Abstract
Ameloblastoma is an uncommon and locally aggressive, benign, odontogenic tumor, with local recurrence when not adequately excised. A rare variant of this neoplasm with the benign features but accompanied with metastases has been described. This rare variant is malignant ameloblastoma and is known to have a poor prognosis. We present the case of a young woman who had recurrent mandibular tumors, which were resected twice and histologically reported as ameloblastoma. Four years later, she presented with pulmonary metastasis and atelectasis. A review of the literature on this very rare neoplasm was also performed.
Keywords: Ameloblastoma, Mediastinum, Neoplasm Metastasis, Pulmonary Atelectasis
CASE REPORT
A 25-year-old female presented to the hospital with a 6-month history of exertional dyspnea and unproductive cough. She denied hemoptysis, chest pain, or orthopnea. Her past medical history included a painless mandibular mass removed through a conservative surgical procedure, in another hospital facility, 5 years before. This relapsed 2 years later and required a re-excision at the same facility. She was told that the excised tissue was reported as ameloblastoma, but did not retain a copy of the histological report. A month before presenting at our facility, she had a mandibular reconstruction of the second excision’s surgical site.
On physical examination, she was mildly tachypneic with the respiratory rate of 22 breaths/min. There was a dull percussion on the entire left hemithorax where no breath sounds were audible. She had a swelling on the right lower jaw measuring 3 cm in diameter with an overlying surgical scar. The remaining physical examination was unremarkable.
The chest x-ray showed a homogenous opacity on the left hemithorax with ipsilateral tracheal deviation and mediastinal shift. A computerized tomography scan showed a left posteromedial soft tissue mass with the upper pulmonary lobe atelectasis. A working diagnosis of a mediastinal tumor — probably of thymic origin — was considered, and the patient was subjected to a left thoracotomy. Intraoperatively, the mass was occupying the space between the descending thoracic aorta and the pericardium with partial left lung collapse. Efforts to remove the tumor provoked profuse and uncontrollable bleeding; the patient developed hemorrhagic shock followed by cardiopulmonary arrest, resulting in death.
Permission for a limited autopsy of the thorax to obtain biopsy samples of the tumor was obtained from the patient’s relatives. The autopsy showed an oval to globular mass in the mediastinum firmly adhered to the pericardium and the mediastinal surface of the left lung (Figure 1A and 1B).
Figure 1. Gross view of the neoplasm. A – The tumor (T) attached to the left lung (L) and the parietal pericardium (P); B – The cut surface of the tumor shows a greyish-white mass attached to the lung surface.
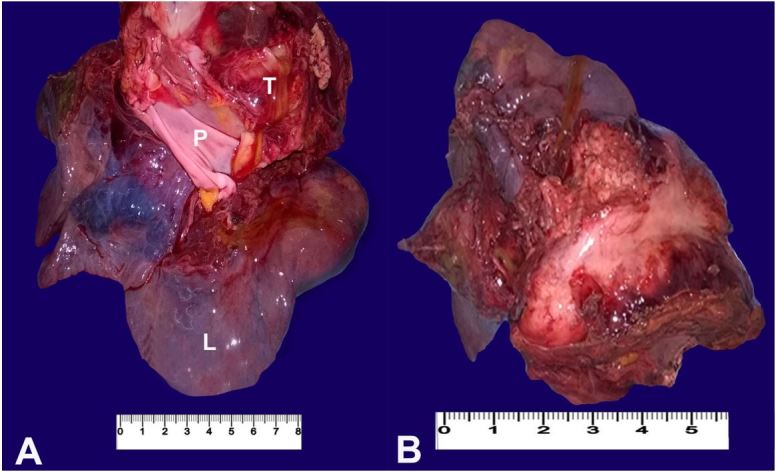
The tumor was firm in consistency, measured 12 × 9 × 8 cm, and weighed 140 g. At the cut surface, the mass showed a greyish and whitish lobulated appearance with areas of anthracotic pigment deposits, and a greenish fluid was draining from its surface. There were multiple nodules on the pleural surface of the left lung. Cut sections of the lung showed consolidations, with extension of the tumor into the lumen of the left bronchus.
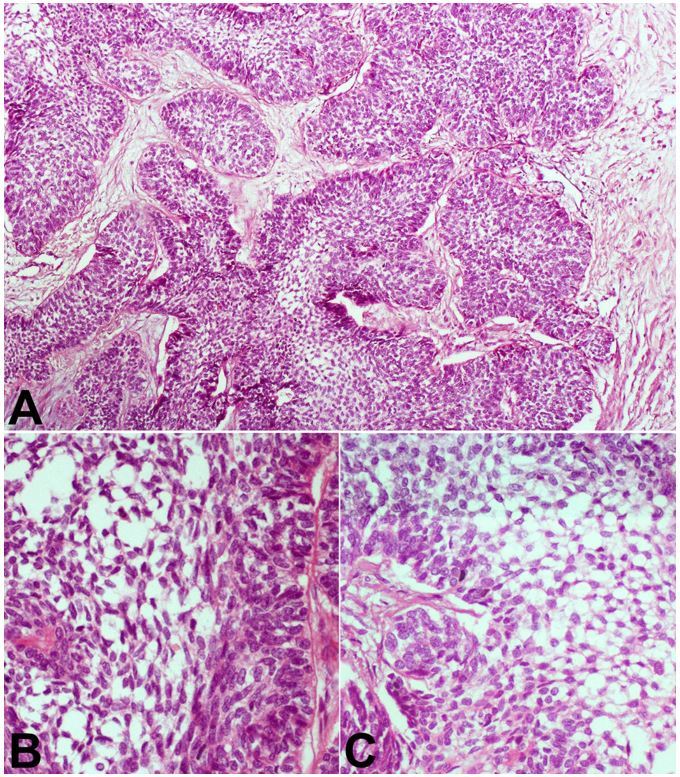
Histology of the tumor showed nodular aggregates of stellate and spindle-shaped cells arranged in a reticular appearance, which were bounded by low columnar cells arranged in a palisade (Figure 2A). These cells have a coarse chromatin nuclear pattern and moderate amphophilic cytoplasm (Figure 2B and 2C). There were very few mitotic figures in the tumor cells (Figure 2B and 2C).
Figure 2. Photomicrography of the tumor. A – Nests and trabeculae of malignant cells show peripheral palisading of darkly staining cells with loose cells in the center (H&E, 250X); B and C – High power view showing stellate cells having a reticular appearance, and palisading columnar to polygonal cells at the periphery (H&E, 400X).
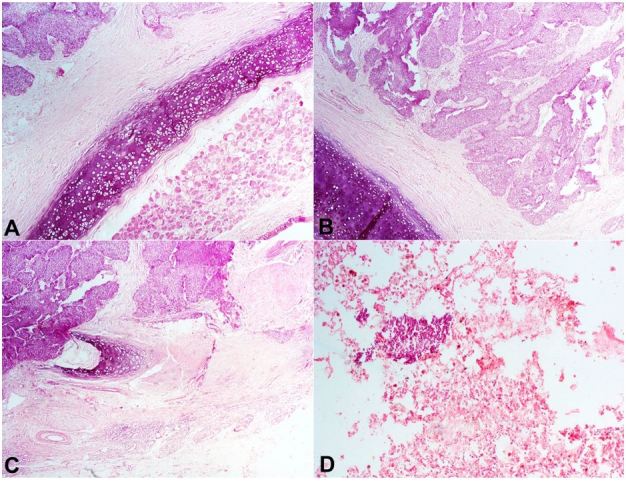
There was infiltration of the trachea by the tumor cells (Figure 3). Focal nests of tumor cells within the lung were also seen (Figure 3D). A diagnosis of metastatic ameloblastoma was made.
Figure 3. Photomicrography of the tumor. A, B and C – Infiltration of the trachea and bronchi by the malignant cells (H&E, 250X); D – A nest of tumor cells within the lung parenchyma (H&E, 250X).
DISCUSSION
Ameloblastoma is a rare, benign, aggressive neoplasm of the odontogenic tissue with a global incidence of 0.5 cases per million person years.1 It is said to be five times more common in blacks compared to whites.1,2 Studies undertaken in Ibadan (Nigeria) have shown that the ameloblastoma is the third commonest tumor of the jaw, with a frequency of 1%–3%.3 Most studies report a slight male predilection 1.1–1.3:1 with the mean presentation age of 32 years (range 2–80 years).3,4 The tumor has a low recurrence rate if adequate excision margins are respected, with subsequent jaw reconstruction. Conservative surgery has a high recurrence rate as was seen in our patient.1
Malignant ameloblastoma is a rare odontogenic tumor accounting for about 2% of the ameloblastic tumors.1 The term “malignant ameloblastoma” describes a tumor having benign histological features but showing evidence of metastasis.1,5 The commonest site of occurrence is the mandible, followed by the maxilla, with a patient age range of 5 to 74 years.5 These features were seen in our patient who presented the primary tumor at the age of 20 years, and the metastases occurred at the age of 25 years.
There is no sex predominance, and metastasis to the lungs is commonly followed by regional lymph nodes, bones, liver, brain, and spinal cord.1,2,6 Metastasis to the mediastinum, as seen in our index case, is very rare. Malignant ameloblastomas appear to have an indolent yet persistent behavior with multiple recurrences, as seen in our patient.6 The average time interval between the diagnosis of ameloblastoma and the detection of metastasis ranges from 3 to 45 years with a mean of 18 years.6 The index patient had a 5-year interval between the primary tumor and the metastasis. There are very few reported cases of mediastinal involvement by malignant ameloblastoma. The tumors in two previously reported cases had biopsies since the tumors were unresectable.7,8 This was due to extensive lymph node involvement, which was similar to that seen in our patient. The metastatic tumors in these two reported cases were described as having the typical nest of odontogenic cells arranged in a loose reticulum network.7,8 The prognosis of malignant ameloblastoma has been described as poor with a median survival time of 2 years if no resection is done.8 The survival increases to 6 years with adequate resection and radiotherapy.8
Recent molecular characterization of the tumor has shown frequent mutations in the BRAF, MAPK, and FGFR2 genetic pathways in ameloblastomas.9 Each of these mutations were found to be mutually exclusive in most of the tumors. The BRAFV600E, a mutation seen in many different other tumors, such as melanoma and glioblastoma, was the commonest mutation seen occurring in about 64% of ameloblastomas.9 Clinical trials of inhibitors of these pathways are currently ongoing and are showing promising results, which may help in better patient management.9
Footnotes
How to cite: Salami A., Ezenkwa U., Salami M., Ajani M., Okolo C. Malignant ameloblastoma: a challenging diagnosis. Autops Case Report [Internet]. 2018;8(4):e2018043. https://doi.org/10.4322/acr.2018.043
Financial support: None
References
- 1.McClary AC, West RB, McClary AC, et al. . Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol. 2016;273(7):1649-61. 10.1007/s00405-015-3631-8. [DOI] [PubMed] [Google Scholar]
- 2.Jayaraj G, Sherlin HJ, Ramani P, et al. . Metastasizing ameloblastoma - a perennial pathological enigma? Report of a case and review of literature. J Craniomaxillofac Surg. 2014;42(6):772-9. 10.1016/j.jcms.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Ajagbe HA, Daramola JO. Ameloblastoma: a survey of 199 cases in the University of College Hospital, Ibadan, Nigeria. J Natl Med Assoc. 1987;79(3):324-7. [PMC free article] [PubMed] [Google Scholar]
- 4.Ladeinde AL, Ogunlewe MO, Bamgbose BO, et al. . Ameloblastoma: analysis of 207 cases in a Nigerian teaching hospital. Quintessence Int. 2006;37(1):69-74. [PubMed] [Google Scholar]
- 5.Kodati S, Majumdar S, Uppala D, Namana M. Ameloblastic carcinoma: a report of three cases. J Clin Diagn Res. 2016;10(10):23-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg. 2010;68(12):2962-74. 10.1016/j.joms.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 7.Dahlgren SE, Ekström C, Mossberg B. Mandibular ameloblastoma with pulmonary and mediastinal lymph node metastases. Acta Otolaryngol. 1971;72(3):220-4. 10.3109/00016487109122476. [DOI] [PubMed] [Google Scholar]
- 8.Georgakas I, Lazaridou M, Dimitrakopoulos I, et al. . Pulmonary metastasis in a 65-year-old man with mandibular ameloblastoma: a case report and review of the literature. J Oral Maxillofac Surg. 2012;70(5):1109-13. 10.1016/j.joms.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Brown NA, Betz BL. Ameloblastoma: a review of recent molecular pathogenetic discoveries. Biomark Cancer. 2015;7(Suppl 2):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


