MOTION ARTIFACTS
Image artifacts caused by cardiac motion appear in different ways, such as blurred edges of anatomies, double contours, distorted shapes, or shadings. They result in images of nondi-agnostic quality and make any quantitative analyses inaccurate and difficult.
The degree and extent of artifacts are a complicated function of the insufficient temporal resolution of the system, the motion pattern of the heart, and the angles at which the heart is scanned. Our study using a digital phantom presented in [1] shows (Figure 1) that artifacts vary significantly depending on the temporal resolution and the scanning angles. Figures 1 and 2 also show that, contrary to conventional belief, a cardiac-gating window with a larger tail (base width) provides fewer image artifacts.
Fig 1.
Reconstructed images of computer-simulated phantom, 4-dimensional extended cardiac-torso phantom [1]. The numbers on the top are the scanner’s rotation speeds and the directions (scan angles) that roughly correspond to the center of the gating window. First, images reconstructed by a cardiac gating window with a wider tail (bottom row) can be seen to introduce fewer artifacts than those with a narrower tail (top row). Second, images with a slower scanner’s rotation speed (left) exhibited stronger motion artifacts than those with a faster speed (middle). Third, the direction and the strength of motion artifacts are affected by the angles in which the heart was scanned (middle vs right column).
Fig 2.
The strength of motion artifacts (ie, the mean and standard deviation of the root mean squared differences that result from the heart motion), measured by the extended cardiac-torso phantom. The measurements were repeated with different scan angles (see Fig 1). The extent of the motion artifacts decreases as the temporal resolution is improved (ie, the full width at half maximum of the cardiac-gating window is decreased). The degree of improvement, however, is not linear. Contrary to conventional belief, the cardiac-gating window with the maximum tail width provides slightly fewer motion artifacts than does the minimum tail width.
A process for reducing motion artifacts is as follows: First, always choose a protocol that provides the best temporal resolution (by a combination of faster scanner rotation speeds and multicycle algorithms [2,3]). Second, find a patient-specific cardiac phase with the least motion. Third, if artifacts are observed, try a wider gating window [4].
CALCIUM-BLOOMING ARTIFACTS
Calcium-blooming artifacts are shadings or smeared whitening typically seen near large amounts of calcium deposits in coronary arteries [5]. These artifacts create the potential risk for a false-positive reading of soft plaque near the calcium because of the decreased pixel values, or a false-negative reading if artifacts mask the plaque. The size of the remaining opening of the blood vessel may also be underestimated.
These artifacts are attributed to 3 factors: the partial-volume effect, beam hardening, and insufficient temporal resolution of the system. Thus, one can mitigate the problem by selecting the narrowest collimation possible, by applying beam-hardening correction and by choosing the protocol with the best temporal resolution.
BEAM-HARDENING ARTIFACTS
Shadings caused by beam-hardening artifacts may appear proximal to the left ventricle, aorta, spine, or ribs. The shadings are especially problematic because they resemble myocar-dial ischemia or perfusion defects [6].
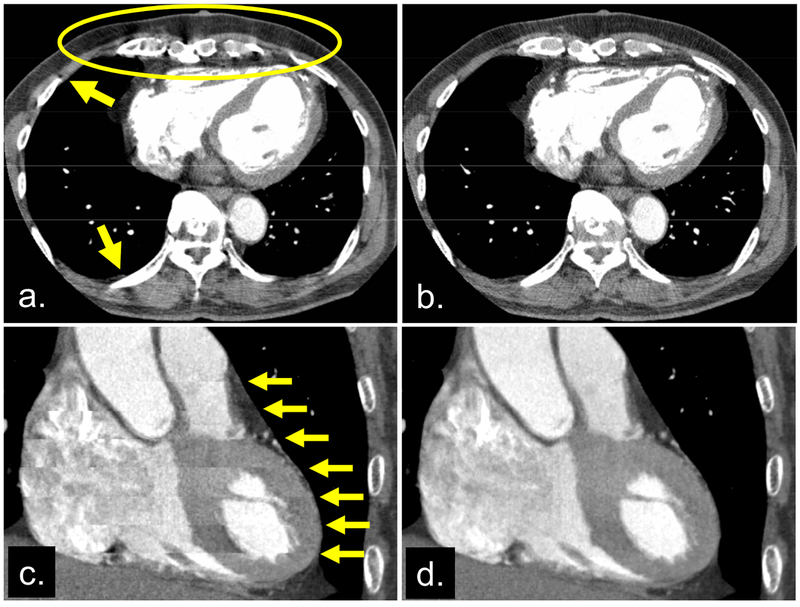
The cause of the artifacts is suboptimal beam-hardening correction. These artifacts are increased by the heart motion and cone-beam geometry because most correction methods have been developed for stationary objects and for fan-beam geometry. The correction may remain suboptimal because of movement of the heart, the nonuniform mixture of blood and iodine in the ventricles and atria (Figure 3), and the x-ray spectrum, which varies depending on the cone angle because of the heel effect.
Fig 3.
Images reconstructed from the same cone-beam projection data. Axial images reconstructed by the fan-beam algorithm (a) and the cone-beam algorithm (b), respectively. Shading artifacts near the stationary ribs (indicated by arrows in [a]) imply that shading in the circle might be due to cone-beam artifacts, not perfusion defects or motion artifacts. This point was proved by applying the cone-beam algorithm (that accurately takes into account the cone-angle) (b). Coronal images reconstructed without (c) and with (d) the cardiac banding artifact correction algorithm [2]. The image without correction (c) exhibited discontinuous banding artifacts (arrows), which are corrected in (d).
Nevertheless, we suggest using beam-hardening corrections implemented for CT scanners while using a protocol that provides the best temporal resolution and uses the smallest detector collimation.
CONE-BEAM ARTIFACTS
Shadings may appear near the spine and ribs when the cone-beam geometry is not correctly handled. The shadings are problematic because they make the detection of perfusion defects difficult (Figures 3a and 3b).
The use of an inaccurate image reconstruction method is usually the cause of the artifacts. If a substantial amount of shading is observed, users should pay attention to shading artifacts near the stationary ribs and spine, away from the heart, and choose a protocol with fewer detector rows (smaller detector apertures). Users should also consult the CT scanner manufacturer because such artifacts may indicate that an inaccurate reconstruction algorithm is implemented in the scanner.
BANDING (OR STEP) ARTIFACTS
Banding artifacts are longitudinal discontinuities that are easily observed in coronal, sagittal, or curved cross-sectional images (Figure 3c). These artifacts result in nondiagnostic images [2].
The cause of these artifacts is a combination of nonperiodic heart motion and a suboptimal gating scheme; most of the electrocardiographically gated image reconstruction methods assume that heart motion is quasiperiodic. The method used then patches data from several heartbeats to obtain a volume image of the entire heart. Thus, discontinuity will result if the heart motion is not periodic because of, for example, a variable heart rate.
Solutions include having the patient take a β-blocker to reduce heart rate variation and, if available, applying gating methods that are robust against heart rate variation [2,3] (Figure 3d) or that use images to find optimal gating phases specific to each heartbeat [7].
SUMMARY
We have reviewed 5 major artifacts that appear in cardiac CT images. We discussed the potential consequences and the causes of artifacts and suggested practical ways to mitigate the problems. With these in mind, one can maximize the potential use of emerging cardiac CT imaging applications.
Questions:
How critical is it to understand the major types of artifacts and their consequences in cardiac computed tomographic (CT) imaging? What are the causes of these artifacts, and how can they be reduced?
Answers:
X-ray cardiac CT imaging is ready for clinical routines, but it is still a relatively new imaging method, and there is much room for improvement. One cannot simply start up a CT scanner, press a few buttons, and expect to obtain beautiful, artifact-free images. Skilled users are needed to maximize the potential of cardiac CT imaging. Thus, it is critical to understand the major types of artifacts in cardiac CT images, their consequences and causes, and ways to mitigate the problems.
The 5 major artifact types are motion artifacts, calcium-blooming artifacts, beam-hardening artifacts, cone-beam artifacts, and banding (or step) artifacts. Below, we discuss each and provide suggestions on how to lessen them.
ACKNOWLEDGMENTS
We acknowledge financial support from the National Institutes of Health (grant R01 HL087918) and the American Heart Association (grant 0865315E). We are grateful to Drs Hirofumi Anno and Be-Shan Chiang for their help with Figure 3 and to Drs W. Paul Segars, George S. K. Fung, and BenjaminM. W. Tsui for their help with the extended cardiac-torso phantom and CT simulator.
REFERENCES
- 1.Segars WP, Mahesh M, Beck TJ, Frey EC, Tsui BMW. Realistic CT simulation using the 4D XCAT phantom. Med Phys 2008;35: 3800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taguchi K, Chiang BS, Hein IA. Direct cone-beam cardiac reconstruction algorithm with cardiac banding artifact correction. Med Phys 2006;33:521–39. [DOI] [PubMed] [Google Scholar]
- 3.Grass M, Manzke R, Nielsen T, et al. Helical cardiac cone beam reconstruction using ret rospective ECG gating. Phys Med Biol 2003;48:3069–84. [DOI] [PubMed] [Google Scholar]
- 4.Bruder HK, Stierstorfer K, Petersilka M, Suess C, Flohr TG, McCollough CH. Retrospective selection of temporal resolution and image noise in cardiac dual source CT (DSCT): applications in obese patients Presented at: Annual meeting of the Radiological Society of North America; 2007; Chicago, Ill. [Google Scholar]
- 5.Schoepf UJ, Becker CR, Ohnesorge BM, Yucel EK. CT of coronary artery disease. Radiology 2004;232:18–37. [DOI] [PubMed] [Google Scholar]
- 6.George R, Lardo A, Lima J. Added value of CT myocardial perfusion imaging. Curr Cardiovasc Imaging Rep 2008;1:96–104. [Google Scholar]
- 7.Sun Z, Segars WP, Fishman EK, Brinker JA, Taguchi K. Banding artifact reduction for cardiac CT In: Nuclear Science Symposium Conference Record, 2008. NSS ‘08 IEEE; 2008; Dresden, Germany; 2008:4838–9. [Google Scholar]