Abstract
Background
The long noncoding RNA (lncRNA) HOTTIP is involved in gastric cancer tumorigenesis, papillary thyroid carcinoma, colorectal cancer, lung adenocarcinoma, and hepatocellular carcinoma, but it is unclear how HOTTIP exerts roles in nasopharyngeal carcinoma (NPC). The present study investigated HOTTIP function during NPC development.
Material/Methods
HOTTIP levels in cancer specimens and cell lines were analyzed using qRT-PCR. HOTTIP function in NPC was determined by Cell Counting Kit-8 (CCK8) and Transwell assay.
Results
HOTTIP expression was increased in NPC tissues. Higher levels of HOTTIP are correlated with lower survival in NPC patients. HOTTIP silencing suppressed the proliferation, cell cycle, migration, and invasion of NPC cells. HOTTIP served as a sponge for miR-4301. miR-4301 expression was significantly inhibited by HOTTIP in NPC cells. miR-4301 overexpression dramatically inhibited NPC cell proliferation, migration, and invasion.
Conclusions
This study showed that HOTTIP acts as an oncogene in NPC by sponging miR-4301.
MeSH Keywords: Cell Proliferation; Nasopharyngeal Neoplasms; RNA, Long Noncoding
Background
Nasopharyngeal carcinoma (NPC) is an aggressive head and neck cancer, originating in the nasopharyngeal epithelium [1]. Due to advances in radiotherapy and chemotherapy, the outcomes of NPC patients have recently improved [2]. However, distant metastasis and recurrence still remain major obstacles to patient survival [3]. In order to better heal NPC, it will be critical to elucidate the potential mechanism underlying NPC pathogenesis.
Long noncoding RNAs (lncRNAs) are more than 200 nucleotides in length and have limited coding potential [4]. Accumulating evidence suggests that lncRNA participates in multiple biological processes, including development, immunity, and cancer [5–7]. Importantly, many studies have shown that dysregulated lncRNA expression is correlated with tumorigenesis [8]. lncRNAs are demonstrated to regulate cancer cell behaviors via regulating gene expression by serving as miRNA sponges or interacting with specific proteins [9,10]. For instance: lncRNA AK096174 enhances growth and metastasis of gastric cancer through WDR66 [11], lncRNA-MEG3 suppresses gastric cancer cell growth and invasion via targeting miRNA-21 [12], lncRNA MALAT1 upregulation promotes NPC progression by ERK/MAPK signaling [13], and lncAKHE promotes liver cancer cell proliferation and invasion by activating the NOTCH2 pathway [14].
The function of lncRNA HOTTIP has been investigated in multiple cancers, including thyroid carcinoma [15] and colorectal cancer [16]. Most studies indicate HOTTIP has oncogenic roles [16]. However, the function of HOTTIP in NPC is unclear. We showed HOTTIP was overexpressed in NPC samples and the expression level of HOTTIP is negatively associated with survival rate of NPC patients. We showed that HOTTIP knockdown suppresses NPC cell proliferation, migration, and HOTTIP binds to miR-4301 and represses its activity. In summary, this study reveals the important function of HOTTIP in NPC progression and elucidates its molecular mechanism.
Material and Methods
Patient samples
We obtained 47 NPC samples and matched adjacent non-tumor tissues from the First Affiliated Hospital of Soochow University from May 2014 to February 2016. Associations between expression of HOTTIP and clinicopathological characteristics in 47 NPC patients are listed in Table 1. Samples were stored immediately in liquid nitrogen. Our study was approved by the Ethics Committee of our hospital. Informed consent was obtained before experiments.
Table 1.
Association between expression of HOTTIP and clinicopathological characteristics in 47 NPC patients.
| Clinical factor | Low (n=23) | High (n=24) | P value |
|---|---|---|---|
| Gender | 0.359 | ||
| Male | 17 | 14 | |
| Female | 6 | 10 | |
| Age | 0.773 | ||
| <45 | 11 | 13 | |
| ≥45 | 12 | 11 | |
| Clinical stage | 0.042 | ||
| I–II | 15 | 8 | |
| III–IV | 8 | 16 | |
| Distant metastasis | 0.017 | ||
| No | 18 | 10 | |
| Yes | 5 | 14 |
(P<0.05 by Chi-square test) is shown in bold.
Cell culture and transfection
Two NPC cell lines (C666-1 and SUNE-1) and the normal nasopharyngeal cell line NP69 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% FBS (Gibco).
siRNA-NC (5′-AAUUCUCCGAACGUGUCACGU-3′), siRNA-HOTTIP (5′-GCGUCUACAUUAACAAAGAUU-3′), miR-4301 mimics (5′-UCCCACUACUUCACUUGUGA-3′) and corresponding negative control (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were synthesized by RiboBio (Guangzhou, China). SUNE-1 cells were cultured in 6-well plates for 24 h and transfected with siRNA-HOTTIP, miR-4301 mimics, or their respective negative controls using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. To determine the efficiency of siRNA-HOTTIP, the expression of HOTTIP was assessed with real-time PCR.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA extraction and qPCR analysis were carried out as described before [17]. U6 was chosen as the internal control. Relative expression was calculated based on the 2ΔΔCt method. The primer sequences were as follows: HOTTIP (Forward, 5′-CCTAAAGCCACGCTTCTTTG-3′; Reverse, 5′-TGCAGGCTGGAGATCCTACT-3′), miR-4301 (Forward, 5′-TCCCACTACTTCACTTGTGA-3′; Reverse, 5′-AACGAGACGACGAC AGAC-3′), U6 (Forward, 5′-GCAAATTCGTGAAGCGTTCCATA-3′; Reverse, 5′-AACGAGACGACGACAGAC-3′).
CCK-8 proliferation assays
This assay was performed as previously reported [18].
Transwell assays
The abilities of tumor cell migration and invasion was assessed using Transwell assay according to a previous report [18].
Cell cycle analysis
Cells were fixed with 70% ethanol overnight at 4°C and stained with propidium iodide, followed by FACS analysis using a previously described protocol [19].
Luciferase assay
The putative binding site for miR-4301 in HOTTIP was predicted using a bioinformatics tool (http://mirdb.org/miRDB/index.html). For luciferase reporter assay, the sequence including the putative binding site or the mutant type was constructed into the pGL3-basic vector (Promega Corp., Madison, WI, USA). Then luciferase reporter assay was conducted as previously reported [20].
Statistical analysis
Statistical analyses were carried out using SPSS 22.0 (IBM, SPSS, Chicago, IL, USA). A t test and one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test was used to compare 2 or more groups for statistical significance. Kaplan-Meier curves combined with log-rank test were used to analyze overall survival rates. All experimental results were from at least 3 independent assays and data are presented as mean ±SD. P<0.05 was considered as a significant difference.
Results
HOTTIP was upregulated in NPC tissues
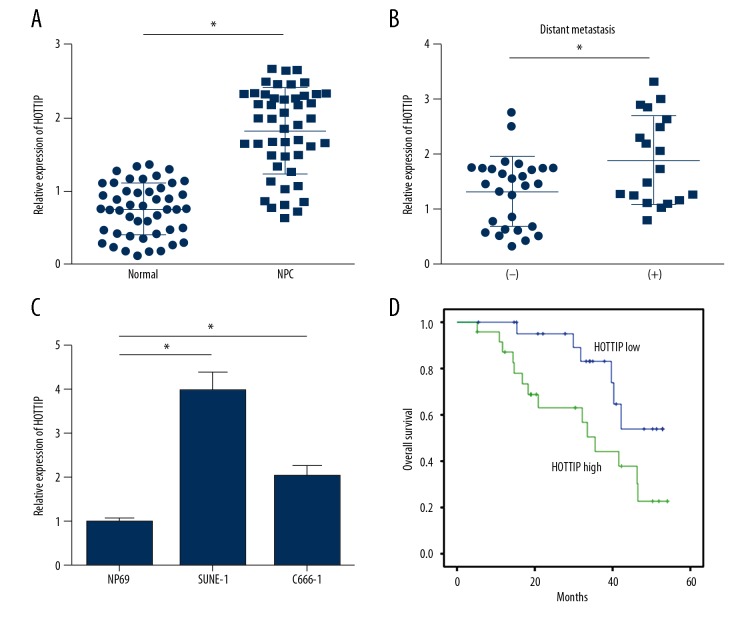
To explore the role of HOTTIP in NPC progression, its expression levels in NPC tissues and normal tissues were determined using qRT-PCR. The results indicated that HOTTIP level was upregulated in cancer tissues compared to non-cancer tissues (Figure 1A). Notably, HOTTIP expression was higher in metastatic NPC tissues than in nonmetastatic tissues (Figure 1B), suggesting that HOTTIP regulates metastasis of NPC. Moreover, HOTTIP expression was higher in NPC cell lines, including SUNE-1 and C666-1 cells, than in NP69 (Figure 1C). To determine whether HOTTIP is an important biomarker, we divided these samples into HOTTIP high expression (n=23) and low expression (n=24) groups according to HOTTIP expression value in tissues (using median value as cutoff). Kaplan-Meier curves were plotted and indicated that high level of HOTTIP was correlated with lower survival rate in NPC patients (Figure 1D).
Figure 1.
HOTTIP was upregulated in NPC tissues. (A) The expression levels of HOTTIP in 47 pairs of NPC tissues and adjacent normal tissues were measured by qRT-PCR. (B) Relative expression of HOTTIP in metastatic NPC tissues (n=19) and nonmetastatic NPC tissues (n=28). (C) Relative expression of HOTTIP in NPC cell lines. (D) Higher expression of HOTTIP predicted poorer prognosis by Kaplan-Meier curve analysis. * P<0.05.
Knockdown of HOTTIP inhibited NPC cell proliferation
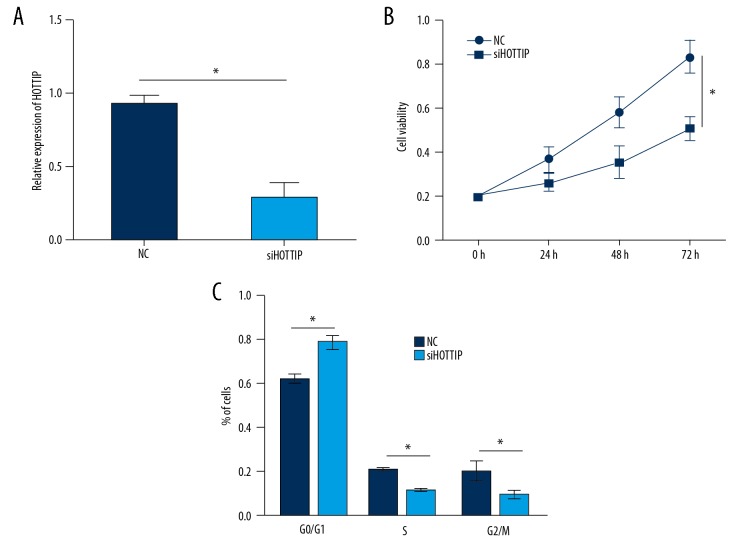
We then investigated the function of HOTTIP in NPC cells. We transduced SUNE-1 cells with siRNAs against HOTTIP, and the qRT-PCR analysis indicated that HOTTIP expression was reduced in SUNE-1 cells after transfection with siHOTTIP (Figure 2A). We then performed CCK8 assay and found that HOTTIP knockdown significantly inhibited SUNE-1 cell proliferation (Figure 2B). Cell cycle progression is a major cause of proliferation; therefore, we assessed whether HOTTIP has a similar effect on the cell cycle progression of SUNE-1 cells by FACS. Results illustrated that HOTTIP knockdown resulted in more cells arrested in G0/G1 phase and decreased numbers of cells in S and G2/M phases (Figure 2C).
Figure 2.
Knockdown of HOTTIP inhibited NPC cell proliferation. (A) Relative expression of HOTTIP in SUNE-1 cells transfected with siHOTTIP or negative control (NC). (B) CCK8 assays were used to measure the proliferation of NPC cells transfected with siHOTTIP or NC. (C) HOTTIP knockdown significantly arrested cell cycle progression in SUNE-1 cells. * P<0.05.
Knockdown of HOTTIP suppressed NPC cell migration and invasion
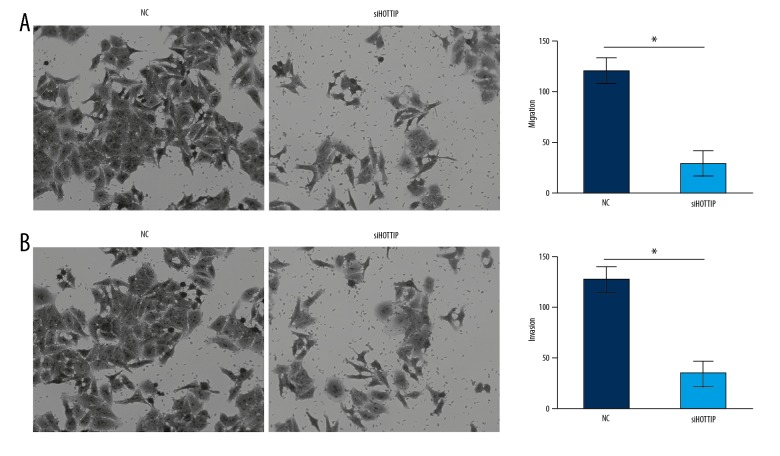
Metastasis is a major cause of poor outcomes of NPC patients, so w next assessed the effect of HOTTIP on metastasis using Transwell assay. The results showed that knockdown of HOTTIP markedly reduced the migrated and invaded SUNE-1 cells (Figure 3A, 3B). These results show that HOTTIP exerts an oncogenic role in NPC by facilitating proliferation, migration, and invasion.
Figure 3.
Knockdown of HOTTIP suppressed NPC cell migration and invasion. (A) Transwell migration assay showed that HOTTIP knockdown inhibited the migration of SUNE-1 cells. (B) Transwell invasion assay showed that HOTTIP knockdown inhibited the invasion of SUNE-1 cells. * P<0.05.
HOTTIP serves as a miR-4301 sponge
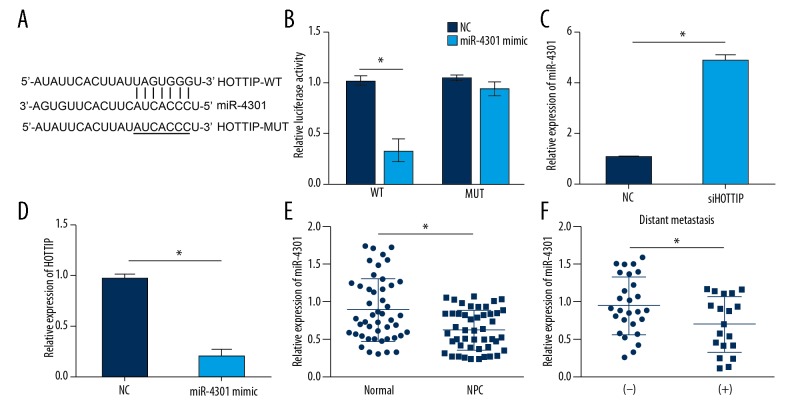
Increasing evidence indicates that lncRNAs serve as miRNA sponges in cancer [9]. To determine the mechanism of HOTTIP, we performed bioinformatics analysis using an online tool (http://mirdb.org/miRDB/custom.html). The results suggested that HOTTIP might be a sponge for miR-4301, and there was a potential binding site of miR-4301 in HOTTIP (Figure 4A). Through luciferase reporter assay, we found that overexpressing miR-4301 impaired the activity of HOTTIP-WT but not HOTTIP-MUT reporter plasmid (Figure 4B), indicating a direct interaction between HOTTIP and miR-4301. Furthermore, HOTTIP knockdown promoted the expression of miR-4301 in SUNE-1 cells (Figure 4C), whereas ectopic expression of miR-4301 repressed the expression of HOTTIP (Figure 4D). Interestingly, miR-4301 expression was downregulated in NPC tissues (Figure 4E) and further decreased in metastatic NPC tissues (Figure 4F), suggesting that miR-4301 might work as a tumor suppressor and negatively regulate NPC metastasis.
Figure 4.
HOTTIP served as a miR-4301 sponge. (A) Predicted binding site of miR-4301 in HOTTIP by bioinformatics analysis. (B) Luciferase reporter assay indicated that miR-4301 overexpression repressed the luciferase activity of HOTTIP-WT but not HOTTIP-MUT. (C) HOTTIP knockdown promoted the expression of miR-4301 in SUNE-1 cells. (D) Overexpression of miR-4301 inhibited the expression of HOTTIP in SUNE-1 cells. (E) The expression levels of miR-4301 in 47 pairs of NPC tissues and adjacent normal tissues were measured by qRT-PCR. (F) Relative expression of miR-4301 in metastatic NPC tissues (n=19) and nonmetastatic NPC tissues (n=28). * P<0.05.
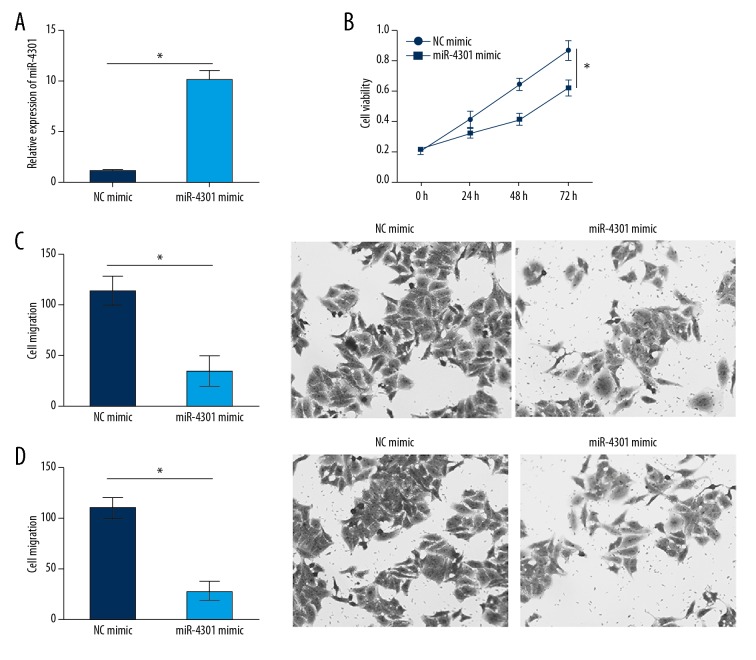
miR-4301 overexpression inhibited NPC cell proliferation, migration, and invasion
The function of miR-4301 in NPC has not been previously elucidated. We sought to determine whether miR-4301 is responsible for the function of HOTTIP in NPC. qRT-PCR analysis showed that miR-4301 expression was significantly upregulated in SUNE-1 cells after transfection with miR-4301 mimics (Figure 5A). According to CCK8 and Transwell assays, miR-4301 upregulation significantly suppressed the proliferation, migration, and invasion of SUNE-1 cells (Figure 5B–5D), suggesting a tumor-suppressive role of miR-4301 in NPC.
Figure 5.
miR-4301 overexpression inhibited NPC cell proliferation, migration, and invasion. (A) Relative expression of miR-4301 in SUNE-1 cells transfected with miR-4301 mimics was determined by qRT-PCR. (B) Overexpression of miR-4301 inhibited the proliferation of SUNE-1 cells. (C, D) Overexpression of miR-4301 suppressed the migration and invasion of SUNE-1 cells by Transwell assays. * P<0.05.
Discussion
As one of the most common malignant head and neck cancers, NPC originating from nasopharyngeal epithelium leads to many cancer-related deaths worldwide [1]. However, the molecular mechanism underlying NPC progression remains elusive. Recently, evidence has indicated that lncRNA expression is associated with tumor progression and can predict disease outcome [21]. In our study, we investigated the role of HOTTIP in NPC progression. HOTTIP expression was elevated in NPC tissues and cell lines. HOTTIP knockdown suppressed the proliferation, migration, and invasion of NPC cells. Mechanistically, we identified HOTTIP as a miRNA sponge for miR-4301. We also demonstrated that HOTTIP promoted NPC progression via inhibiting miR-4301. Our study contributes to understanding of the mechanism of NPC progression.
LncRNAs are a large group of noncoding RNAs with no protein-coding ability. Increasing evidence indicates a close relationship between lncRNA expression and cancer development. For instance, lncRNA NEAT1 regulates ovarian cancer cell resistance to paclitaxel through promoting ZEB1 expression [22], and lncRNA ZFAS1 targets miR-484 to increase proliferation and metastasis of colorectal cancer [23]. Previous studies suggest HOTTIP is a key regulator of cancer development and drug resistance in various cancers, including thyroid carcinoma [15], small cell lung cancer [24], and colorectal cancer [16]. In addition, Fu et al. reported that HOTTIP regulates the stem cell property of pancreatic cancer cells [25]. Zhang et al. showed that HOTTIP accelerates epithelial-mesenchymal transition in glioma through the miR-101/ZEB1 pathway [26]. Lin et al. reported that HOTTIP promotes growth and invasion in esophageal squamous carcinoma [27]. This evidence demonstrates that HOTTIP serves as oncogene. However, the role of HOTTIP in NPC is unclear. We showed that HOTTIP promoted NPC cell proliferation, migration, and invasion, indicating HOTTIP also acts as an oncogene in NPC. Moreover, our results indicate that HOTTIP might be a potential prognostic marker for NPC patients.
MicroRNAs (miRNAs) are another class of noncoding RNAs, with a length of about 22 nucleotides. miRNAs regulate gene expression via binding to the complementary site of the 3′-UTR of target mRNA. Dysregulation of miRNA expression levels leads to carcinogenesis [28], and the expression of miRNAs is inhibited by lncRNAs [9]. In our study, we found that HOTTIP serves as a sponge of miR-4301 and that HOTTIP knockdown significantly promoted the expression of miR-4301, but miR-4301 upregulation suppressed the HOTTIP level in NPC cells. A previous study showed that miR-4301 promotes cell death in breast cancer [29], suggesting miR-4301 acts as a tumor suppressor. However, the role of miR-4301 in NPC remains elusive. To determine whether HOTTIP regulates NPC progression via inhibiting miR-4301, we analyzed the effects of miR-4301 on NPC cells. By CCK8 and Transwell assays, we found that miR-4301 overexpression significantly suppressed NPC cell proliferation, migration, and invasion.
Conclusions
In conclusion, our study, for the first time, reveals the function of HOTTIP in NPC. Our results demonstrated that HOTTIP facilitates NPC progression by serving as a miR-4301 sponge, suggesting that the HOTTIP/miR-4301 axis is a potential target for NPC therapy.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Gong Z, Zhang S, Zeng Z, et al. LOC401317, a p53-regulated long non-coding RNA, inhibits cell proliferation and induces apoptosis in the nasopharyngeal carcinoma cell line HNE2. PLoS One. 2014;9:e110674. doi: 10.1371/journal.pone.0110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–78. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Chen MH, Wu MY, Wu XY. Correlation between expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 and cervical lymph node metastasis of nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2013;122:210–15. doi: 10.1177/000348941312200311. [DOI] [PubMed] [Google Scholar]
- 4.Xing YH, Bai Z, Liu CX, et al. Research progress of long noncoding RNA in China. IUBMB Life. 2016;68:887–93. doi: 10.1002/iub.1564. [DOI] [PubMed] [Google Scholar]
- 5.Ye B, Liu B, Yang L, et al. LncKdm2b controls self-renewal of embryonic stem cells via activating expression of transcription factor Zbtb3. EMBO J. 2018;37(8) doi: 10.15252/embj.201797174. pii: e97174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Ye B, Yang L, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18:499–508. doi: 10.1038/ni.3712. [DOI] [PubMed] [Google Scholar]
- 7.Du M, Huang T, Wu J, et al. Long non-coding RNA n326322 promotes the proliferation and invasion in nasopharyngeal carcinoma. Oncotarget. 2018;9:1843–51. doi: 10.18632/oncotarget.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nie GH, Li Z, Duan HF, et al. lncRNA C22orf32-1 contributes to the tumorigenesis of nasopharyngeal carcinoma. Oncol Lett. 2017;13:4487–92. doi: 10.3892/ol.2017.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Lu Z, Wang N, et al. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp Mol Med. 2018;50:57. doi: 10.1038/s12276-018-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu YC, Liang CJ, Zhang DX, et al. LncSHRG promotes hepatocellular carcinoma progression by activating HES6. Oncotarget. 2017;8:70630–41. doi: 10.18632/oncotarget.19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Yu S, Zhang Z, et al. Long non-coding RNA AK096174 promotes cell proliferation and invasion in gastric cancer by regulating WDR66 expression. Biosci Rep. 2018;38(4) doi: 10.1042/BSR20180277. pii: BSR20180277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Dan J, Wang J, Wang Y, et al. LncRNA-MEG3 inhibits proliferation and metastasis by regulating miRNA-21 in gastric cancer. Biomed Pharmacother. 2018;99:931–38. doi: 10.1016/j.biopha.2018.01.164. [DOI] [PubMed] [Google Scholar]
- 13.Du M, Chen W, Zhang W, et al. TGF-? regulates the ERK/MAPK pathway independent of the SMAD pathway by repressing miRNA-124 to increase MALAT1 expression in nasopharyngeal carcinoma. Biomed Pharmacother. 2018;99:688–96. doi: 10.1016/j.biopha.2018.01.120. [DOI] [PubMed] [Google Scholar]
- 14.Huang G, Jiang H, Lin Y, et al. lncAKHE enhances cell growth and migration in hepatocellular carcinoma via activation of NOTCH2 signaling. Cell Death Dis. 2018;9:487. doi: 10.1038/s41419-018-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Q, Liu Y, Fan Y, et al. LncRNA HOTTIP promotes papillary thyroid carcinoma cell proliferation, invasion and migration by regulating miR-637. Int J Biochem Cell Biol. 2018;98:1–9. doi: 10.1016/j.biocel.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Yu T, Hu H, He K. Knockdown of the long non-coding RNA HOTTIP inhibits colorectal cancer cell proliferation and migration and induces apoptosis by targeting SGK1. Biomed Pharmacother. 2018;98:286–96. doi: 10.1016/j.biopha.2017.12.064. [DOI] [PubMed] [Google Scholar]
- 17.Zhu PP, Wang YY, Wu JY, et al. LncBRM initiates YAP1 signalling activation to drive self-renewal of liver cancer stem cells. Nature Commun. 2016;7:13608. doi: 10.1038/ncomms13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Yang X, Bao W, et al. Circular RNA circMAN2B2 facilitates lung cancer cell proliferation and invasion via miR-1275/FOXK1 axis. Biochem Biophys Res Commun. 2018;498:1009–15. doi: 10.1016/j.bbrc.2018.03.105. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Liu Y, Zhang H, et al. Hsa-miR-125b suppresses bladder cancer development by down-regulating oncogene SIRT7 and oncogenic long non-coding RNA MALAT1. FEBS Lett. 2013;587:3875–82. [PubMed] [Google Scholar]
- 20.Zeng K, Chen X, Xu M, et al. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9:417. doi: 10.1038/s41419-018-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–49. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–90. doi: 10.2147/OTT.S147586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie S, Ge Q, Wang X, et al. Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle. 2018;17:154–61. doi: 10.1080/15384101.2017.1407895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Hu B, Wang Q, et al. Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR-216a. Cell Death Dis. 2018;9:85. doi: 10.1038/s41419-017-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Z, Chen C, Zhou QW, et al. LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett. 2017;410:68–81. doi: 10.1016/j.canlet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Wang W, Liu G, et al. Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis. Biomed Pharmacother. 2017;95:711–20. doi: 10.1016/j.biopha.2017.08.133. [DOI] [PubMed] [Google Scholar]
- 27.Lin C, Wang Y, Wang Y, et al. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene. 2017;36:5392–406. doi: 10.1038/onc.2017.133. [DOI] [PubMed] [Google Scholar]
- 28.Song Q, Liu B, Li X, et al. MiR-26a-5p potentiates metastasis of human lung cancer cells by regulating ITGbeta8- JAK2/STAT3 axis. Biochem Biophys Res Commun. 2018;501(2):494–500. doi: 10.1016/j.bbrc.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Gholipour N, Ohradanova-Repic A, Ahangari G. A novel report of MiR-4301 induces cell apoptosis by negatively regulating DRD2 expression in human breast cancer cells. J Cell Biochem. 2018;119(8):6408–17. doi: 10.1002/jcb.26577. [DOI] [PubMed] [Google Scholar]