Abstract
New evidence suggests that low serum Vitamin D may cause nonalcoholic fatty liver disease (NAFLD). Hypovitaminosis D is associated with the severity and incidence of NAFLD. The objective of this study was to conduct a systematic review on randomized controlled trials (RCTs) assessing the effect of Vitamin D on serum metabolic profile among NAFLD patients. Databases including PubMed, Institute for Scientific Information Web of Science, Scopus, and Google Scholar were searched up to November 2016. RCTs which studied Vitamin D effect on metabolic profiles and liver function, and conducted among adults were included. Six articles were eligible to be considered in this systematic review. According to the result, Vitamin D supplementation might improve lipid profile and inflammatory mediators when compared with placebo. No article indicated significant effect of Vitamin D on liver enzymes except one article which revealed that Vitamin D together with calcium carbonate can reduce liver enzymes. Vitamin D supplementation may not improve anthropometric measures and glycemic index variables among patients with NAFLD. Vitamin D supplement might improve NAFLD symptoms, especially inflammatory mediators. More RCTs in different parts of world with different forms and doses of Vitamin D are necessary.
Keywords: Nonalcoholic fatty liver disease, systematic review, Vitamin D
Introduction
Nonalcoholic fatty liver disease (NAFLD) is considered as the most common cause of liver disease.[1] NAFLD has a broad spectrum of diseases that vary from nonalcoholic steatohepatitis (NASH) to fibrosis and cirrhosis.[2] The prevalence of NAFLD in the general population is 20%, but in patients with type 2 diabetes, it is 90%.[2,3] During the last decades, different pharmacological and nutraceutical interventions for the treatment of NAFLD were assessed, but none indicated significant results; therefore, no medically approved medicine for NAFLD is available.[4] Due to the fact that obesity causes inflammation and insulin resistance which are the main causes of NAFLD, weight reduction is considered as a standard treatment for this disease.[5]
New evidence suggested that low serum Vitamin D may cause metabolic diseases[6,7,8] and NAFLD.[9] Hypovitaminosis D is associated with the severity[9] and incidence of NAFLD among patients who have normal liver enzymes.[10] According to the result of a recent meta-analysis, patients with NAFLD have a 26% additional risk for Vitamin D deficiency as compared to controls.[11]
Vitamin D through Vitamin D receptor (VDR) can affect the liver. VDR exists in hepatic cells, and its expression can reduce inflammation in chronic hepatic diseases.[12] In vitro studies indicated that VDR by the enhancement of glucose transporter-4 muscular expression and modulation of free fatty acids (FFAs) increased insulin sensitivity.[13] Vitamin D also has antifibrotic, antiproliferative, and anti-inflammatory effects on the liver.[14] Furthermore, Vitamin D can reduce the concentration of cytokeratin 18 apoptotic fragment M30 as a marker of hepatic damage.[15,16,17,18]
Moreover, some articles indicated that low concentration of serum 25-hydroxyvitamin D (25(OH)D) enhances the body, which is a risk factor for hepatic steatosis.[2] Animal studies have shown that high level of calcium can reduce body weight through the reduction of fatty acid synthesize and the enhancement of lipolysis.
All these effects make Vitamin D a promising supplement for the treatment of NAFLD by the inhibition of lipid accumulation in liver cells. Studies that assessed the effects of Vitamin D on NAFLD have controversies. Therefore, through a systematic review, the authors proposed to assess the effectiveness of Vitamin D in the treatment of NAFLD.
Methods
Systematic search was performed up to July 2016 by searching databases, including PubMed, Institute for Scientific Information Web of Science, Scopus, and Google Scholar. A combination of two groups of MeSH and non-MeSH terms was used. These terms included: (1) “Vitamin D,” “calcitriol,” “Cholecalciferol,” “Vitamin D 3,” “Cholecalciferols,” “Cholecalciferol,” “1 alpha, 25-Dihydroxyvitamin D3,” “alpha, 25-Dihydroxyvitamin D3, 1”, “1, 25-Dihydroxyvitamin D3,” “D3, 1, 25-Dihydroxyvitamin,” “1 alpha, 25-Dihydroxycholecalciferol,” “1 alpha, 25 Dihydroxycholecalciferol,” “1, 25-Dihydroxycholecalciferol,” and (2) “Non-alcoholic Fatty Liver Disease,” “Non alcoholic Fatty Liver Disease,” “NAFLD,” “Nonalcoholic Fatty Liver Disease,” “Fatty Liver, Nonalcoholic,” “Liver, Nonalcoholic Fatty,” “Nonalcoholic Fatty Liver,” “Nonalcoholic Steatohepatitis,” “Steatohepatitides, Nonalcoholic,” and “Steatohepatitis.” To search the exact expression or terms, quotation marks were used; to locate a group of search terms, parentheses were used; and asterisks were used to search all the words derived from one key word, respectively. Boolean operators (AND and OR) were used for designing the search strategy. The search was performed without any limitation on language, publication time, and study design. Software endnote version X6 was used for managing the search results.
To find relevant articles, title and/or the abstract of all articles were screened. Furthermore, reference lists of all found articles were searched to find further relevant articles. Discrepancies were solved by a group discussion.
Inclusion criteria
Articles were considered in our review only if they satisfied the following criteria: (1) original article; (2) designed as a controlled trial; (3) conducted on a human of age 18 years or more; (4) used Vitamin D for intervention; (5) study outcomes: fatty liver enzyme, lipid profile, anthropometric index, and glycemic index; and (6) intervention or control group did not receive any food or supplement.
Exclusion criteria
Articles were excluded if they had one of the exclusion criteria such as (1) articles with unclear table or not reporting values for leptin; (2) used Vitamin D in the control group; (3) used other supplements beside Vitamin D in the intervention group; and (4) took Vitamin D through diet.
Data extraction
The primary extracted data was the efficacy of Vitamin D in NAFLD including (a) lipid profile: Low-density lipoprotein (LDL), high-density lipoprotein (HDL), triacylglycerol (TG), and total cholesterol (TC); (b) anthropometric variables: body weight, body mass index (BMI), fat mass, waist-to-hip ratio (WHR), waist circumference (WC); (c) glycemic index: insulin, fasting blood sugar (FBS), homeostatic model assessment-insulin resistance (HOMA-IR); and (d) liver enzyme: alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The secondary data was first author's name, publication year, study location, sample size in each group, gender, age, disease, type of placebo, and intervention duration. Any disagreement was solved by group discussion and unclear data was clarified by contacting the corresponding author.
Quality assessment
Assessing the risk of bias for articles was performed by Cochrane collaboration's tool.[17] The bias of six trials in the study and outcome level were assessed with the following criteria: random sequence generation, allocation concealment, blinding of the examiner and/or patient, blinding of outcome assessment, incomplete data outcome, selective outcome reporting, and other sources of bias [Figure 1]. Each article was rated “low,” “high,” and “unclear.” “Low” risk meant performing appropriate blinding and random method. “High” risk referred to no blinding, no allocation concealment, and incorrect random method. “Unclear” referred to no description in the text to assess risk.
Figure 1.
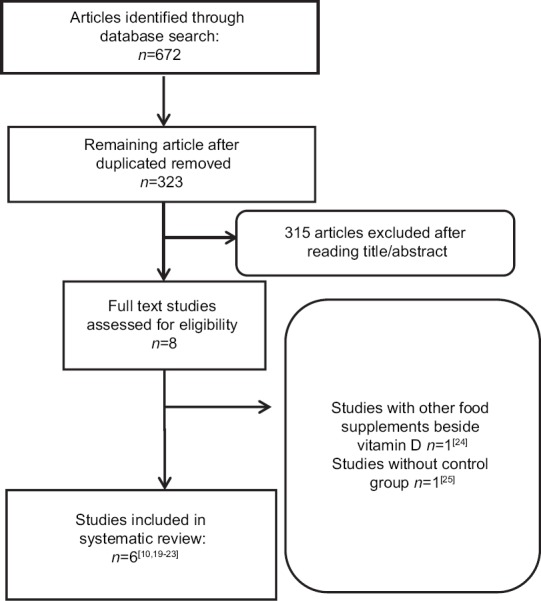
Study selection process
Results
Database search
The initial database search retrieved 672 articles. After removing duplicate articles, 322 were left. By reading titles and abstracts, 315 articles were removed and 8 were selected. Six articles fulfilled the criteria for inclusion[10,19,20,21,22,23,24,25] and two were removed due to the following reasons: using other food supplements beside Vitamin D[24] and not having control group[25] [Figure 1].
Characteristics of included studies
Among the six included articles, one trial was only on type 2 diabetic patients diagnosed with NAFLD. In four trials, Vitamin D was administrated at a dose of 50000 IU every week and 2 weeks, in one trial, 2000 IU/day, and in other trial, 25 μg/day. Intervention duration lasted from 6 to 24 weeks. In all the studies, the participants were asked to maintain their usual lifestyle and habitual dietary intake. Participants in the trials were adults and studies were conducted on both men and women [Table 1].
Table 1.
Randomized controlled trial studies included in the systematic review and meta-analysis
| First author (year), country | Subjects and gender | Age | Study design | Duration (week) | Intervention | Control | Subjects | Jadad score | Results |
|---|---|---|---|---|---|---|---|---|---|
| Barchetta, Italy[10] | n=55 | 57.4±10.7* | Randomized, double-blind, placebo- controlled trial | 24 | Cholecalciferol (2000 IU/day) | Not mention | Type 2 diabetic patients with NAFLD | 2 | There were not any significant differences in anthropometric and metabolic variables |
| Female=20 | |||||||||
| Male=35 | |||||||||
| Foroughi, Iran[20] | n=60 | 48.5±4.8 | Randomized double-blind placebo- controlled clinical trial | 10 | 50,000 IU Vitamin D every week | Not mention | Nonalcoholic fatty liver patients | 3 | Anthropometric and metabolic variables did not change significantly, but TG and CRP decreased significantly in intervention group |
| Female=31 | |||||||||
| Male=29 | |||||||||
| Foroughi, Iran[19] | n=60 | 46.7±5.1 | Randomized double-blind placebo- controlled clinical trial | 6 | 50,000 IU Vitamin D every week | Not mention | Nonalcoholic fatty liver patients | 2 | FBS and HOMA-IR decreased in the intervention group compared to the control group |
| Female=31 | |||||||||
| Male=29 | |||||||||
| Lorvand Amiri, Iran[21] | n=73 | 44±10.8 | Randomized, placebo- controlled, double-blind clinical trial | 12 | 25 µg calcitriol/day | Not mention | Nonalcoholic fatty liver patients | 2 | FBS, WHR, HOMA-IR, TG, ALT, and AST decreased significantly and HDL increased significantly |
| Female=34.3% Male: 64.7% | |||||||||
| Sharifi, Iran[23] | n=26 | 31-48 | Double-blind placebo- controlled study | 16 | 50,000 IU vitamin D3 every 14 days | Not mention | NAFLD | 3 | TC, LDL-C, and CRP decreased significantly in intervention group but other metabolic variables and anthropometric measures did not change significantly |
| Male=0 | |||||||||
| Female=27 | |||||||||
| Sharifi, Iran[23] | n=26 | 31-48 | Double-blind placebo- controlled study | 16 | 50,000 IU vitamin D3 every 14 days | Not mention | NAFLD | 3 | TC decreased significantly in intervention group but other metabolic variables and anthropometric measures did not change significantly |
| Male=26 | |||||||||
| Female=0 | |||||||||
| Sharifi, Iran[22] | n=53 | 40.33±8.65 | Double-blind, randomized- controlled design | 16 | 50,000 IU vitamin D3 every 14 days | Not mention | NAFLD | 2 | MDA decreased significantly and CRP indicated near significant changes in intervention group |
| Female=26 | |||||||||
| Male=27 |
*Mean±SD. TC=Total cholesterol, LDL-C=Low-density lipoprotein cholesterol, TG=Triglycerides, HDL=High-density lipoprotein, CRP=C-reactive protein, MDA=Malondialdehyde, WHR=Waist-hip ratio, FBS=Fasting blood sugar, HOMA-IR=Homeostatic model assessment-insulin resistance, ALT=Alanine aminotransferase, AST=Aspartate aminotransferase, NAFLD=Nonalcoholic fatty liver disease, SD=Standard deviation
Quality of the included studies
The quality of the included studies is from moderate-to-high quality according to the Cochrane risk of bias assessment tool. The summary of quality assessment domains of included studies is shown in Figure 2.
Figure 2.
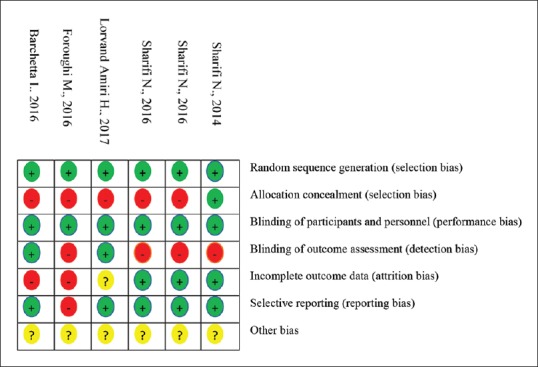
Risk of bias assessment for RCTs
Anthropometric variables
Out of six eligible studies, five reported on BMI[10,20,21,22,23] and three reported on WC,[10,22,23] fat mass, weight, and WHR.[21,22,23] Overall, 270 participants were assessed on BMI (range, 26–73) and 154 were assessed on fat mass, weight, and WHR (range, 26–73) and 134 were assessed on WC (range, 26-55). Intervention duration lasted for 10–24 weeks for BMI, 12–16 weeks for fat mass, weight, and WHR, and 16–24 weeks for WC. All the trials were on obese participants with BMI ≥30. The study design was randomized, double-blind, placebo-controlled trial for all articles. Vitamin D supplement for WC was in cholecalciferol form, but for other anthropometric variables, it was in both calcitriol and cholecalciferol form.
In one study by Barchetta et al.,[10] type 2 diabetic patients with NFLD took 2000 IU/day Vitamin D for 24 weeks. In this randomized, double-blind, placebo-controlled trial, Vitamin D could not reduce WC and BMI. Foroughi et al.[20] indicated that taking 50000 IU/week Vitamin D could not change BMI. In this study, patients with NAFLD took Vitamin D supplement at dose of 50000 IU/week for 10 weeks. The authors measured BMI before and after intervention and their results revealed that Vitamin D did not change BMI significantly in comparison with the control group. In another double-blind placebo-controlled trial by Sharifi et al.,[23] the result of taking Vitamin D supplementation was reported on men and women separately. Patients with NAFLD took 50000 IU Vitamin D3 every 14 days for 16 weeks. At the end of the study, within- and between-group comparisons for each subgroup of men and women for weight, body fat percentage, and BMI was not significant. However, decreases in BMI, WC, and weight were more pronounced among men in the Vitamin D group. Taking 25 μg calcitriol/day for 12 weeks decreased WHR among NAFLD patients, but fat mass, weight, and BMI did not change significantly.[21] In another trial by Sharifi et al.,[22] it was indicated that 50000 Vitamin D3 every 15 days for 16 weeks could not change fat mass, weight, BMI, WC, and WHR.
Lipid profile variables
Out of the 6 selected RCTs, four studies measured TG[10,20,21,23] and three measured low-density cholesterol (LDL-c), high-density cholesterol (HDL-c), and TC.[10,21,23] Totally, 277 patients with NAFLD were enrolled in these studies. Two studies had intervention duration ≥16;[10,23] in these studies, Vitamin D dose was 2000 IU/day and 50,000 IU every 14 days. In one trial with duration of 24 weeks, 55 patients (female = 20, male = 35) with type 2 diabetic and NAFLD were allocated to intervention and placebo groups.[10] Participants in the intervention group took 2000 IU/day cholecalciferol. Their results after 24 weeks indicated no significant effect on LDL, HDL, TG, and TC. Another study with duration ≥16 weeks revealed reduced effect of Vitamin D on TC among male and female and LDL-c among female.[23] In this double-blind placebo-controlled study, 57 NAFLD patients were allocated to intervention group to receive 50,000 IU Vitamin D3 every 14 days for 16 weeks (n = 27, male = 13 and female = 14), or placebo group (n = 26, male = 13 and female = 13). The authors reported Vitamin D effect separately on men and women, and they believed that Vitamin D may respond differently among men and women regarding lipid profile.
In the study of Lorvand Amiri et al.,[21] Vitamin D was used in the form of calcitriol. Participants were assigned to Vitamin D (n = 37), calcium carbonate + Vitamin D 25 μg calcitriol (n = 37), and placebo (n = 36) groups. After 12-week intervention, there was significant difference in HDL-c and TG between the three groups. Calcium carbonate with Vitamin D showed a significant increase in HDL levels in comparison with calcitriol group. A study by Foroughi et al.[20] assessed Vitamin D effect on TG as a cardiometabolic biomarker. This randomized, double-blind, placebo-controlled clinical trial involved 60 patients with NAFLD, allocated equally to two Vitamin D and placebo groups. During 10 weeks, participants in the Vitamin D group received 50,000 IU Vitamin D weekly. After 10 weeks, significant decrease in TG was seen in Vitamin D group when compared with baseline values.
Liver enzymes
In total, five studies[10,20,21,22,23] assessed the effect of Vitamin D on liver enzymes. Five studies reported ALT and AST; one study reported ALT, AST, and alkaline phosphatase (ALP)[22] and one reported on gamma-glutamyltransferase (γ-GT), ALT, and AST.[10] Overall, 293 patients (ranging from 52 to 73) were assessed on ALT and AST, 53 patients on ALT, AST, ALP, and 55 patients on γ-GT, ALT, and AST. The study duration lasted for 10–24 weeks. Vitamin D dose ranged from 25 μg/day in the form of calcitriol to 50000 IU in the form of cholecalciferol every 2 weeks. Two studies which used 50000 IU Vitamin D every 14 days for 16 weeks did not show any significant effects on liver enzymes.[22,23] Both studies were performed by Sharifi et al., in one study, Vitamin D effect on liver damage was assessed by measuring ALT, AST,[23] and in the other study, in addition to ALT and AST, ALP was measured.[22] In these studies, Vitamin D supplement could not change any of the liver enzymes. In a study by Barchetta et al.[10] using Vitamin D3 (2000 IU/day) for a long duration (24 weeks) could not change the result of two previous studies. In another study by Foroughi et al.,[20] 50,000 IU/week Vitamin D could not change liver enzyme. In this study, participants with NAFLD took Vitamin D every week for 10 weeks, but after 10 weeks, their result on liver enzymes (ALT, AST) was still nonsignificant. In a study by Lorvand Amiri et al.,[21] taking 25 μg/day Vitamin D with 500 mg calcium carbonate and without calcium carbonate could decrease ALT and AST when compared with the placebo. According to their result, Vitamin D with calcium carbonate was more effective in liver enzyme reduction in comparison with Vitamin D alone.
Glycemic Index
Out of six selected articles, 5 reported Vitamin D effect on HOMA-IR,[10,19,20,21,22] 4 assessed Vitamin D effect on fasting blood sugar (FBS),[10,19,21,23] and 3 measured insulin.[10,19,21] In total, 293 patients were assessed on HOMA-IR, 241 on FBS, and 188 on insulin.
Among all the articles, one reported significant reduction in FBS, insulin, and HOMA-IR[21] and one indicated marginally significant reduction in FBS and HOMA-IR.[19] Other studies revealed nonsignificant effect of Vitamin D on the glycemic index. In a study by Lorvand Amiri et al.,[21] 25 μg/day calcitriol for 16 weeks could decrease FBS, insulin, and HOMA-IR, while in other studies with same duration, 50,000 IU Vitamin D3 every 14 days could not change HOMA-IR and FBS. In Barchetta et al.,[10] study, intervention duration was 24 weeks and Vitamin D3 dose was 2000 IU/day. In this study, after 24 weeks, there was no significant change in FBS, insulin, and HOMA-IR. In Fouroughi's study,[19] sixty patients with NAFLD were enrolled in the intervention (n = 30, Vitamin D3 50000 IU/week) and placebo groups (n = 30). Patients took Vitamin D supplements or placebo every week for 6 weeks. Their result at the end of the study revealed marginally significant changes in FBS and HOMA-IR.
Inflammatory Mediators
Four studies measured Vitamin D effect on C-reactive protein (CRP)[10,20,22,23] as a variable for assessing anti-inflammatory effect of Vitamin D and one study measured both tumor necrosis factor-α (TNF-α) and CRP.[22] Three studies indicated significant reduction in CRP after taking Vitamin D[20,22,23] and one study did not indicate any significant effect.[10] In total, 220 participants were assessed on CRP and 55 were assessed on TNF-α. Intervention duration lasted for 10–24 weeks. In a double-blind, randomized-controlled study by Sharifi et al.,[22] anti-inflammatory effect of vitamin was indicated by CRP reduction. In this study, 53 NAFLD patients (female = 26 male = 27) were assigned to intervention (n = 27) and placebo (n = 26) groups. Participants in the intervention group took 50,000 IU Vitamin D3 every 14 days. For assessment of Vitamin D effect on inflammation, CRP and TNF-α were measured before and after the intervention. Their result indicated that after 16 weeks of intervention, TNF-α did not change, but CRP decreased significantly.
In another study by Sharifi et al.,[23] taking 50,000 IU Vitamin D for 16 weeks significantly reduced CRP in women, but results among men were nonsignificant. Participants with NAFLD were randomly assigned to take one pearl of Vitamin D consisting of 50,000 IU Vitamin D3 (n = 27, 13 males and 14 females) or a placebo (n = 26, 13 males and 13 females) every 14 days for 16 weeks. Data analysis separately between men and women showed different effects of Vitamin D on CRP among male and female. In another randomized, double-blind placebo-controlled clinical trial by Foroughi et al.,[20] anti-inflammatory effect of Vitamin D was indicated by CRP reduction. NAFLD patients were assigned to Vitamin D (n = 30 one pearl of Vitamin D consisting of 50,000 IU Vitamin D every week for 10 weeks) and placebo groups (n = 30). After 10 weeks, there was a significant reduction in CRP concentration. Although there was a long intervention duration in Barchetta et al.,[10] study, taking daily 2000 IU Vitamin D for 24 weeks could not reduce CRP.
Discussion
In the present systematic review, it was found that Vitamin D supplementation might improve lipid profile and inflammatory mediators when compared with placebo. No article indicated significant effect of Vitamin D on liver enzymes except one which revealed that Vitamin D together with calcium carbonate can reduce liver enzymes. It was also found that Vitamin D supplementation may not improve anthropometric measures and glycemic index variables among patients with NAFLD.
According to the result of recent systematic reviews and meta-analysis on cross-sectional and case–control studies, patients with NAFLD had significantly lower levels of 25(OH)D than controls.[11,26] Scientists believe that lower levels of 25(OH)D in patients with NAFLD might contribute to the progression of NAFLD. The exact mechanism of Vitamin D deficiency and NAFLD is not fully indicated.
One animal study indicated that feeding obese rats with a Vitamin D-deficient Western diet can deteriorate the progress of NAFLD. Authors suggested that the enhancement of inflammation might cause the progress of NAFLD.[27] Another animal study revealed that increasing serum 25(OH)D levels by phototherapy can inhibit the development of NAFLD in rat models by the reduction of hepatocyte inflammation, apoptosis, and fibrosis.[28] Furthermore, Vitamin D, through its effect on peroxisome proliferator-activated receptor, can modulate FFA metabolism, thereby Vitamin D reduces FFA-induced insulin resistance in bloodstream. Hence, Vitamin D deficiency due to FFAs enhancements in bloodstream and hepatocytes can deteriorate the progression of NAFLD.[10] The result of our systematic review might prove this hypothesis. Out of the four studies that measured Vitamin D effect on inflammatory mediators, three indicated significant reduction in inflammation. In a study by Barchetta et al.,[10] using 2000 IU/day cholecalciferol could not change inflammatory mediators. Participants in this trial were type 2 diabetic patients with NAFLD, while other studies were on NAFLD patients without other diseases. Different study population may cause nonsignificant effect of Vitamin D on inflammatory mediators.
New evidence revealed that Vitamin D can prevent hepatic stellate cell (HSC) activation which increases cellular transformation and proliferation.[29] Animal study showed that Vitamin D effect on HSC proliferation is associated with antifibrotic effects.[30] One in vitro study also indicated the inhibition of HSCs activity by Vitamin D even with FFAs enhancement.[14] The expression of VDR in inflammatory cells of liver biopsies was determined among patients with NASH and result showed polymorphism of VDR gene, which showed that Vitamin D synthesis and activation might be associated with the status of Vitamin D and NAFLD development.[31,32] In a study by Lorvand Amiri et al.,[21] using Vitamin D in the form of calcitriol was more effective in comparison with other studies that used Vitamin D in the form of cholecalciferol. Out of five studies that assessed Vitamin D effect on liver enzymes, only that of Lorvand Amiri et al., indicated that Vitamin D supplement can reduce liver enzymes. Using Vitamin D in active form might cause useful effect of Vitamin D on liver enzymes.
Obesity can be considered as a risk factor for NAFLD.[33] There is a positive correlation between BMI enhancement and increased NAFLD incidence.[33] Therefore, measuring the anthropometric variables changes has an important role in assessing Vitamin D efficacy in NAFLD treatment. According to the result of a recent meta-analysis, Vitamin D deficiency is associated with BMI enhancement.[34] The results of the current study indicated that Vitamin D could not change anthropometric indexes among NAFLD patients. The result of one meta-analysis indicated that Vitamin D in the absence of calorie restriction cannot reduce adiposity measures.[35] No study in this systematic review used weight reduction diet together with Vitamin D supplement. All the studies that evaluated Vitamin D effect on lipid profile revealed significant effect of Vitamin D on at least, lipid profiles variables, except Barchetta et al. study, which reported nonsignificant effect of Vitamin D on lipid profile. The study population in this study was different in comparison with other trials. All the studies were performed in Iran, but Barchetta's study was performed in Italy. According to molecular evidence, polymorphisms of VDR gene is significantly different between different populations.[36]
This review has certain limitations. First of all, using liver enzymes as markers for NAFLD diagnosis is controversial, and there are no other noninvasive methods to use in trials. The limited number of included studies was also another limitation. Third, most of the included studies were performed in Iran, therefore, the effect of VDR polymorphism was not considered. Effects of different doses of Vitamin D might be remarkable, but dose was the same in most of the articles. The effect of sex was not investigated in this review because only one study reported its result separately on female and male.
Conclusions
This review indicated that Vitamin D supplement might improve NAFLD by inflammation reduction, but the exact mechanism of inflammation reduction on NAFLD was not found. It seems Vitamin D in active form might directly affect NAFLD development. More RCTs in different parts of the world with different forms and doses of Vitamin D is necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ (Clin Res Ed) 2014;349:g4596. doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 4.Del Ben M, Polimeni L, Baratta F, Pastori D, Loffredo L, Angelico F, et al. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:8341–50. doi: 10.3748/wjg.v20.i26.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: The dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 6.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A Cross-Sectional Study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 7.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barchetta I, De Bernardinis M, Capoccia D, Baroni MG, Fontana M, Fraioli A, et al. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One. 2013;8:e68689. doi: 10.1371/journal.pone.0068689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–24. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, et al. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:246–54. doi: 10.1111/apt.12377. [DOI] [PubMed] [Google Scholar]
- 12.Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: Relationship with liver histology and Vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–7. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- 13.Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X, et al. 1,25-dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev. 2008;24:459–64. doi: 10.1002/dmrr.873. [DOI] [PubMed] [Google Scholar]
- 14.Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–37. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology. 2009;50:1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joka D, Wahl K, Moeller S, Schlue J, Vaske B, Bahr MJ, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55:455–64. doi: 10.1002/hep.24734. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–2. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, et al. No effects of oral Vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92. doi: 10.1186/s12916-016-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foroughi M, Maghsoudi Z, Askari G. The effect of Vitamin D supplementation on blood sugar and different indices of insulin resistance in patients with non-alcoholic fatty liver disease (NAFLD) Iran J Nurs Midwifery Res. 2016;21:100–4. doi: 10.4103/1735-9066.174759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G. Effect of Vitamin D supplementation on C-reactive protein in patients with nonalcoholic fatty liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- 21.Lorvand Amiri H, Agah S, Tolouei Azar J, Hosseini S, Shidfar F, Mousavi SN, et al. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin Nutr. 2017;36:1490–7. doi: 10.1016/j.clnu.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does Vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease?. A randomized clinical trial. Endocrine. 2014;47:70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi N, Amani R, Hajiani E, Cheraghian B. Women may respond different from men to Vitamin D supplementation regarding cardiometabolic biomarkers. Exp Biol Med (Maywood) 2016;241:830–8. doi: 10.1177/1535370216629009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Della Corte C, Carpino G, De Vito R, De Stefanis C, Alisi A, Cianfarani S, et al. Docosahexanoic acid plus Vitamin D treatment improves features of NAFLD in children with serum Vitamin D deficiency: Results from a single centre trial. PLoS One. 2016;11:e0168216. doi: 10.1371/journal.pone.0168216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papapostoli I, Lammert F, Stokes CS. Effect of short-term Vitamin D correction on hepatic steatosis as quantified by controlled attenuation parameter (CAP) J Gastrointestin Liver Dis. 2016;25:175–81. doi: 10.15403/jgld.2014.1121.252.cap. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Li W, Zhang Y, Yang Y, Qin G. Association between Vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Results from a meta-analysis. Int J Clin Exp Med. 2015;8:17221–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, et al. Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol. 2016;7:498. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, et al. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol. 2011;55:415–25. doi: 10.1016/j.jhep.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Seydel S, Beilfuss A, Kahraman A, Aksoy K, Gerken G, Akkiz H, et al. Vitamin D ameliorates stress ligand expression elicited by free fatty acids in the hepatic stellate cell line LX-2. Turk J Gastroenterol. 2011;22:400–7. doi: 10.4318/tjg.2011.0254. [DOI] [PubMed] [Google Scholar]
- 30.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–58. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of Vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grünhage F, Hochrath K, Krawczyk M, Höblinger A, Obermayer-Pietsch B, Geisel J, et al. Common genetic variation in Vitamin D metabolism is associated with liver stiffness. Hepatology. 2012;56:1883–91. doi: 10.1002/hep.25830. [DOI] [PubMed] [Google Scholar]
- 33.Chang Y, Jung HS, Yun KE, Cho J, Ahn J, Chung EC, et al. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity (Silver Spring) 2016;24:1996–2003. doi: 10.1002/oby.21580. [DOI] [PubMed] [Google Scholar]
- 34.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and Vitamin D deficiency: A systematic review and meta-analysis. Obes Rev. 2015;16:341–9. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 35.Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: A systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014;15:528–37. doi: 10.1111/obr.12162. [DOI] [PubMed] [Google Scholar]
- 36.Fan L, Tu X, Zhu Y, Zhou L, Pfeiffer T, Feltens R, et al. Genetic association of Vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the chinese. J Gastroenterol Hepatol. 2005;20:249–55. doi: 10.1111/j.1440-1746.2005.03532.x. [DOI] [PubMed] [Google Scholar]


