 136. Chan S, Debono M. Replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Ther Adv Endocrinol Metab 2010; 1: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Debono M, Price JN, Ross RJ. Novel strategies for hydrocortisone replacement. Best Pract Res Clin Endocrinol Metab 2009; 23: 221–232. [DOI] [PubMed] [Google Scholar]
- 138. Duocort Plenadren. European public assessment reports (EPAR), EMEA/H/C/2185, 2011. Available at: https://www.ema.europa.eu/documents/assessment-report/plenadren-epar-public-assessment-report_en.pdf
- 139. Johannsson G, Bergthorsdottir R, Nilsson AG, et al. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol 2009; 161: 119–130. [DOI] [PubMed] [Google Scholar]
- 140. Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab 2012; 97: 473–481. [DOI] [PubMed] [Google Scholar]
- 141. Giordano R, Guaraldi F, Marinazzo E, et al. Improvement of anthropometric and metabolic parameters, and quality of life following treatment with dual-release hydrocortisone in patients with Addison’s disease. Endocrine 2016; 51: 360–368. [DOI] [PubMed] [Google Scholar]
- 142. Isidori AM, Venneri MA, Graziadio C, et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2018; 6: 173–185. [DOI] [PubMed] [Google Scholar]
- 143. Venneri AM, Hasenmajer V, Fiore D, et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J Clin Endocrinol Metab 2018; 103: 2998–3009. [DOI] [PubMed] [Google Scholar]
- 144. Nilsson AG, Bergthorsdottir R, Burman P, et al. Long-term safety of once-daily, dual-release hydrocortisone in patients with adrenal insufficiency: a phase 3b, open-label, extension study. Euro J Endocrinol 2017; 176: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 2009; 94: 1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Whitaker M, Debono M, Huatan H, et al. An oral multi-particulate, modified release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin Endocrinol 2014; 80: 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Quinkler M, Miodini Nilsen R, Zopf K, et al. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur J Endocrinol 2015; 172: 619–626. [DOI] [PubMed] [Google Scholar]
- 148. Verma S, VanRyzin C, Sinaii N, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (ChronocortTM) versus conventional hydrocortisone (CortefTM) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol 2010; 72: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 2015; 100: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf) 2018; 88: 21–29. [DOI] [PubMed] [Google Scholar]
- 151. Løvås K, Husebye ES. Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur J Endocrinol 2007; 157: 109–112. [DOI] [PubMed] [Google Scholar]
- 152. Gagliardi L, Nenke MA, Thynne TLRJ, et al. Continuous subcutaneous hydrocortisone infusion therapy in addison’s disease: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab 2014; 99: 4149–4157. [DOI] [PubMed] [Google Scholar]
- 153. Øksnes M, Björnsdottir S, Isaksson M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of addison’s disease: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 154. Björnsdottir S, Øksnes M, Isaksson M, et al. Circadian hormone profiles and insulin sensitivity in patients with Addison’s disease: a comparison of continuous subcutaneous hydrocortisone infusion with conventional glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2015; 83: 28–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_PDF for Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency by Alina Oprea, Nicolas C. G. Bonnet, Olivier Pollé and Philippe A. Lysy in Therapeutic Advances in Endocrinology and Metabolism