Abstract
Background
Obstructive sleep apnea (OSA) is associated with many cardiovascular disorders. Intermittent hypoxia (IH) is a key pathological hallmark of OSA. This study was conducted to evaluate the potential therapeutic effects and the associated mechanisms of adiponectin (APN) on IH induced human adult cardiac myocytes (HACMs) injury.
Material/Methods
HACMs were exposed to normoxia or IH (1% to 21% O2) using a novel cell culture bio-reactor with gas-permeable membranes. Cell viability was detected by Cell Counting Kit-8 assay. Cell membrane integrity was assessed by the detection of lactate dehydrogenase (LDH) release. Cell apoptosis was analyzed by flow cytometry. Malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) levels were determined using specific assay kits. P-AMPK (AMP-activated protein kinase), p-LKB1, and p-p65 protein levels were measured by western blotting. Pro-inflammatory factors including interleukin (IL)-1β, IL-6, IL-8 expressions were detected by enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction.
Results
The results showed that APN had no cytotoxic to HACMs. Compared with the control group, HACMs cell viability significantly decreased, LDH release increased and cell apoptosis increased in the IH group. The levels of IL-1β, IL-6, IL-8, MDA, and p-p65 were higher, while the levels of SOD, GSH-Px, p-AMPK, and p-LKB1 were lower in HACMs cells in the IH group than that in the control group. However, APN treatment significantly rescued these effects compared with the IH group in a dose-dependent manner.
Conclusions
In conclusion, these results indicated that APN protected against IH induced HACMs injury possibly mediated by AMPK and NF-κB pathway.
MeSH Keywords: Adiponectin; Apoptosis; Inflammation; Myocytes, Cardiac; Sleep Apnea, Obstructive
Background
Obstructive sleep apnea syndrome (OSAS) is a sleep breathing disorder that has comorbidities of multiple disorders such as cardiovascular disease. OSAS is an independent risk factor for increased cardiovascular morbidity and mortality, and several major epidemiological studies have confirmed its importance [1]. Intermittent hypoxia (IH) is one of the most important pathological hallmark of OSAS. Previous reports showed that IH was associated with cardiovascular disease [2,3]. IH has also been shown to induce left ventricular remodeling [4]. However, until now, few studies have reported on the role of IH in cardiomyocytes [5].
Oxidative stress and inflammation are important pathogenic factors in the development of cardiovascular diseases [6,7]. Previous studies have reported that IH could increase the level of reactive oxygen species and other signaling molecules that promote myocardial inflammation and injury [8,9]. Clinical studies have shown that the levels of circulating pro-inflammatory cytokines and oxidative stress bio-markers were higher in patients with OSAS compared with patients without OSAS [10,11].
Adiponectin (APN) is an adipocyte-specific protein that is synthesized by adipose tissue and secreted into the blood through adipose tissue, and plays a vital role in regulation of energy metabolism and adipocyte differentiation [12], specifically inflammatory and oxidative stress [13,14]. A number of studies have shown that APN plays a key role in vivo [15,16]. Studies have shown that the genioglossal mitochondrial dysfunction in rats exposed to IH could be alleviated by APN [16]. APN protects the kidney against chronic IH induced injury through inhibiting endoplasmic reticulum stress [17]. APN also has cardioprotective effects [18]. However, the effect of APN on human adult myocardial cells under IH conditions is still unclear.
The aim of the present in vitro study was to investigate the effects of APN on human adult cardiac myocytes (HACMs) under IH conditions and reveal its potential mechanisms.
Material and Methods
Isolation and culture of HACMs
The heart tissue samples were obtained from heart transplanted patients in our hospital (from January 2015 to June 2017) and used to prepare the primary culture of HACMs following a protocol previously described [17,19]. The present study was approved by the ethics committee of the Affiliated Hospital of Jiangnan University. All in vitro experiments were performed using passage 2 to 4 of the HACMs. Briefly, HACMs (5×104 cells/plate) were seeded in a 1% gelatin-coated (SigmaAldrich, St. Louis, MO, USA) OptiCell™ plates (Nunc, Thermo Scientific, Waltham, MA, USA) and cultured in Medium 199 (M199, Gibco, Invitrogen, Carlsbad, CA, USA) containing 20% fetal bovine serum (FBS superior, Biochrom, Berlin, Germany) and 1% penicillin/streptomycin (Gibco, Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2.
For passaging and seeding, cells were removed from M199 and incubated with 5 mL of 0.25% trypsin-EDTA solution (Sigma-Aldrich) at 37°C for 2 minutes. Cells were detached by agitation and then M199 containing 20% FBS was added to stop the trypsin activity. At 80% to 90% concentration, HACMs (0.6×105 cells/plate) were seeded into OptiCell (OC) plates precoated with 1% gelatin. The HACMs were cultured for 6 days before experimental treatments. Experiments were carried out at different O2 concentrations (0% to 21% O2) after starvation, for 24-hour incubation in serum-free M199 followed with 0.1% bovine serum albumin (BSA, Sigma-Aldrich), 1% penicillin, and 1% streptomycin.
HACMs were treated with 100 μmol/L APN for 4 hours, then, 24 hours later, Cell Counting Kit-8 (CCK-8) assay, lactate dehydrogenase (LDH) release assay, and flow cytometry assay were performed. Cells without any treatment were used as the control.
Intermittent hypoxia process
IH exposure was conducted using a custom-designed computer-controlled incubator chamber connected to a BioSpherix OxyCycler (BioSpherix, Redfield, NY, USA) as previously described [20]. Briefly, cells were cultured in the hypoxic chamber in which O2 levels were alternated between 1% for 5 minutes and 21% for 10 minutes for 64 cycles. Cells in the control group were cultured in normoxic conditions (21% O2, 5% CO2, and balance N2). To analyze the role of oxygen concentrations in the HACMs, the cells were maintained in using the cycle of 1% O2 for 5 minutes and 21% O2 for 10 minutes, cycle of 1% O2 for 5 minutes and 21% O2 for 10 minutes, cycle of 5% O2 for 5 minutes and 21% O2 for 10 minutes, and cycle of 10% O2 for 5 minutes and 21% O2 for 10 minutes or normoxia, respectively [20].
HACMs were treated with or without a series of concentrations of APN (25, 50, or 100 μmol/L) for 4 hours, then IH was conducted or not for 72 hours. Then, CCK-8 assay, LDH release assay, and flow cytometry assay were performed. Cells without any treatment were used as the control.
CCK-8 assay
CCK-8 assay [21] was conducted to detect the cell viability of HACMs following the manufacturer’s manual. After treatment with a series of concentrations of APN (25, 50, or 100 μmol/L), the HACMs cells (1×104 cells per well) were seeded into 96-well plates. The cells were exposed to IH or normoxia for 72 hours. Then we added CCK-8 reagents (WST-8,(2-(2-methoxy-4-nitrophenyl)-3-(40nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt) and incubated for 1.5 hours in the dark at 37°C. The optical density of each well was measured at 450 nm and quantified using a micro-plate reader (Bio-Rad).
LDH release assay
LDH activity in the culture medium was measured by a commercial LDH assay kit (Roche, Mannheim, Germany) according to the supplier’s manual [22]. Absorbance of the samples was detected at 490 nm on a spectrophotometer (Victor3, Perkin Elmer, Waltham, MA, USA).
Flow cytometry assay
Cell apoptosis was detected using flow cytometry [23]. HACMs were plated into 6-well plates and treatment with a series of concentrations of APN (25, 50, or 100 mmol/L) for 4 hours. Then the cells were exposed to IH or normoxia for 72 hours. Cells after IH were harvested and stained with Annexin V-fluorescein isothiocyanate (V-FITC) binding buffer for 60 minutes at 37°C, at 5% CO2. Then cells were stained with propidium iodide (PI) single staining solution for 5 minutes at room temperature without light (Annexin V-FITC/PI apoptosis detection kit; Cat no. 70-AP101-100; MultiSciences, Hangzhou, China). The apoptosis of HACMs was analyzed by flow cytometer (FACSCalibur, Becton Dickinson).
Enzyme-linked immunosorbent assay (ELISA)
The expression of released interleukin (IL)-1β, IL-6, and IL-8 were detected by enzyme-linked immunosorbent assays kit (ELISA, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions for each kit. Optical density readings for each protein were compared with standard curves to quantify the amount of protein in the original samples. The values were normalized to cell count and presented as the percentage of baseline.
Measurement of antioxidant activity
To determine the antioxidant activity of APN, the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were detected using assay kits (Bio-Plex Multiplex System, Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol.
Western blot analysis
After IH treatment, proteins were extracted from the HACMs with RIPA buffer (Beyotime Institute of Biotechnology), supplemented with 1 mM of PMSF (Roche, Germany) and a phosphatase inhibitor cocktail (Roche, Germany). The concentration of protein was detected by the bicinchoninic acid (BCA) assay kit (Pierce Chemical, USA). Equal quantities of protein (40 μg/line) were separated on 10% sodium dodecyl sulfate PAGE (SDS-PAGE) and then transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Then, the membranes were incubated with the specific primary antibodies p-LKB1, p-AMPK (AMP-activated protein kinase), p-p65, and β-actin at 4°C overnight, followed by incubation with horseradish peroxidase labeled secondary antibody for 2 hour at room temperature. The bands were visualized by electrochemiluminescence ECL kit (Thermo Scientific, USA); and ImageJ 2.0 software (Bio-Rad Laboratories Inc., USA) was used to quantify the intensity of band.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the HACMs using TRIzol reagent (Takara Bio Inc., Japan) following the manufacturer’s instructions. RNA (2 μg) was reverse transcribed to complementary DNA (cDNA) using Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). Quantitative detection of mRNA expression was carried out using SYBR Green quantification method (Thermo Scientific, USA) with an ABI 7900 Real Time PCR System (Applied Biosystems Inc., USA). Quantities of these genes were normalized to the GAPDH and calculated by the 2−ΔΔCt method [24].
Statistical analysis
All data analysis was performed using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Statistical analysis between all groups was performed with Student’s t-test or one-way analysis of variance (one-way ANOVA) followed by Tukey’s test. Data were presented as the mean ± standard deviation. P<0.05 was considered statistically significant.
Results
Effects of APN on HACMs, without other treatments
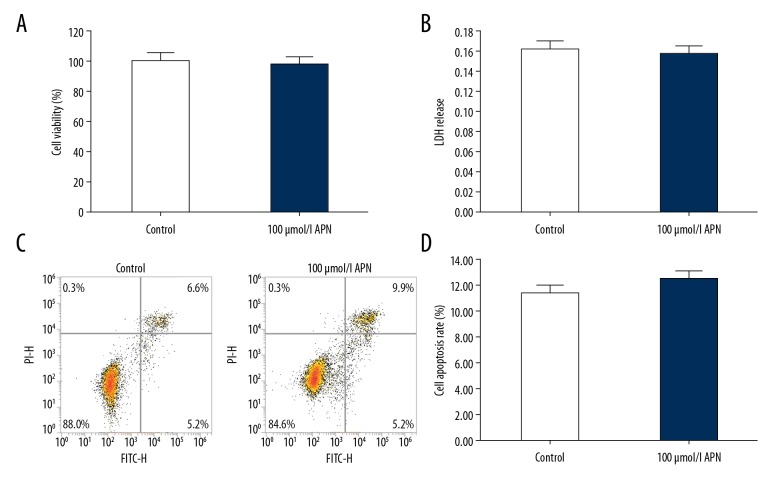
As shown in Figure 1, compared with the control group, 100 μmol/L APN treatment had no effect on cell viability, plasma membrane integrity, or apoptosis of HACMs. There was no significant difference in cell viability (Figure 1A), LDH release (Figure 1B), or cell apoptosis (Figure 1C, 1D) between the control group and the APN treatment group.
Figure 1.
Effects of adiponectin on human adult cardiac myocytes that were treated with 100 μmol/L adiponectin for 4 hours. Cell viability (A), lactate dehydrogenase release (B), and cell apoptosis (C, D) were determined respectively. Data were displayed as mean ± standard deviation.
Effects of APN on IH induced cell death
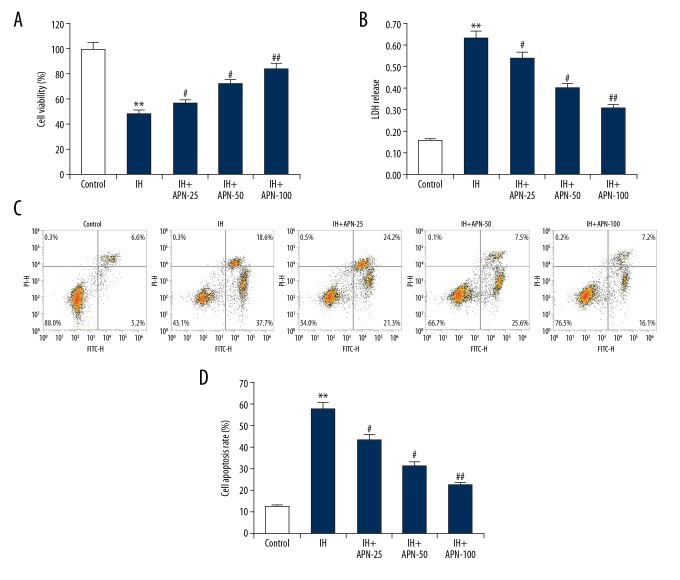
As compared with the control group, HACMs treated with IH had reduced cell vitality (Figure 2A, P<0.01), increased LDH release (Figure 2B, P<0.01), and increased proportions of apoptotic cells (Figure 2C, 2D, P<0.01), while APN treatment significantly reversed these effects in a dose-independent manner compared with the IH group (P<0.05, P<0.01, respectively).
Figure 2.
Effects of adiponectin on intermittent hypoxia treated human adult cardiac myocytes. The human adult cardiac myocytes were treated with a series of concentrations of adiponectin (25, 50, or 100 μmol/L) for 4 hours, then intermittent hypoxia for 72 hours. Cell viability (A), lactate dehydrogenase release (B), and cell apoptosis (C, D) were determined respectively. Data were displayed as mean ± standard deviation. ** P<0.01 versus the control group; #, ## P<0.05, 0.01 versus intermittent hypoxia group.
Effects of APN on IH induced inflammatory response
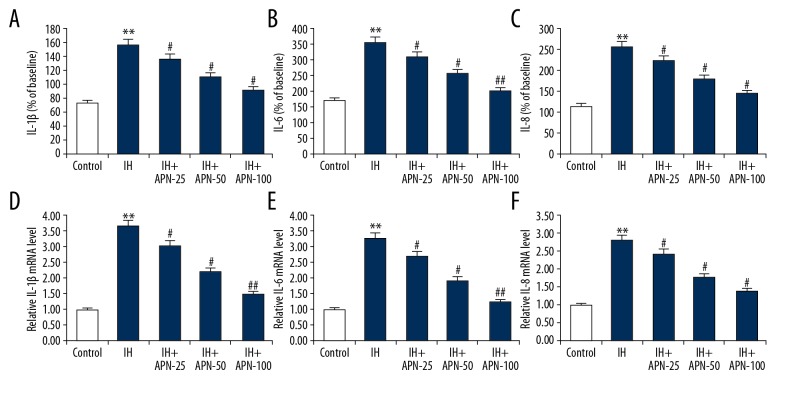
ELISA (Figure 3A–3C) and quantitative real-time polymerase chain reaction (qRT-PCR) (Figure 3D–3F) results showed that compared with the control group, the levels of IL-1β, IL-6, and IL-8 were significantly increased in the IH group (P<0.05, P<0.01, respectively). The levels of IL-1β, IL-6, and IL-8 in APN group had a significant decrease in a dose-independent manner when compared with the IH group (P<0.05, P<0.01, respectively).
Figure 3.
Effects of adiponectin on inflammatory factors expression in intermittent hypoxia treated human adult cardiac myocytes. The human adult cardiac myocytes were treated with a series of concentrations of adiponectin (25, 50, or 100 μmol/L) for 4 hours, then intermittent hypoxia for 72 hours. The protein (A–C) and mRNA (D–F) levels of interleukin (IL)-1β, IL-6, and IL-8 were detected using enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction respectively. Data were displayed as mean ± standard deviation. *, ** P<0.05, 0.01 versus the control group; #, ## P<0.05, 0.01 versus the intermittent hypoxia group.
Effects of APN on IH induced oxidative injury
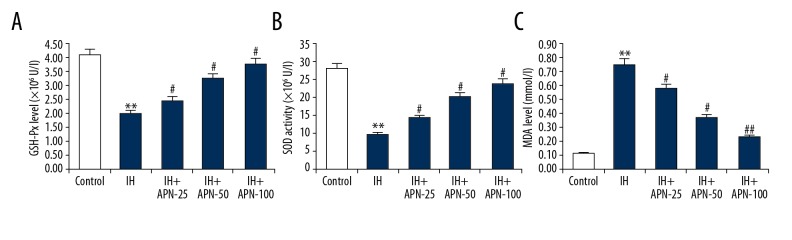
Compared with the control group, the activities of GSH-Px and SOD were significantly decreased in the IH group (P<0.01), while the content of MDA was significantly increased (P<0.01). However, APN significantly restored the activities of these enzymes in a dose-independent manner compared with the IH group in a dose-independent manner (Figure 4) (P<0.05, P<0.01, respectively).
Figure 4.
Effects of adiponectin on oxidative stress in intermittent hypoxia treated human adult cardiac myocytes. The human adult cardiac myocytes were treated with a series of concentrations of adiponectin (25, 50, or 100 μmol/L) for 4 hours, then intermittent hypoxia for 72 hours. The activities of glutathione peroxidase (A) and superoxide dismutase (B), and the level of malondialdehyde (C) were measured. Data were displayed as mean ±SD. ** P<0.01 versus the control group; #, ## P<0.05, 0.01 versus the intermittent hypoxia group.
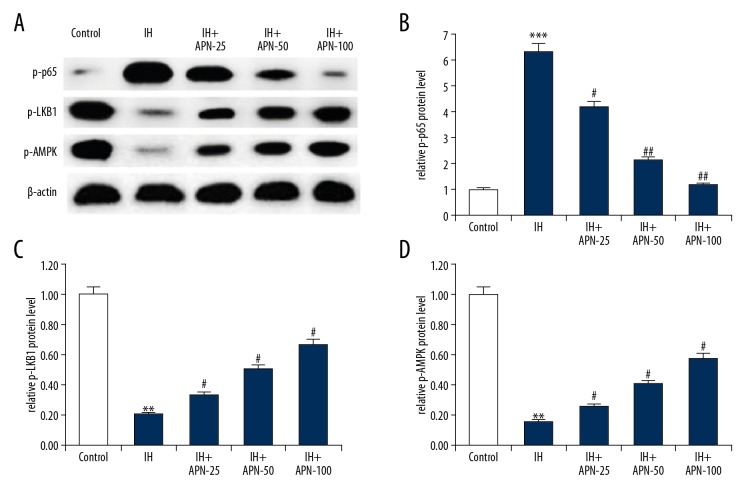
Effects of APN on AMPK and NF-κB pathway
To further study the protective mechanism of APN against IH-induced HACMs injury, we analyzed the expression of p-LKB1, p-AMPK, and p-p65 proteins by western blot assay (Figure 5). After IH exposure, p-LKB1 (P<0.01) and p-AMPK (P<0.01) protein levels were reduced and the protein level of p-p65 (P<0.001) was increased compared with those in the control group. Compared with the IH group, APN treatment significantly increased p-LKB1 and p-AMPK protein levels and decreased p-p65 protein level in a dose-independent manner (P<0.05, P<0.01, respectively).
Figure 5.
Effects of adiponectin on the AMPK and NF-κB pathways in intermittent hypoxia treated human adult cardiac myocytes. The human adult cardiac myocytes were treated with a series of concentrations of adiponectin (25, 50, or 100 μmol/L) for 4 hours, then intermittent hypoxia for 72 hours. (A) The protein level of p-p65, p-LKB1, and p-AMPK was determined using western blotting. And relative protein levels of p-p65 (B), p-LKB1 (C), and p-AMPK (D) were calculated and presented as fold of the control. Data were displayed as mean ± standard deviation. **, *** P<0.01, 0.001 versus the control group; #, ## P<0.05, 0.01 versus the intermittent hypoxia group.
Discussion
In the present study, we found that APN was not cytotoxic to HACMs. IH-induced cell vitality reduction, LDH release increase, and apoptosis in HACMs were significantly inhibited by APN treatment. The increased levels of IL-1β, IL-6, IL-8, and MDA, and the decreased GSH-Px and SOD activities caused by IH were eliminated by APN treatment. Besides, the upregulated protein levels of p-LKB1 and p-AMPK, and the reduced p-p65 protein level caused by IH were reversed by APN administration. These results indicated that APN protected against IH-induced HACMs injury, possibly mediated by AMPK and NF-κB pathway.
OSAS is characterized by repetitive upper airway obstruction during sleep. IH is a key pathophysiological characteristic of OSAS. IH induces HACMs dysfunction by enhancing inflammatory mechanisms [25]. However, the effect of APN on IH-induced HACMs damage has remained elusive. In a previous study, we found that APN could reduce HACMs inflammation, inhibit cell apoptosis and oxidative damage, and activate the AMPK and NF-κB pathway after IH exposure.
As a previous study demonstrated that IH could induce inflammatory and oxidative stress [26], we then examined the antioxidant activities of APN in IH-induced HACMs, and the activities of antioxidant enzymes (SOD and GSH-Px) as well as the levels of MDA in the HACMs. SOD and GSH-Px are the enzymes that protect the organism from oxidative damage, while MDA is an important marker of lipid peroxidation [27]. In the present study, APN treatment markedly enhanced the levels of GSH-Px and SOD as well as decreased the levels of MDA induced by IH. Therefore, it is indicated that APN effectively protected the HACMs oxidative damage induced by IH. In addition, studies have indicated that IH could promote the production of ROS, which in turn might activate the proinflammatory transcription factor NF-κB pathway [27,28], resulting in increased expressions of downstream targeted genes such as IL-1β, IL-6, and IL-8. Many clinical studies have found the levels of serum pro-inflammatory cytokines (IL-1β, IL-6, and IL-8) were higher in OSAS patients than non-OSAS controls [29,30]. Consistent with previous reports [17,19], our results showed that IH caused elevation of the levels of IL-1β, IL-6, IL-8, and p-p65 in HACMs. APN treatment markedly reduced the levels of IL-1β, IL-6, IL-8, and p-p65.Therefore, these results indicated that APN effectively attenuates HACMs inflammation induced by IH.
The AMP-activated protein kinase (AMPK) is a sensor of conserving cellular energy status, and it has been reported that the AMPK pathway plays a pivotal role in regulating energy balance, oxidative stress, inflammatory response, and apoptosis [31,32]. Activation of AMPK can result in enhanced oxidative capacity [33]. Previous studies have reported that APN could stimulate glucose utilization and fatty acid oxidation by activating the AMPK pathway [34,35]. APN rescues IH-induced reductions in mitochondrial synthesis and oxidative capability through the AMPK pathway [16]. In the present study, our results showed that APN treatment could elevate the levels of p-LKB1 and p-AMPK in HACMs induced by IH, indicating that APN could activate AMPK pathway in IH-induced HACMs.
In summary, the present study demonstrated that APN treatment could enhance cell viability, reduce cell apoptosis, and alleviate inflammatory response and oxidative stress damage in HACMs induced by IH. APN protected the HACMs function through regulating the AMPK and NF-κB pathways. However, further studies are still needed to explore the detailed cardioprotective mechanisms of APN during IH.
Conclusions
APN protected against IH-induced HACM injury was possibly mediated by the AMPK and NF-κB pathways. Our data strongly suggested that APN is a feasible novel therapeutic agent to prevent IH-induced HACMs dysfunction.
Footnotes
Conflict of interests.
None.
Source of support: The present study was supported by the National Natural Science Foundation (No. 81500071)
References
- 1.Kim SM, Cho KI, Kwon JH, et al. Impact of obstructive sleep apnea on left atrial functional and structural remodeling beyond obesity. J Cardiol. 2012;60:475–83. doi: 10.1016/j.jjcc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Archontogeorgis K, Voulgaris A, Nena E, et al. Cardiovascular risk assessment in a cohort of newly diagnosed patients with obstructive sleep apnea syndrome. Cardiol Res Pract. 2018;2018 doi: 10.1155/2018/6572785. 6572785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muxfeldt ES, Margallo VS, Guimaraes GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–78. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 4.Inamoto S, Yoshioka T, Yamashita C, et al. Pitavastatin reduces oxidative stress and attenuates intermittent hypoxia-induced left ventricular remodeling in lean mice. Hypertens Res. 2010;33:579–86. doi: 10.1038/hr.2010.36. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Stefaniak J, Hafner C, et al. Intermittent hypoxia causes inflammation and injury to human adult cardiac myocytes. Anesth Analg. 2016;122:373–80. doi: 10.1213/ANE.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 6.Uno K, Nicholls SJ. Biomarkers of inflammation and oxidative stress in atherosclerosis. Biomark Med. 2010;4:361–73. doi: 10.2217/bmm.10.57. [DOI] [PubMed] [Google Scholar]
- 7.Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. doi: 10.1159/000479254. [DOI] [PubMed] [Google Scholar]
- 8.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia – revisited – the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lyons OD, Bradley TD. Heart failure and sleep apnea. Can J Cardiol. 2015;31:898–908. doi: 10.1016/j.cjca.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Vatansever E, Surmen-Gur E, Ursavas A, Karadag M. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath. 2011;15:275–82. doi: 10.1007/s11325-010-0378-8. [DOI] [PubMed] [Google Scholar]
- 11.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Li F, Zhang Y, et al. Silencing of ADIPOQ efficiently suppresses preadipocyte differentiation in porcine. Cell Physiol Biochem. 2013;31:452–61. doi: 10.1159/000343381. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–42. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: Regulation of its production and its role in human diseases. Hormones (Athens) 2012;11:8–20. doi: 10.1007/BF03401534. [DOI] [PubMed] [Google Scholar]
- 15.Ding WX, Dong YB, Ding N, et al. Adiponectin protects rat heart from left ventricular remodeling induced by chronic intermittent hypoxia via inhibition of TGF-beta/smad2/3 pathway. J Thorac Dis. 2014;6:1278–84. doi: 10.3978/j.issn.2072-1439.2014.07.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Jiang X, Dong Y, et al. Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS One. 2014;9:e109284. doi: 10.1371/journal.pone.0109284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding W, Cai Y, Wang W, et al. Adiponectin protects the kidney against chronic intermittent hypoxia-induced injury through inhibiting endoplasmic reticulum stress. Sleep Breath. 2016;20:1069–74. doi: 10.1007/s11325-016-1321-4. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–46. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafner C, Wu J, Tiboldi A, et al. Hyperoxia induces inflammation and cytotoxicity in human adult cardiac myocytes. Shock. 2017;47:436–44. doi: 10.1097/SHK.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 20.Zhen YQ, Wu YM, Sang YH, et al. 2,3-Oxidosqualene cyclase protects liver cells from the injury of intermittent hypoxia by regulating lipid metabolism. Sleep Breath. 2015;19:1475–81. doi: 10.1007/s11325-015-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Y, Liu Y, Yan Q, et al. Inhibition of Mus81 by siRNA enhances sensitivity to 5-FU in breast carcinoma cell lines. Onco Targets Ther. 2014;7:1883–90. doi: 10.2147/OTT.S64339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y, Kim D, Yagi M, et al. Application of LDH-release assay to cellular-level evaluation of the toxic potential of harmful algal species. Biosci Biotechnol Biochem. 2013;77:345–52. doi: 10.1271/bbb.120764. [DOI] [PubMed] [Google Scholar]
- 23.Telford WG. Multiparametric analysis of apoptosis by flow cytometry. Methods Mol Biol. 2018;1678:167–202. doi: 10.1007/978-1-4939-7346-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Stefaniak J, Hafner C, et al. Intermittent hypoxia causes inflammation and injury to human adult cardiac myocytes. Anesth Analg. 2016;122:373–80. doi: 10.1213/ANE.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 26.Ding W, Zhang X, Huang H, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: Cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43:167–88. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- 28.Ota H, Itaya-Hironaka A, Yamauchi A, et al. Pancreatic beta cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013;93:664–72. doi: 10.1016/j.lfs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Carpagnano GE, Kharitonov SA, Resta O, et al. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–67. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovitch RC, Samborska B, Faubert B, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casanova E, Baselga-Escudero L, Ribas-Latre A, et al. Chronic intake of proanthocyanidins and docosahexaenoic acid improves skeletal muscle oxidative capacity in diet-obese rats. J Nutr Biochem. 2014;25:1003–10. doi: 10.1016/j.jnutbio.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–19. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 35.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–12. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]