Abstract
Atrial fibrillation (AF) can be initiated from arrhythmogenic foci within the muscular sleeves that extend not only into the pulmonary veins but also into both vena cavae. Patients with SVC-derived AF have the common clinical and genetic risk factors. Bayesian network analysis is a probabilistic model in which a qualitative dependency relationship among random variables is represented by a graph structure and a quantitative relationship between individual variables is expressed by a conditional probability.
We used data of meta-analysis of 2170 AF patients with and without SVC arrhythmogenicity in the previous article. Bayesian Networking analysis was performed using the software “bnlearn”. Using the clinical and genetic factors associated with SVC arrhythmogenicity in the previous article, we investigated a Bayesian networking structure to determine the probabilitic causation of variants to clinical parameters and found that the rate of recurrence depended on SVC arrhythmogenicity and LA diameter, and that SVC arrhythmogenicity was conditionally dependent on gender, body mass index, and genetic risk score. We found the possibility of prediction model generated from three factors. Receiver-operation characteristic analysis showed the area under the curve was 0.84.
Using the clinical/genetic factors associated with SVC arrhythmogenicity through the previous meta-analysis of over 2000 patients, Bayesian networking analysis indicated the probabilistic causation of SVC arrhythmogenicity and associated clinical/genetic factors.
Keywords: Superior vena cava arrhythmogenicity, Atrial fibrillation, Bayesian networking structure
1. Introduction
Bayesian network analysis is a probabilistic model in which a qualitative dependency relationship among random variables is represented by a graph structure and a quantitative relationship between individual variables is expressed by a conditional probability. The model is defined by a graph structure representing dependency relationships between nodes derived from a set of conditional probabilities. The conditional probability with the state of the node adjacent by the arrow propagates one after another through the arrow. The graph structure is obtained from the probabilities of all the nodes. The effective network automatically generated from the data reveals the influence relationship between the data items. It is a dynamic model in which the probability of all nodes in this network changes by changing the state of one node. This model can be used for intelligent information processing such as probabilistic causation of clinical parameters and profile of cell signaling including uncertainty by using probability calculation. Recently, Bayesian network analysis is applied for clinical practice with electric health record [1,2] and intricate hierarchical analysis [3]. Bayesian network analysis in studies of a single disease such as Alzheimer's disease [4] and hip fracture [5] facilitates the probability causation and hierarchical structure.
Atrial fibrillation (AF) can be initiated from arrhythmogenic foci derived from muscular sleeves that extend not only into the pulmonary veins (PV) but also both vena cavae including the superior vena cava (SVC) [6]. Patients with arrhythmogenic SVC have been reported to have the common clinical and genetic risk factors [7]. Interestingly, patients with arrhythmogenic SVC have long myocardial sleeves around the SVC and high amplitude electrical potentials within them [8]. These common phenotypes in arrhythmogenic SVC suggest the possible existence of genetic factors. Next, we confirmed the positions of clinical/genetic factors in the network under the probabilistic causation.
Using Bayesian network structure analysis, the aim of the current study was to investigate the probabilistic causation of clinical characteristics and genetic factors associated with SVC arrhythmogenicity in consecutive AF patients through the generated graphical network.
2. Methods
2.1. Study dataset
We used the existing data of our previous study [7]. In brief, we divided 2170 AF patients who underwent catheter ablation including PV isolation into two groups: patients recruited from Saitama Red Cross Hospital (Panel 1) and ones from National Disaster Medical Center (Panel 2) and Tsuchiura Kyodo Hospital (Panel 3). We showed the association between SVC arrhythmogenicity and clinical/genetic factors: genetic risk score (GRS), gender, body-mass index (BMI), and left atrial diameter (LAD). The GRS was calculated for participants by multiplying risk allele dosages of rs2634073 and rs6584555 weighted with the respective natural logarithm of odds ratio. SVC arrhythmogenicity was assessed with drug challenge and pacing maneuvers in Panel 1. After PV isolation, the induction of atrial arrhythmias was attempted by infusion of high-dose isoproterenol (ISP), followed by atrial burst pacing from the pacing catheter in the coronary sinus. In Panel 2 and 3, SVC arrhythmogenicity was simply defined as ectopy in the SVC initiating AF in spite of the repetitive procedure for PV isolation. The types of atrial fibrillation (paroxysmal, persistent or long-standing ones) and recurrence rate after the catheter procedure including PV and SVC isolation were available only in Panel 1.
All patients provided their written informed consent. The study protocol was approved by the ethical committees of Tokyo Medical and Dental University (TMDU) and all associated hospitals. The study complied with the Declaration of Helsinki.
2.2. Statistical analysis
We studied the probabilistic causation among clinical and genetic factors through Bayesian network structure learning with the “bnlearn” package in the R statistical software environment [9,10]. The network was trained using a score-based “greedy search” algorithm. We calculated the score with Hill-Climbing method using Akaike Information Criterion (AIC) for model-fitting. This algorithm ranks network structures based on these scores with respect to a goodness-of-fit model, the increase/decrease of score (Akaike Information Criterion; AIC) caused by the removal of the arc. All the scores between two factors (nodes) were calculated and the arcs between two nodes were generated by the network score if there were the direct relations. The value < 0 of arc strength would be significant. The operator then evaluated all of the arcs including their direction. Arcs that were illogical were “blacklisted” and omitted from analysis to improve the model-fitting, preventing from circular structure in the structure learning process. There were three of them, the arc from SVC to BMI, from LA diameter to BMI and from BMI to Gender. For example, BMI cannot influence gender.
We also validated the strength of arcs in structured Bayesian network using Bootstrap method in the same R package. The program generated a bootstrap matrix and estimated the network. This process was repeated 1000 times and the value of probability was calculated.
We made a prediction model of SVC arrhythmogenicity of AF patients with logistic regression model, selecting factors which Bayesian network analysis indicated as probability causation. We used Panel 1 and Panel 2/3 as training data and validating data, respectively. We calculated individual values of validating data with the prediction model and generated receiver-operator characteristic curve.
3. Result
3.1. Bayesian network structure learning
We investigated the network of clinical and genetic factors related to SVC arrhythmogenicity in the meta-analysis [7]. We employed Bayesian network analysis to investigate any probabilistic causation in Panel 1. The network structure learned by the dataset showed that the rate of AF recurrence after catheter ablation depended on the LA diameter and SVC arrhythmogenicity. SVC arrhythmogenicity was conditionally dependent on gender, BMI, and GRS [Fig. 1 (A), Table (A)]. We then integrated Panels 2/3 and applied Bayesian network analysis for this second group. Intriguingly, the arcs from GRS, Gender, and BMI to SVC were replicated [Fig. 1 (B), Table (B)].
Fig. 1.
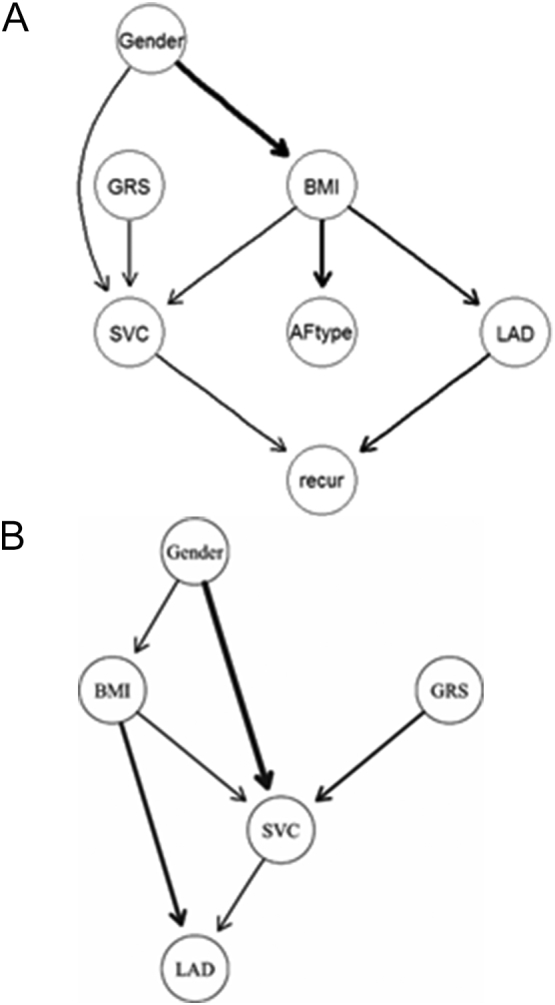
Bayesian network analysis with a score-based learning algorithm (Hill-Climbing greedy search). A) Bayesian networking of clinical and genetic factors in the first panel. The arc indicates the direction of the probabilistic causation. We measured the strength of the probabilistic relationships expressed by the arc of a Bayesian network. The line width indicates the strength of the probability. B) We confirmed Bayesian network of clinical and genetic factors in the 1st panel with the 2nd and the 3rd panels. Three directions (“SVC to BMI”, “LAD to BMI”, “BMI to Gender”) were omitted as “blacklist”, following the author's instruction because these probabilistic causations are impossible.
Table A.
Arc strength of factors in Panel 1.
| From | To⁎ | Strength⁎⁎ | Probability⁎⁎⁎ | |
|---|---|---|---|---|
| 1 | Gender | BMI | −12.465741 | 1.000 |
| 2 | BMI | AFtype | −9.4877596 | 0.790 |
| 3 | BMI | SVC | −2.3935417 | 0.745 |
| 4 | BMI | LAD | −4.074912 | 1.000 |
| 5 | LAD | recur | −3.309854 | 0.685 |
| 6 | SVC | recur | −1.6888468 | 0.585 |
| 7 | Gender | SVC | −0.6974259 | 0.725 |
| 8 | GRS | SVC | −2.5013679 | 0.860 |
The arcs between two factors shows the direction from a factor to another.
The criteria of the strength is less than zero.
Probability was calculated with bootstrap analysis. The criteria is >0.80.
Table B.
Arc strength of factors in Panel 2/3.
| From | To⁎ | Strength⁎⁎ | Probability⁎⁎⁎ | |
|---|---|---|---|---|
| 1 | Gender | SVC | −131.5850631 | 1.000 |
| 2 | BMI | LAD | −48.2886403 | 1.000 |
| 3 | GRS | SVC | −21.0736531 | 1.000 |
| 4 | SVC | LAD | −3.2513704 | 0.740 |
| 5 | Gender | BMI | −0.3654641 | 0.145 |
| 6 | BMI | SVC | −0.2508458 | 0.450 |
The arcs between two factors shows the direction from a factor to another.
The criteria of the strength is less than zero.
Probability was calculated with bootstrap analysis. The criteria is >0.80.
We assessed this result with Bootstrap method and confirmed that the probability of arc strength of three arcs (gender → BMI, BMI → LAD, and GRS → SVC) were >0.80. In Panel 2/3, three arcs (gender → SVC, BMI → LAD, and GRS → SVC) were >0.80. BMI → LAD and GRS → SVC were validated as common probability causation in two Panels.
3.2. Prediction model for SVC arrhythmogenicity in AF patients
The prediction model of SVC arrhythmogenicity in AF patients was made with Panel 1. The factors associated with SVC arrhythmogenicity were selected Next, this model was applied for individuals in Panel 2/3. ROC curve was drawn in Fig. 2 and the AUC of ROC was 0.84.
Fig. 2.
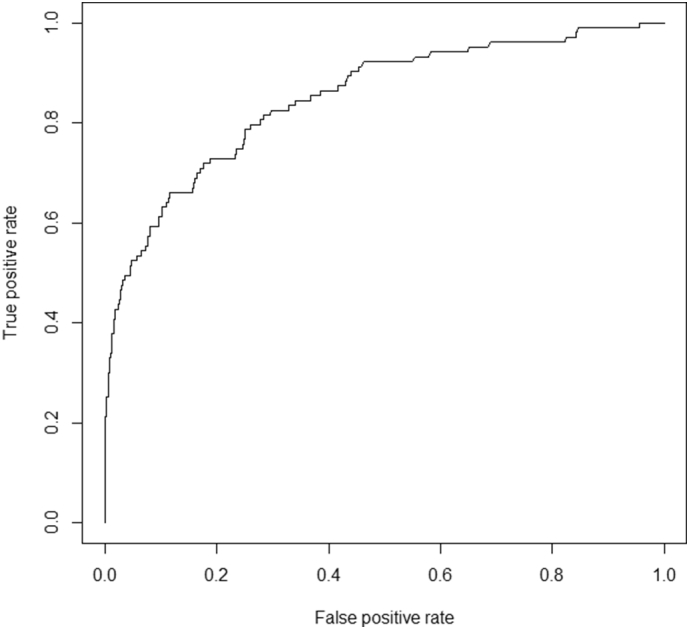
Receiver-operation characteristics analysis of prediction model for SVC arrhythmogenicity. Prediction model was generated from training data (Panel 1), and validated with Panel 2/3. The area under curve (AUC) was 0.84.
4. Discussion
Recent studies have shown that AF patients with arrhythmogenic SVC have genetic [7] and clinical [8,11] characteristics that differ from AF patients without arrhythmogenic SVC. In the current study, we confirmed the probabilistic causation of arrhythmogenic SVC with graphical network among the associated factors in the previous study. Bayesian network analysis combining clinical and genetic factors generated a directed acyclic graph which consisted of nodes of the associated factors and edges according to conditional independence and showed that as probabilistic causation, SVC arrhythmogenicity depended on three factors: gender, BMI, and GRS which had been indicated as associated. Bayesian network analysis of two independent datasets drew the arrow directed from gender, BMI, and GRS to SVC. Among them, arc of GRS → SVC was confirmed in the robustness with bootstrap method. Arcs of gender → SVC in two datasets differed in strength. This difference was derived from the degree of model fitting. The criteria of SVC arrhythmogenicity in Panel 2/3 was stricter than one in Panel 1, which affected the calculation of model-fitting. The stronger probability causation from gender to SVC arrhythmogenicity in the group whose SVC arrhythmogenicity criteria was strict was shown.
The prediction model generated from training data with three factors was confirmed through validating data. Bayesian network analysis showed solid probability causation of GRS → SVC. Limited number of variants identified in the previous study was used in this study. SVC arrhythmogenicity was intricate pathogenesis, including further factors, especially genetic factors. Further analysis and solid data are required.
Intriguingly, the arrow direction of probabilistic causation between SVC and LA diameter was not consistent: in Panel 2/3 group, the arrow directed from SVC to LA diameter, while in Panel 1 group, SVC and LA diameter were associated, but did not directly intact with each other. The criteria of SVC arrhythmogenicity with or without challenge test were slightly different in Panel 1 and Panel 2/3. In the facility of panel 1, patients underwent one-time procedure, evaluating Challenge test. On the other hand, the patients of Panel 2/3 who had SVC arrhythmogenicity underwent two-four times of procedure. As a result, we thought that the different strategy of Panel 1 and Panel 2/3 caused the difference of exposure time and that it made some difference of relationship of SVC to LAD. The exposure time and the frequency of AF initiation in SVC change the conduction environment in not only left but right atrium. This discrepancy means that while direct effects of gender, BMI and GRS on SVC arrhythmogenicity were consistent. On the other hand, because LAD is influenced by various factors during AF, the direct effect of SVC arrhythmogenicity on LAD is diminished in spite of the association with LAD.
Using the clinical/genetic factors associated with SVC arrhythmogenicity through the previous meta-analysis of over 2000 patients, Bayesian networking analysis indicated the probabilistic causation of SVC arrhythmogenicity and associated clinical/genetic factors through graphical network structure.
Study limitation
The prediction model in this study includes gender, MI, and GRS with limited numbers of variants. It is required to find further genetic and clinical factors and validate this model with large-scale study.
Fund support
This work was supported by a Grant-in-Aid for Scientific Research (C) (17K07251) from Japan Society for the Promotion of Science (JSPS).
Acknowledgments
Acknowledgement
We would also like to express our gratitude to the study participants and the doctors and research staff at TMDU and the associated hospitals.
Disclosure
No conflicts of interest to disclose.
References
- 1.Lucasa Peter J.F., van der Gaag Linda C., Abu-Hanna Ameen. Bayesian networks in biomedicine and health-care. Artif. Intell. Med. 2004;30(3):201–214. doi: 10.1016/j.artmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Chao Yi-Sheng, Scutari Marco, Chen Tai-Shen, Wu Chao-Jung, Durand Madeleine, Boivin Antoine, Wu Hsing-Chien, Chen Wei-Chih. A network perspective of engaging patients in specialist and chronic illness care: the 2014 international health policy survey. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0201355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lappenschaar Martijn, Hommersom Arjen, Lucasa Peter J.F., Lagro Joep, Visscher Stefan. Multilevel Bayesian networks for the analysis of hierarchical health care data. Artif. Intell. Med. 2013;57(3):171–183. doi: 10.1016/j.artmed.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Rembach Alan, Stingo Francesco C., Peterson Christine, Vannucci Marina, Do Kim-Anh, Wilson William J., Macaulay S. Lance, Ryan Timothy M., Martins Ralph N., Ames David, Masters Colin L., Doecke James D., The AIBL Research Group Bayesian graphical network analyses reveal complex biological interactions specific to Alzheimer's disease. J. Alzheimers Dis. 2015;44(3):917–925. doi: 10.3233/JAD-141497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillet Pascal, Klemm Sarah, Ducher Michel, Aussem Alexandre, Schott Anne-Marie. Hip fracture in the elderly: a re-analysis of the EPIDOS study with causal Bayesian networks. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goya M., Ouyang F., Ernst S., Volkmer M., Antz M., Kuck K.H. Electroanatomic mapping and catheter ablation of breakthroughs from the right atrium to the superior vena cava in patients with atrial fibrillation. Circulation. 2002;106:1317–1320. doi: 10.1161/01.cir.0000033115.92612.f4. [DOI] [PubMed] [Google Scholar]
- 7.Ebana Y., Nitta J., Takahashi Y., Miyazaki S., Suzuki M., Liu L., Hirao K., Kanda E., Isobe M., Furukawa T. Association of the clinical and genetic factors with superior vena cava arrhythmogenicity in atrial fibrillation. Circ. J. 2017;82(1):71–77. doi: 10.1253/circj.CJ-17-0350. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi K., Yamauchi Y., Hirao K., Sasaki T., Hachiya H., Sekiguchi Y. Superior vena cava as initiator of atrial fibrillation: factors related to its arrhythmogenicity. Heart Rhythm. 2010;7:1186–1191. doi: 10.1016/j.hrthm.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Scutari M. Learning Bayesian networks with the bnlearn R package. J. Stat. Softw. 2010;35(3):1–22. [Google Scholar]
- 10.Beal M.J., Falciani F., Ghahramani Z., Rangel C., Wild D.L. A Bayesian approach to reconstructing genetic regulatory networks with hidden factors. Bioinformatics. 2005;21(3):349–356. doi: 10.1093/bioinformatics/bti014. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki S., Taniguchi H., Kusa S., Ichihara N., Nakamura H., Hachiya H. Factors predicting an arrhythmogenic superior vena cava in atrial fibrillation ablation: insight into the mechanism. Heart Rhythm. 2014;11:1560–1566. doi: 10.1016/j.hrthm.2014.06.016. [DOI] [PubMed] [Google Scholar]


