Abstract
Background and Aims:
Dexmedetomidine has a promising role as an intrathecal adjuvant. However it's role as an adjuvant to ropivacaine has not been evaluated extensively. This study is designed to find out the effect of addition of dexmedetomidine 5 μg to isobaric ropivacaine 18.75 mg on block characteristics and hemodynamic parameters in patients undergoing surgeries for fracture neck of femur under subarachnoid block (SAB).
Material and Methods:
Sixty-one American Society of Anesthesiologists (ASA) Class I or II patients between 18–60 years undergoing surgeries for fracture neck of femur under SAB were recruited and randomized into two groups. Thirty patients in Group RN received 2.5 ml isobaric ropivacaine 0.75% (18.75 mg) with 0.5 ml normal saline (NS) to make a total volume of 3 ml, while 31 patients in Group RD received 2.5 ml isobaric ropivacaine 0.75% with dexmedetomidine 5 μg diluted with NS to make a total volume of 3 ml. The block characteristics, hemodynamic parameters, and side effects were recorded in both the groups.
Results:
Patients in Group RD had significantly longer duration of sensory block (202.90 ± 50.2 min) compared to Group RN (157.33 ± 31.6 min), P < 0.001. Time to first rescue analgesia request was significantly longer in the Group RD compared to Group RN (265.16 ± 71.4 min vs 203.67 ± 35.6 min, respectively) (P < 0.001). However, the sensory block onset, maximum block height, time to two dermatomal regression, and motor block intensity remained unaltered. Incidence of side effects like hypotension, bradycardia, nausea, vomiting, and shivering were statistically similar in both the groups.
Conclusion:
Addition of 5 μg dexmedetomidine enhances the analgesic effect of intrathecal 18.75 mg isobaric ropivacaine for the conduct of fracture neck of femur surgeries with minimal adverse events.
Keywords: Anesthesia, dexmedetomidine, femoral neck fractures, ropivacaine, spinal
Introduction
Subarachnoid block (SAB) is a commonly used, safe, and effective technique which provides rapid and reliable anesthesia with good muscle relaxation for patients undergoing surgery for lower limb.[1] It offers advantages over general anesthesia (GA) by providing superior intraoperative and postoperative analgesia, reduced risk of postoperative deep vein thrombosis and confusion, commonly seen in patients emerging from GA.[1]
Ropivacaine is a local anesthetic agent which, when administered as an intrathecal (IT) drug, provides effective anesthesia with early motor recovery, thereby leading to decreased incidence of venous thromboembolism, early mobilization, and shorter hospitalization. It is less cardiotoxic and neurotoxic compared to bupivacaine.[2] Opioids and non-opioids such as alpha-2 agonists can be added to the IT local anesthetic agents to prolong the duration and quality of block.[3] Alpha-2 agonists like clonidine block conduction of C and A delta fibers.[4] Dexmedetomidine hyperpolarizes the membrane potentials of substantia gelatinosa neurons by G-protein-mediated activation of K+ channels through alpha (2A)- and alpha (2C)-adrenoceptors.[5] There is a probability of a direct anti-nociceptive effect as well.[5,6] It shortens the onset and prolongs the sensory blockade produced by ropivacaine in plexus blocks[7] and bupivacaine in neuraxial blocks.[8] In addition, it is cardioprotective, neuroprotective, and has minimal respiratory depression.[9] The dose of IT dexmedetomidine as an adjuvant to local anesthetic agents varies from 3 μg–15 μg.[10]
Considering the above facts, we hypothesized that IT dexmedetomidine should be able to prolong the duration of sensory blockade of isobaric ropivacaine. Though there are a handful of studies of bupivacaine with IT dexmedetomidine, there are very few studies in the literature on IT dexmedetomidine as an adjuvant to isobaric ropivacaine. The results of these studies on the efficacy of the combination of dexmedetomidine with ropivacaine are contradictory. In a dose of 3 μg and 5 μg with 15 mg isobaric ropivacaine, dexmedetomidine was found to produce an inadequate anesthetic effect for the conduct of abdominal hysterectomy.[11] However, the dose of 5 μg dexmedetomidine with 22.5 mg ropivacaine was effective in other two studies for the conduct of lower limb surgeries and vaginal hysterectomies.[12,13] So, the role of dexmedetomidine as an adjuvant to isobaric ropivacaine needs to be evaluated further. Hence, this study was planned to find out the effect of addition of 5 μg dexmedetomidine to IT isobaric ropivacaine 0.75% (2.5 ml; 18.75 mg) on the sensory and motor block characteristics in patients undergoing surgeries for fracture neck of femur under SAB.
Material and Methods
This double-blind randomized controlled study was conducted after approval from the Institutional Ethical Committee: Human Research. All the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was taken from all the participating patients.
Sixty-one patients, of either sex between 18–60 years of age, belonging to the American Society of Anesthesiologists (ASA) physical status I or II, undergoing elective surgery for fracture neck of femur were included. Patients with contraindication to SAB, known hypersensitivity to any of the drugs used in the study, height <150 cm or >180 cm, and with bilateral lower limb fractures were excluded.
In the operating room, monitors including ECG, pulse oximeter, non-invasive blood pressure were attached. Intravenous (IV) access was secured with 18G cannula and baseline systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were recorded. Co-loading with 10 ml/kg of IV lactated Ringer's solution was done. Using a computer-generated random number table, patients were randomly allocated to one of the two groups depending upon the drug they were to receive for SAB as under.
Group RN: 2.5 ml isobaric ropivacaine 0.75% (18.75 mg) with 0.5 ml normal saline (NS).
Group RD: 2.5 ml isobaric ropivacaine 0.75% (18.75 mg) with 5 μg of dexmedetomidine in 0.5 ml NS.
The study drug was prepared by an anesthesiologist not involved in block assessment. The investigator assessing the block characteristics and other variables was blinded to the group allocation.
Under all aseptic precautions L3–L4 vertebral interspace was palpated with the patient in the sitting position and SAB was performed with 25G Quincke's needle. Once the free flow of cerebrospinal fluid was recognized, IT test drug solution was injected slowly. Patients were made supine thereafter. Hemodynamic variables including SBP, DBP and HR were recorded at T0 and every 5 min till 30 min, every 15 min for the next 30 min and every 30 min till the end of the study, i.e., time to demand of rescue analgesic or Visual Analogue Scale (VAS) ≥3. Sensory dermatomal level was assessed every 2 min for the first 10 min and then every 5 min till the same level was obtained in three consecutive readings. This was considered as the maximum height of sensory block achieved (Smax). Subsequently, the sensory block was assessed every 30 min. Onset of sensory block was defined as the time taken from the IT drug administration up to the time taken to block sensory stimuli at T10. Duration of sensory block was calculated from the time of onset till recession of block to T12. During the intraoperative period if the level receded to T12, GA was given for completion of surgery. At this point, the study was terminated. Failure of SAB was defined as non-achievement of T10 sensory level within 20 min of administration of IT drug. The intensity of motor block was assessed in the non-fractured limb using modified Bromage scale.
Hypotension was defined as 25% decrease in SBP from the baseline.[14] It was treated with fluids and vasopressors (Inj. mephentermine 6 mg IV). Bradycardia was defined as HR <50/min[15] and was treated with 0.6 mg of atropine IV. Pain score was evaluated every 30 min in the postoperative period using a 0–10 cm VAS at which 0 represented “no pain” and 10 represented the “worst imaginable pain.” Rescue analgesia in the form of inj. paracetamol in a dose of 15 mg/kg IV was given when VAS was ≥3. Any episode of nausea, vomiting, shivering, or pruritus intraoperatively or postoperatively were recorded.
Sample size and statistical analysis
Considering a standard deviation of 40 min from the previous literature,[16] a sample size of 29 patients in each group was found to be sufficient to detect a difference of 30 min in the duration of sensory blockade at 80% power and 5% level of significance. So, a minimum of 30 patients were included in each group.
Qualitative variables such as ASA status, gender distribution, and incidence of side effects were compared using the Chi-square and Fisher's exact test. Quantitative variables such as age, height, weight, and duration of surgery were compared using the Student's t-test. Within group and intergroup comparison of HR, SBP, and DBP was done using repeated measure analysis of variance followed by Dunnett's test. Parameters like sensory block onset, time to achieve maximum sensory block, and time to two dermatomal regression did not follow a normal distribution. Therefore, non-parametric test the Mann–Whitney U test was applied. A P value <0.05 was considered significant.
Results
A total of 83 patients undergoing surgery for fracture neck of femur under SAB were enrolled and screened for eligibility to participate in this trial. Sixteen patients did not meet the inclusion criteria and six patients did not give consent. The remaining 61 patients were allocated to one of the two study groups using a computer-generated random number table. Thirty-one patients received 2.5 ml isobaric ropivacaine 0.75% with dexmedetomidine 5 μg diluted with NS to a total volume of 3 ml (Group RD). In one of the patients, there was a leakage of drug solution while injecting in subarachnoid space. Hence this patient was excluded from the study, leaving a total of 30 patients in this group. Thirty patients received 2.5 ml isobaric ropivacaine 0.75% (18.75 mg) with NS to a total volume of 3 ml (Group RN). Finally, a total of 60 patients were analyzed [Figure 1]. The demographic data and duration of surgery were comparable in both the groups [Table 1].
Figure 1.
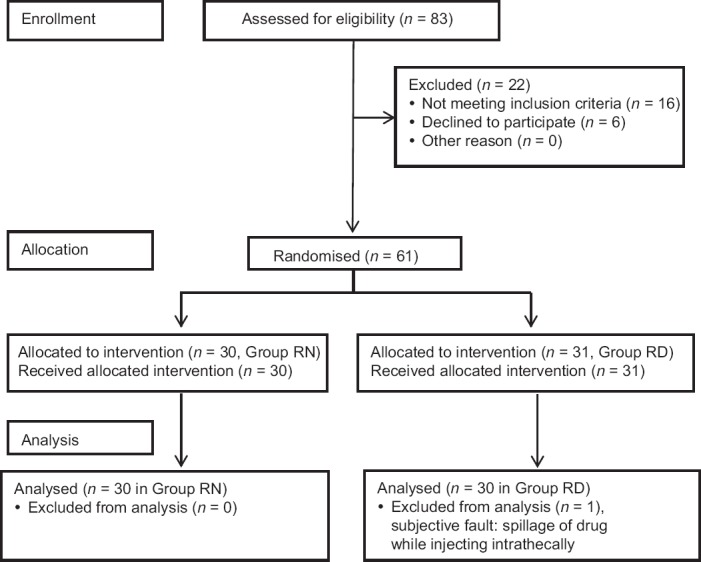
CONSORT flow diagram
Table 1.
Demographic profile
| Parameters | Group RD (n=30) | Group RN (n=30) | P |
|---|---|---|---|
| Age* (years) | 38.9±15.4 | 34.3±14.2 | 0.235 |
| Gender# (M:F) | 20:10 | 23:7 | 0.298 |
| ASA# (I:II) | 27:3 | 27:3 | 1.000 |
| Height* (cm) | 156.0±3.2 | 156.0±2.3 | 0.999 |
| Weight* (kg) | 58.8±6.7 | 58.4±9.2 | 0.857 |
| Duration of surgery* (min) | 155.16±43.07 | 140.17±35.0 | 0.183 |
*Values are expressed as mean±SD, SD=Standard deviation, #no. of cases. M=Male, F=Female, ASA=American Society of Anesthesiologists, P<0.05 significant
The sensory block onset time was comparable in both the groups (P = 0.123). The maximum cephalad spread was up to T4 dermatome in both the groups with the inter-quartile range between (T6–T8) in both the groups [Figure 2]. Time taken to achieve maximum sensory block level (Tmax) and time to two dermatomal regression (T2dr) were also comparable (P = 0.077 and 0.461, respectively). A significantly longer duration of sensory block was found in Group RD (202.90 ± 50.2 min) compared to Group RN (157.33 ± 31.6 min), P value <0.001. Similarly time to rescue analgesic request was significantly longer in Group RD (265.16 ± 71.4 min) compared to Group RN (203.67 ± 35.6 min), P value <0.001. The maximum intensity of motor block attained was similar in both the groups [Table 2].
Figure 2.
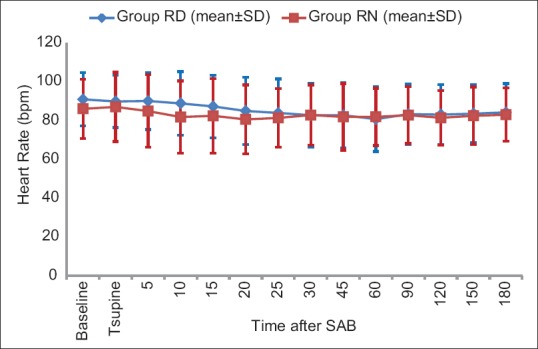
Comparison of heart rate in both the groups
Table 2.
Sensory and motor block characteristics
| Parameter | Group RD (n=30) | Group RN (n=30) | P |
|---|---|---|---|
| Sensory block onset (min) | 9.94±6.7 | 7.60±4.7 | 0.123 |
| Max. sensory block level (Smax)* | T6 [T6-T8] | T8 [T6-T8] | 0.344 |
| Tmax (min) | 21.03±14.4 | 15.73±7.2 | 0.077 |
| T2dr (min) | 51.40±33.0 | 57.13±26.5 | 0.461 |
| Sensory block duration (min) | 202.90±50.2 | 157.33±31.6 | 0.000 |
| Time to rescue analgesia (min) | 265.16±71.4 | 203.67±35.6 | 0.000 |
| Maximum intensity of motor block | I | I | 1.000 |
Values expressed as mean±SD. SD=Standard deviation. Tmax=Time to achieve maximum sensory block, T2dr=Time to two dermatomal regression. P<0.05 - not significant. *Values expressed as median and values in [ ] are interquartile range
Baseline hemodynamic parameters were comparable in the two groups. There was a general trend of decrease in HR compared to baseline after SAB in both groups. Overall, there was no significant difference in HR at the corresponding time points between the two groups (P value = 0.489) [Figure 3]. There was statistically significant fall in SBP and DBP from baseline value within both the groups after administration of IT drug [Figures 4 and 5] but SBP and DBP values at corresponding time points were comparable between the two groups (P = 0.157 and P = 0.654, respectively).
Figure 3.
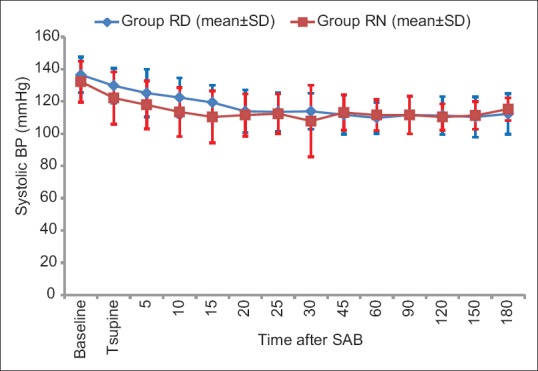
Comparison of systolic blood pressure in both the groups
Figure 4.
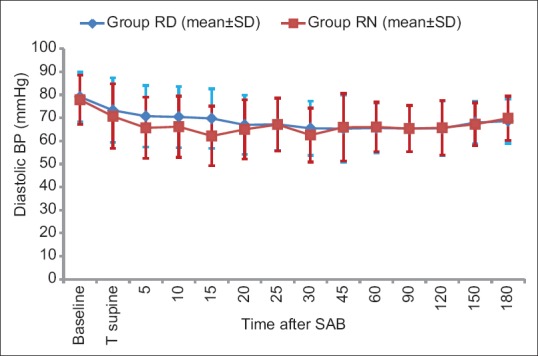
Comparison of diastolic blood pressure in both the groups
Figure 5.
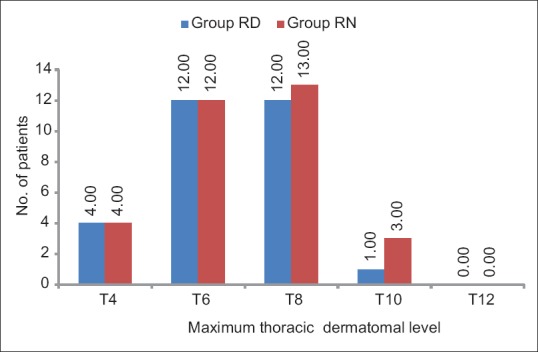
Comparison of thoracic dermatomal level in both the groups
Intraoperative hypotension was a frequent finding in both the groups but the incidence was comparable (P = 0.09). The total dose of mephentermine administered to treat hypotension was also comparable (P = 0.711) [Table 3].
Table 3.
Side effects
| Side effects | Group RD (n=30) | Group RN (n=30) | P |
|---|---|---|---|
| Hypotension | 23 (74.3) | 16 (53.3) | 0.090 |
| Bradycardia | 03 (9.7) | 02 (6.6) | 1.000 |
| Nausea and vomiting | 03 (9.7) | 01 (3.3) | 0.612 |
| Shivering | 03 (9.7) | 02 (6.7) | 1.000 |
| Urinary retention | 0 | 0 | 1.000 |
| Pruritus | 0 | 0 | 1.000 |
The values are number of patients (percentage of patients), P<0.05 - not significant
Only three patients in Group RD and two in Group RN developed bradycardia. A single bolus dose of inj. atropine 0.6 mg IV was effective in managing bradycardia. The incidence of nausea, vomiting, and shivering were comparable in both the groups. Urinary retention and pruritus were not observed in any of the patients included in the study [Table 3].
Discussion
The aim of the present study was to evaluate whether addition of 5 μg dexmedetomidine has a favorable effect on the sensory and motor block characteristics of IT isobaric ropivacaine 0.75% (2.5 ml; 18.75 mg) in patients undergoing fracture neck of femur surgeries under SAB.
Ropivacaine is commercially available as an isobaric solution in a concentration of 0.2, 0.5, 0.75, and 1%. Hyperbaric ropivacaine has been found to produce more predictable and reliable sensory and motor block, with faster onset than isobaric ropivacaine for a wide range of surgical procedures, but commercial preparation of hyperbaric ropivacaine is not yet available. Isobaric solution has been administered as an IT local anesthetic agent in dose between 15–30 mg in various scientific researches. Choice of dosage of ropivacaine in the present study was based on a previous study where 2.5 ml isobaric ropivacaine 0.75% and 1% (18.75 mg and 25 mg) was shown to have a good cardiovascular stability profile.[17]
Previous literature reported that a dose of 5 μg dexmedetomidine as an additive to isobaric ropivacaine provided intraoperative and postoperative hemodynamic stability with good postoperative analgesia.[18] Therefore, we chose a dose of 5 μg dexmedetomidine for our study to explore its effect on the sensory and motor block characteristics of ropivacaine.
The key findings of our study were as follows. Addition of dexmedetomidine significantly prolonged the duration of sensory block and time to rescue analgesic request without affecting the hemodynamic profile, sensory block onset time, maximum cephalad spread, time taken to achieve maximum sensory block level, maximum intensity of motor block, time to two dermatomal regression, and side effect profile.
All the patients who received the intervention had a successful block height of at least T10, so 18.75 mg isobaric ropivacaine with or without the addition of 5 μg dexmedetomidine was sufficient to carry out surgery for fixation of fracture neck of femur. In our study, the onset of sensory block of ropivacaine was not affected by adding dexmedetomidine. Similar to our study results, Parmar et al. also did not find any difference in the time of onset of sensorimotor block with the addition of IT dexmedetomidine to ropivacaine.[12] Dexmedetomidine, however, was found to hasten the onset time of hyperbaric bupivacaine in a dose-dependent manner in the study by Al-Mustafa et al.[8] We found that dexmedetomidine did not alter the maximum height of sensory block and time to two dermatomal regression from highest sensory level achieved. Similar findings were reported in the studies by Parmar et al.,[12] Jain et al.,[19] and Gupta et al.[13] where the maximum dermatomal block height was T5 or T6.
The addition of IT dexmedetomidine to isobaric ropivacaine in the present study significantly prolonged the duration of sensory blockade by approximately 45 min at T12 dermatome. Our results correspond to the results of a meta-analysis by Wu et al.,[20] which included 16 randomized controlled trials, wherein it was stated that addition of dexmedetomidine to IT drug in a dose of ≤5 μg prolonged the mean duration of sensory blockade by 43 min. The study by Jain et al.[19] found that the addition of dexmedetomidine prolonged the sensory duration by approximately 2 h. The difference in the duration of sensory blockade from our study is because of the difference in the dermatomal level at which the sensory block duration was assessed (i.e., T12 in our study vs S2 in the study by Jain et al.).
The duration of analgesia was significantly prolonged with the addition of 5 μg dexmedetomidine as compared to ropivacaine alone (P < 0.001). Three patients in group that received ropivacaine alone and one patient in group that received dexmedetomidine with ropivacaine required GA for completion of surgical procedure, as there was intraoperative regression to T12 dermatome. In all these cases, the surgery was deemed difficult by the surgeon and so the surgical duration exceeded the mean duration of sensory block provided by the IT drug.
The incidence of hypotension in the ropivacaine only group in our study (53.3%) was higher than that reported by McNamee et al.[17](24%) with a similar dose of isobaric ropivacaine 0.75% (18.75 mg). This may be because the volume of drug that was administered in our study was 3.0 ml compared to 2.5 ml in the study by McNamee et al. However, the addition of dexemdetomidine did not alter the hemodynamic profile of ropivacaine. This can be derived from the fact that a similar incidence of hypotension and bradycardia was found in both the groups. This finding is in concurrence with the results of previous studies.[18,19] Incidence of bradycardia in our study was comparable to that observed by McNamee et al.[17]
In our study, all the patients were kept supine after the SAB. No table tilt was required to establish the block and there was no case of erratic spread of the drug resulting in higher sensory levels or block of cervical dermatomes as has been previously reported with isobaric ropivacaine.[17] The reason for this difference may be due to the difference in position of patient while doing the SAB, lateral decubitus position in their study in contrast to sitting position in our study.
The side effects like nausea, vomiting, and shivering were observed with similar incidence in both the groups. Since our trial was not powered enough to study the side effects following SAB, we cannot comment on the significance of these findings. In addition, the anti-shivering effect of alpha-2 adrenergic agonists as reported by Moawad and Elawdy[21] also cannot be commented upon. None of the patient complained of urinary retention and pruritus following SAB.
In our study, we could not assess the intraoperative motor block characteristics, duration, and recovery as the patients were operated on a fracture table with limbs tied and draped. The strength of the study was that time to two dermatomal regression from the highest level of sensory block achieved as well as time to regression up to T12 level were measured which has not been done in previous studies.
Conclusion
Thus, we conclude that the addition of dexmedetomidine (5 μg) to isobaric ropivacaine (18.75 mg) provides effective anesthesia for conducting fracture neck of femur surgeries under SAB and is beneficial by prolonging the duration of sensory block and time to first rescue analgesic request without adversely affecting the side effect profile. Further studies are required to find out the duration and recovery of motor block of ropivacaine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kettner SC, Willschke H, Marhofer P. Does regional anaesthesia really improve outcome? Br J Anaesth. 2011;107(Suppl 1):90–5. doi: 10.1093/bja/aer340. [DOI] [PubMed] [Google Scholar]
- 2.Leone S, Di Cianni S, Casati A, Fanelli G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed l'Ateneo Parm. 2008;79:92–105. [PubMed] [Google Scholar]
- 3.Shukla D, Verma A, Agarwal A, Pandey HD, Tyagi C. Comparative study of intrathecal dexmedetomidine with intrathecal magnesium sulfate used as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:495–9. doi: 10.4103/0970-9185.86594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenach J, De Kock M, Klimscha W. Alpha2-Adrenergic agonists for regional anesthesia. Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27:3182–90. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalso EA, Pöyhiä R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol. 1991;68:140–3. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 7.Kathuria S, Gupta S, Dhawan I. Dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block. Saudi J Anaesth. 2015;9:148–54. doi: 10.4103/1658-354X.152841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 9.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]
- 10.Sudheesh K, Harsoor SS. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naithani U, Meena M, Gupta S, Meena K, Swain L, Pradeep D. Dose-dependent effect of intrathecal dexmedetomidine on isobaric ropivacaine in spinal anesthesia for abdominal hysterectomy: Effect on block characteristics and hemodynamics. J Anaesthesiol Clin Pharmacol. 2015;31:72–9. doi: 10.4103/0970-9185.150549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmar NK, Bhandari G, Shahi KS, Chand G, Rani D, Sharma G, et al. Effect of intrathecal ropivacaine with dexmedetomidine for operative and post operative analgesia: A prospective randomized study. J Evol Med Dent Sci. 2014;3:2917–25. [Google Scholar]
- 13.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55:347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halder S, Das A, Mandal D, Chandra M, Ray S, Biswas MR, et al. Effect of different doses of dexmedetomidine as adjuvant in bupivacaine -induced subarachnoid block for traumatized lower limb orthopaedic surgery: A prospective, double-blinded and randomized controlled study. J Clin Diagnostic Res. 2014;8:1–6. doi: 10.7860/JCDR/2014/9670.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erturk E, Tutuncu C, Eroglu A, Gokben M. Clinical comparison of 12 mg ropivacaine and 8 mg bupivacaine, both with 20 μg fentanyl, in spinal anaesthesia for major orthopaedic surgery in geriatric patients. Med Princ Pract. 2010;19:142–7. doi: 10.1159/000249581. [DOI] [PubMed] [Google Scholar]
- 16.Salhotra R, Mohta M, Agarwal D, Sethi AK. Intrathecal ropivacaine with or without tramadol for lower limb orthopedic surgeries. J Anaesthesiol Clin Pharmacol. 2016;32:483–6. doi: 10.4103/0970-9185.173323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamee DA, Parks L, Mcclelland AM, Scott S, Milligan KR, Ahlén K, et al. Intrathecal ropivacaine for total hip arthroplasty: Double-blind comparative study with isobaric 7.5 mg/ml and 10 mg/ml solutions. Clin Invest. 2001;87:743–7. doi: 10.1093/bja/87.5.743. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Patel I, Gandhi R. Haemodynamic effects of intrathecal dexmedetomidine added to ropivacaine intraoperatively and for postoperative analgesia. Int J Basic Clin Pharmacol. 2013;2:26–9. [Google Scholar]
- 19.Jain G, Verma R, Singh A, Singh Y. Comparison of two different doses of intrathecal dexmedetomidine as adjuvant with isobaric ropivacaine in lower abdominal surgery. Anesth Essays Res. 2015;9:343–7. doi: 10.4103/0259-1162.158009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Yu, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia. A systematic review and meta-analysis? PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moawad HE, Elawdy MM. Efficacy of intrathecal dexmedetomidine in prevention of shivering in patients undergoing transurethral prostatectomy: A randomized controlled trial. Egypt J Anaesth. 2015;31:181–7. [Google Scholar]


