Abstract
Introduction
Metformin and glucagon‐like peptide‐1 (GLP‐1) agonists are widely used for treating type two diabetes mellitus (T2DM). While recent studies suggest these drugs might modify the gastrointestinal tract (GIT) microbiome, further confirmation is required from human clinical trials.
Materials and methods
Here, we compare, in patients with T2DM, the effects of metformin (n = 18 subjects) and liraglutide (n = 19), a GLP‐1 agonist, on their GIT microbiomes over a 42 day period (n = 74 samples) using 16S ribosomal RNA (rRNA) sequencing.
Results
We found that these drugs had markedly different effects on the microbiome composition. At both baseline and Day 42, subjects taking metformin had a significant increase (Baseline adj. P = .038, Day 42 adj. P = .041) in the relative abundance of the bacterial genus Sutterella, whereas liraglutide dosing is associated with a significant increase (Baseline adj. P = .048, Day 42 adj. P = .003) in the genus Akkermansia, a GIT bacteria positively associated with gut barrier homoeostasis. Bacteroides and Akkermansia relative abundances were also significantly associated with duration of subject diabetes (adj P < .05). Specifically, there was a significantly higher abundance of Akkermansia in subjects with short and medium durations than those with long duration of diabetes.
Discussion
To our knowledge, this is the first report of GLP‐1 agonist‐associated changes in the human microbiome and its differentiating effects to metformin. Our study suggests that modulation of the GIT microbiome is a potentially important component in the mechanism of action of these drugs.
Keywords: liraglutide, metformin, microbiota
1. INTRODUCTION
Gastrointestinal tract (GIT) microbiome dysbiosis is associated with increased severity of type two diabetes mellitus (T2DM), yet interactions between diabetes medicines and bacterial communities are just being investigated. While metformin is the most widely prescribed drug for T2DM, its mechanism of action remains unclear. Mitochondrial function and AMPK activity in liver and skeletal muscle as well as facilitation of active glucagon‐like peptide‐1 (GLP‐1) secretion have been suggested as potential mechanisms 1. In contrast to oral dosing, intravenously administered metformin does not improve glucose metabolism, suggesting that the gastrointestinal tract may be the principal site of drug action.2 Recent studies suggest that metformin treatment does alter the human GIT microbiome and could be an important pharmacological effect of this drug.3, 4, 5
Another pharmaceutical approach to treat T2DM is to enhance GLP‐1 function, either by the administration of GLP‐1 peptide mimetics (ie liraglutide) or suppressing its degradation by dipeptidyl peptidase‐4 (DPP‐4) (ie saxagliptin, sitagliptin). Short‐chain fatty acids (SCFAs) produced through bacterial metabolism also induce GLP‐1 expression via binding with the G‐coupled protein receptor, FFAR2 (formerly GPR43). Studies in mice show that liraglutide but not saxagliptin alters the gut microbiome suggesting a relationship between GIT microbes and GLP‐1 agonism.6, 7 In patients with T2DM taking liraglutide, sitagliptin or placebo, only liraglutide increased serum levels of deoxycholic acid, a secondary bile acid produced by bacterial metabolism.8
Here, we describe the first comparative analysis of microbiome changes in a T2DM human cohort taking either metformin or liraglutide.9 Originally, these data were collected in a clinical study designed to test the efficacy and safety of a novel combination of four nutritional agents (GSK2890457) intended to be used as a metabolic potentiate in combination with existing treatments for obesity and T2DM (ClinicalTrials.gov NCT01725126). While GSK2890457 showed significant weight loss and reduction in glucose levels in diet‐induced obese mouse models, these preclinical effects did not translate into human. However, during this clinical trial, we found specific evidence for the novel effects of liraglutide and metformin on the human microbiome, which we present herein.
2. MATERIALS AND METHODS
2.1. Study design
Detailed methods are provided in Appendix S1. Briefly, the study population, clinical trial design and ethics statement (SMP116623; www.clinicaltrials.gov NCT01725126) were previously described.9 Subject enrolment was in accordance with ICH Good Clinical Practice guidelines, subject privacy requirements and the principles of the Declaration of Helsinki. One site participated in Part A (Quintiles Early Clinical Development, Overland Park, KS, USA), and two sites participated in Parts B and C (Elite Research Institute, Miami, FL, USA; Profil Institute for Clinical Research, Inc. Chula Vista, CA, USA). The study protocol was approved by Schulman Associates Institutional Review Board, and a copy is available as Appendix S1 in Hodge et al.9 All subjects provided written informed consent before enrolment.
T2DM subjects (n = 37) who were stable on metformin were randomized into two study arms, Part B and Part C (Figure 1A). Part A was comprised of only health volunteers. Microbiome analysis was not performed on these subjects; therefore, data pertaining to this subgroup are not presented here. After a single‐week familiarization period, Part B subjects (n = 19) were switched from oral metformin to subcutaneous once daily injections of liraglutide. Measurements and samples were taken after the completion of a twelve‐week stabilization period. Part C subjects (n = 18) remained on metformin for the duration of the study with a 4‐week stabilization period. The metformin‐liraglutide substitution design was used because a prestudy feasibility assessment determined that it was impossible to recruit sufficient subjects with liraglutide as their primary T2DM medication.9 After the stabilization period, subjects were further randomized to receive either placebo or GSK2890457, a mixture of four nutritional ingredients: oligofructosaccharide (OFS), apple pectin, black‐current extract and oleic acid in a ratio of 5:5:2:3. Each ingredient is a generally regarded as safe (GRAS) substance and present in normal diet. Subjects were fed standardized meals on pharmacodynamic profiling days. Age, sex, race, BMI, weight change and years duration of T2DM were recorded along with measured glucose levels.
Figure 1.
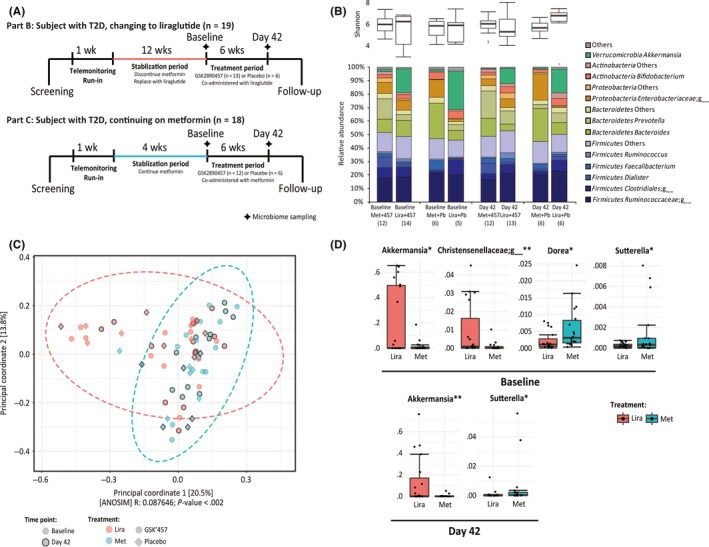
Difference in microbiome composition in metformin or liraglutide‐treated T2DM subjects. A, Study design and stool sampling periods at baseline and Day 42 as adapted from Hodge et al.9 B, Comparison of alpha diversity (Shannon) and major bacterial taxa between samples from liraglutide and metformin‐treated patients, both at baseline and Day 42 and in concert with GSK2890457 and placebo treatment. C, PCoA plot of all samples based on weighted UniFrac distance showed significant differential distribution of metformin and liraglutide groups. D, Significantly differentially represented bacterial genera between metformin and liraglutide groups, at baseline and Day 42
Considerable effort was made to standardize medications and diet as to minimize potential inter‐subject variation in pharmacodynamic and microbiome measurements. The study protocol has an extensive list of exclusion criteria based on co‐morbidity diseases and concomitant medications. Particularly relevant to reducing biases in measurements of the microbiome are the medications prohibited within 14 days to the randomization of placebo and GSK2890457 dosing which include dietary supplements, fibre supplements, oral antibiotics, bile acid sequestrants, protein‐pump inhibitors, H2 antagonists, probiotics, herbal and nutraceutical products intended to impact gut health and use of stomach “coating agents,” for example Pepto‐Bismol™ and Kaopectate™. Patients were excluded from the study if there was the presence or symptoms of an active infection at the time of enrolment. If an enrolled subject presented with an active infection 2 days prior to stool sampling, the visit was rescheduled once the signs/symptoms were absent for at least 5 days and/or antibiotic therapy ceased for at least 4 weeks.
At the beginning of the treatment period, all subjects were given a Block Brief Food Frequency Questionnaire (a validated questionnaire obtained from and analysed by Nutrition Quest Inc., Berkeley, CA, USA). Mann‐Whitney‐Wilcoxon FDR P‐values are nonsignificant for all diet variables in comparisons between subjects on liraglutide and metformin or subjects treated with either placebo or GSK2890457 (Appendix S2). Throughout the study, subjects were on the diet recommended by their primary care physician. Subjects were also made to visit the clinical centre during weeks 7, 14 and 28 for evaluation and monitoring compliance. Noncompliance with the medication, self‐monitoring and/or expected lifestyle regimens lead to subject exclusion from the study.
2.2. Microbiome sampling and analysis
Stool samples (n = 74) were taken just prior to commencement of placebo or GSK2890457 dosing (baseline or Day 0) and at the end of the 6‐week trial period (Day 42). Prior to the two stool collections, subjects were placed on a standardized diet beginning with dinner at Day ‐2 (2 days before stool sampling) and breakfast, lunch and dinner during Day ‐1 (1 day before stool sampling). Stool samples were collected with a sterile spoon, transferred into a prelabelled tube containing 8 mL of Stool DNA Stabilizer, mixed by shaking and then immediately stored frozen at −20°C prior to shipment. Once the samples arrived in the laboratory, they were immediately stored at −80°C until DNA extraction. Following DNA extraction from stool samples, multiplex barcoded primers were used for paired‐end sequencing of the 16S rRNA hypervariable V4 region on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) with the appropriate controls for detecting potential sample contamination.
2.3. Bioinformatics and statistical analysis
As previously described by us,4 quality filtering and analyses of multiplexed DNA sequencing reads were performed using QIIME 1.8.10 The remaining reads were subject to a 97% identity cut‐off open‐reference OTU picking using the UCLUST method.11 The OTU table was rarefied to a depth of 51 967 sequences. Beta diversity was estimated using the weighted UniFrac distance and visualized by principal coordinate analysis (PCoA). Analysis of similarities (ANOSIM) was used to assess the significance of separation between the tested groups of samples. Differentially represented bacterial genera for all comparisons were identified using edgeR in R bioconductor.12 Correlation between bacterial genera and patient demographic variables were calculated using Spearman Rank Correlation. Sequence data are available from the National Center for Biotechnology Information Sequence Read Archive (SRP107313).
3. RESULTS
High‐quality 16S rRNA gene sequencing data were obtained for all 74 stool samples. A total of 12 531 456 DNA sequencing reads were left after demultiplexing and quality control filtering. A rarefaction depth of 51 967 reads per sample was selected, and 11 800 OTUs were identified across 74 samples.
Comparisons of microbiome composition for samples collected at baseline or Day 42 showed markedly different microbial profiles between subjects switched to liraglutide (Part B) and those remaining on metformin (Part C), irrespective of placebo or GSK2890457 regimen (Figure 1B, Figure S1). PCoA of all samples based on weighted UniFrac distance showed significantly differential distribution of samples from liraglutide subjects compared to those taking metformin (Figure 1C, ANOSIM, P < .002), but not for other comparisons (ie baseline and Day 42, GSK2890457 and placebo). The similar distribution pattern was observed for both baseline and Day 42 samples (Figure S2). Comparisons of samples from subjects on metformin or liraglutide revealed significant differences in the relative abundances among several bacterial genera. At baseline, the genus Akkermansia and an unknown genus in the family Christensenellaceae showed a significant increase in liraglutide relative to metformin subjects and the genera Dorea and Sutterella increased significantly in metformin subjects (adj. P < .05, Figure 1D). At Day 42, when controlling for GSK2890457 and placebo treatments in the statistical model, a significant increase in Akkermansia and a significant decrease in Sutterella were observed in liraglutide relative to metformin subjects (adj. P < .05, Figure 1D). No significant differences in any bacterial genera were observed in GSK2890457 relative to placebo subjects. The Akkermansia difference remained significant in liraglutide compared with metformin at Day 42, in patient subgroups with GSK2890457 or placebo, respectively (adj. P < .05).
To assess the stability of microbiome over time, we looked for any significant changes in bacterial genera between baseline and Day 42 within their respective liraglutide and metformin groups. We found that bacterial compositions were highly consistent between the time points. No significant changes in any bacterial genera were observed among liraglutide or metformin group, whether co‐administered with GSK2890457 or placebo. Likewise, no significant changes in any bacterial genera were observed among GSK2890457 or placebo group, irrespective of the concurrent liraglutide or metformin treatment. This is consistent with the lack of overall clinical response to GSK2890457 treatment.9
No statistically significant correlations were found between bacterial phyla or genera abundances and patient epidemiological variables (gender, race, age, body mass index and weight change). However, baseline fasting 24‐hour glucose AUC levels were significantly positively correlated with the baseline relative abundance of Methanobrevibacter (Spearman's rho = 0.590; adj. P = .009, Figure S3A). Bacteroides and Akkermansia were significantly associated with patient duration of diabetes (adj P < .05, Figure S3B). Specifically, there was a significantly higher abundance of Akkermansia in patients with short and medium durations than those with long duration of diabetes.
4. DISCUSSION
Our study shows that metformin and liraglutide treatments are associated with distinct GIT microbiome communities in T2DM human subjects. In a previous study, we found significant differences in bacterial composition, serum bile acid levels and GLP‐1 associated with temporary metformin withdrawal in T2DM subjects.4 In a cross‐sectional study, de la Cuesta‐Zuluaga et al. compared microbiomes of 28 T2DM human subjects taking metformin to those of 84 nondiabetic participants and found enrichment of microbial species associated with short‐chain fatty acid production as well as the species Akkermansia municiphila.13 More recently, Wu et al. compared treatment‐naïve T2DM individuals taking either placebo or metformin and found significant changes in the genera Escherichia and Intestinibacter as well enrichment of Bifidobacterium in a subgroup of individuals switched onto metformin after 6 months.5 Akkermansia sp. were also found to be enriched in their study but only after a focused search for gene signatures in metagenomic data and Akkermansia sp. relative abundance was not found to be significantly correlated with % haemoglobin A1c. Therefore, the overall view of specific microbiota modulated by metformin in humans with T2DM is somewhat inconsistent across current studies.
Here, we found that subjects receiving a GLP‐1 agonist had higher Akkermansia abundances than those on metformin. We also showed a significant reduction in Akkermansia in patients with long durations of diabetes. Interestingly, metabolic syndrome subjects were found to have an enrichment of Sutterella and depletion of Akkermansia compared to healthy individuals, which is the opposite pattern we observed in liraglutide subjects.14 A. muciniphila has been shown to support the integrity of gut barrier function in rodents and likely plays a role in maintaining a healthy mucosal interface between the luminal cavity and the epithelial cellular layer.6, 15, 16 Increased abundance of Akkermansia sp. is correlated with elevated GLP‐1 levels in patients after gastric bypass surgery 17 and dietary intervention for obesity.18 Active GLP‐1 likely plays a broad role in supporting gut homoeostasis as prophylactic administration of liraglutide has been shown to be protective in a T cell‐driven adoptive transfer colitis mouse model.19 Our study suggests that GLP‐1 agonism, maintenance of gut homoeostasis and Akkermansia species occurrence are inter‐related factors for T2DM aetiology.
Our study has several caveats. First, the microbiome composition of patients at the onset of metformin‐liraglutide substitution is unknown, which could be important to further attribute the microbiota differences in our cross‐sectional comparison to the effect of liraglutide or metformin, or both. Ideally, one would also need to sample the microbiota in treatment‐naïve subjects to more precisely understand the effects of liraglutide and metformin treatments. Second, the 16S rRNA sequencing has limitations in understanding the species/strain level diversity and functional capability of the gut microbiota. Further studies with shotgun metagenomic profiling are necessary to investigate biomarkers of improved gut barrier function and hyperglycaemic control. Our work supports a growing body of evidence that metformin and GLP‐1 agonists modulate the gut microbiome. Further studies to better understand the role of GIT pharmacology in the mechanisms of action of these drugs will assist in the development of future T2DM therapies.
CONFLICT OF INTEREST
All authors were employees of GlaxoSmithKline during this study. This funding source had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
AUTHOR CONTRIBUTIONS
Z.W., D.K.R and J.R.B conceived and designed the study. S.VH., E.T., C.T. and G.S. performed DNA extractions from subject stool samples, 16S rRNA sequencing and initial data quality controls. Z.W. and S.S. performed in‐depth computational analysis. Z.W. and J.R.B. wrote the manuscript. All co‐authors commented, revised and approved the final version.
Supporting information
ACKNOWLEDGEMENTS
Contributions by subject volunteers to this study are gratefully acknowledged.
Wang Z, Saha S, Van Horn S, et al. Gut microbiome differences between metformin‐ and liraglutide‐treated T2DM subjects. Endocrinol Diab Metab. 2018;1:e9 10.1002/edm2.9
Funding information
Research was supported by GlaxoSmithKline.
REFERENCES
- 1. Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898‐1906. 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonora E, Cigolini M, Bosello O, et al. Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C‐peptide, glucagon and growth hormone in non‐diabetic subjects. Curr Med Res Opin. 1984;9:47‐51. 10.1185/03007998409109558. [DOI] [PubMed] [Google Scholar]
- 3. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262‐266. 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Napolitano A, Miller S, Nicholls AW, et al. Novel gut‐based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE. 2014;9:e100778 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment‐naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850_858. 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 6. Everard A, Belzer C, Geurts L, et al. Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci USA. 2013;110:9066‐9071. 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes. 2012;61:364‐371. 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smits MM, Tonneijck L, Muskiet MH, et al. Biliary effects of liraglutide and sitagliptin, a 12‐week randomized placebo‐controlled trial in type 2 diabetes patients. Diabetes Obes Metab. 2016;18:1217‐1225. 10.1111/dom.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodge RJ, Paulik MA, Walker A, et al. Weight and glucose reduction observed with a combination of nutritional agents in rodent models does not translate to humans in a randomized clinical trial with healthy volunteers and subjects with type 2 diabetes. PLoS ONE. 2016;11:e0153151 10.1371/journal.pone.0153151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7:335‐336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460‐2461. 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 12. Robinson MD, McCarthy DJ, Smyth GK. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139‐140. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de la Cuesta‐Zuluaga J, Mueller NT, Corrales‐Agudelo V, et al. Metformin is associated with higher relative abundance of mucin‐degrading Akkermansia muciniphila and several short‐chain fatty acid‐producing microbiota in the gut. Diabetes Care. 2017;40:54‐62. 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 14. Lim MY, You HJ, Yoon HS, et al. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2017;66:1031‐1038. 10.1136/gutjnl-2015-311326. [DOI] [PubMed] [Google Scholar]
- 15. Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107‐113. 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 16. Schneeberger M, Everard A, Gomez‐Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan M, Song MM, Bai RX, Cheng S, Yan WM. Effect of Roux‐en‐Y gastric bypass surgery on intestinal Akkermansia muciniphila . World J Gastrointest Surg. 2016;8:301‐307. 10.4240/wjgs.v8.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dao MC, Everard A, Aron‐Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426‐436. 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 19. Bang‐Berthelsen CH, Holm TL, Pyke C, et al. GLP‐1 induces barrier protective expression in Brunner's glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22:2078‐2097. 10.1097/MIB.0000000000000847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


