Abstract
Oncology and cerebrovascular disease constitute two of the most common diseases afflicting the central nervous system. Standard of treatment of these pathologies is based on multidisciplinary approaches encompassing combination of interventional procedures such as open and endovascular surgeries, drugs (chemotherapies, anti-coagulants, anti-platelet therapies, thrombolytics), and radiation therapies. In this context, therapeutic ultrasound could represent a novel diagnostic/therapeutic in the armamentarium of the surgeon to treat these diseases. Ultrasound relies on mechanical energy to induce numerous physical and biological effects. The application of this technology in neurology has been limited due to the challenges with penetrating the skull, thus limiting a prompt translation as has been seen in treating pathologies in other organs, such as breast and abdomen. Thanks to pivotal adjuncts such as multiconvergent transducers, magnetic resonance imaging (MRI) guidance, MRI thermometry, implantable transducers, and acoustic windows, focused ultrasound (FUS) is ready for prime-time applications in oncology and cerebrovascular neurology. In this review, we analyze the evolution of FUS from the beginning in 1950s to current state-of-the-art. We provide an overall picture of actual and future applications of FUS in oncology and cerebrovascular neurology reporting for each application the principal existing evidences.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-00683-3) contains supplementary material, which is available to authorized users.
Key Words: Neuro-oncology, cerebrovascular neurology, focused ultrasound, therapeutic ultrasound, neurosurgery
Introduction
Ultrasound (US) is defined as mechanical waves with frequency higher than human audible sound (conventionally 20,000 Hz) able to travel through all types of matter: gases, liquids, solids, and plasma. Mechanical waves can subsequently be reflected, refracted, or attenuated by the medium.
The history of US application dates back to 1880, when Curie brothers described for the first time the piezoelectric effect [1]. Their outstanding study demonstrated that some crystalline materials, when subjected to mechanical pressure, generate an electric potential. Furthermore, when these materials are exposed to rapidly changing electric potential, a mechanical vibration is generated which consequently led to the concept of US.
The first practical application of reverse piezoelectric effect consisted of a sandwich of quartz crystals engineered to reveal submarines during World War I [2]. Since then, US has been used in a number of fields, ranging from everyday use devices to industrial and medical applications. Undeniably, the most relevant application of US in medicine is represented by its use for diagnostic imaging: diagnostic US is non-invasive, real-time in nature, and has a high spatial and temporal resolution with the possibility to conduct multimodal studies [3]. The second, less widespread, but rapidly growing medical application is represented by therapeutic US. The first study focusing on this application date back in 1938, from Raimar Pohlman, who described the “therapeutic effect” of ultrasound waves when exposing human tissue to US. Few years later, Jerome Gersten described the US treatment of muscle disorders and rheumatoid arthritis [4] while other groups described the treatment of Meniere’s disease and gastric ulcer [5, 6]. Lynn and Putnam, in 1942, treated 37 animals with high-frequency and short-wavelength ultrasound waves. They obtained highly specific damage in targeted cortical and subcortical regions in the brain with only marginal effects on the neighboring areas; they observed both reversible and irreversible neurological deficits [7]. William Fry and Russell Meyers [8] demonstrated the possibility to treat patients affected by different brain pathologies, and in particular Parkinson’s disease, using focused ultrasound (FUS) performed through a craniotomy.
On the basis of these successes, in the 1970s, a computer-controlled focus ultrasound system guided by B-mode ultrasound was engineered (“The Candy machine”) in order to treat brain tumor patients. This device could not be used through the skull and its application required a craniectomy. Treatment was then performed through the skin once healed. The results were controversial, although the safety and feasibility of the procedure was demonstrated [9].
Relying on these preliminary experiences, in 1980s, a new FUS device guided by computed tomography (CT) or magnetic resonance imaging (MRI) was introduced but its application was limited to animal (canine) models of disease.
The real limitation for FUS treatment was the presence of the skull and consequently the need for a craniectomy. Indeed, the skull distorts the US waves, absorbs energy (leading to skull heating), and attenuates the US beam. In the following years, numerous experiments tried to overcame skull limitations leading to the introduction of phased arrays US transducers and the use of pre-operative CT scan to take into account the distortion [10].
The second major improvement in FUS was the introduction of MRI guidance. MRI has an excellent spatial resolution and sensitivity and allows to assess tumor volumes. Furthermore, it permits to measure temperature changes with high accuracy [11, 12]. Actual FUS devices are based on these two improvements: MRI guidance for targeting the volume and temperature control and phased arrays to correct the skull distortion.
Principal Biological Effects
Thermal Effect
Heat generation is mainly due to pressure variations related to US waves mechanical property. This leads to microscopic tissue shearing and consequently conversion of mechanical energy into heat [13]. Another source of temperature increase is nonlinear wave propagation [14].
The theory behind FUS relies upon employing spherical transducer or phased arrays of small single elements to concentrate US energy in a small volume representing the focus. In the focal region, the intensity of US energy is several orders higher than in neighbor areas allowing to confine the effects in the focus, leading to heat generation and temperature rising in a few seconds (Figs. 1 and 2) [15]. Notably, thermal lesion could be extremely defined, with lethal and sub-lethal effects being divided by several cells thickness [16]. Depending on transducer characteristics and US frequency the focal volume dimensions could range from 1 mm to several time this length [17]. Treatment of bigger volumes is possible through repeated US expositions of multiple overlapping focal volumes [18] leading to elongation of treatment time. Pulsed FUS represents an alternative to this modality: it employs short duty cycles reducing the overall temporal average intensity. As a consequence, the temperature is only slightly elevated, allowing the nonthermal effects to overcome: acoustic cavitation, acoustic radiation forces, and acoustic streaming [19].
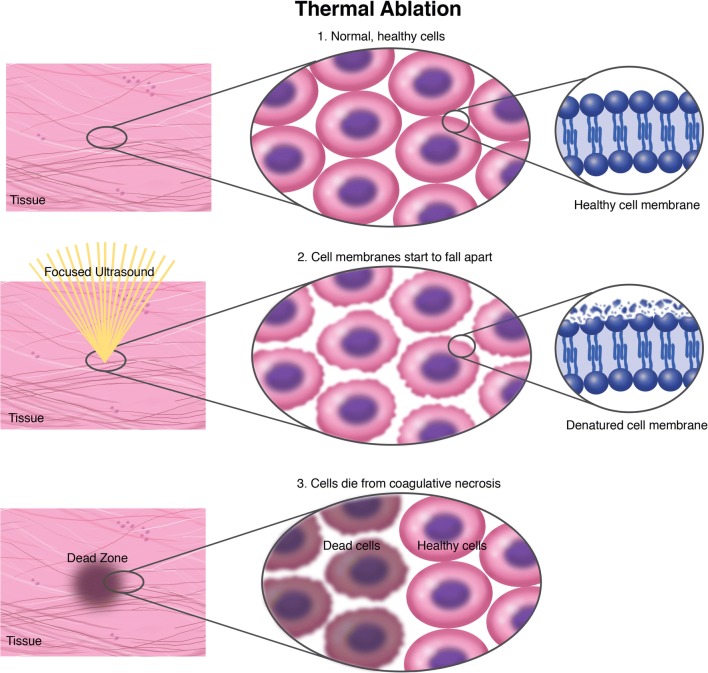
Fig. 1.
Thermal ablation. Schematic representation of thermal ablation mechanism and specificity.
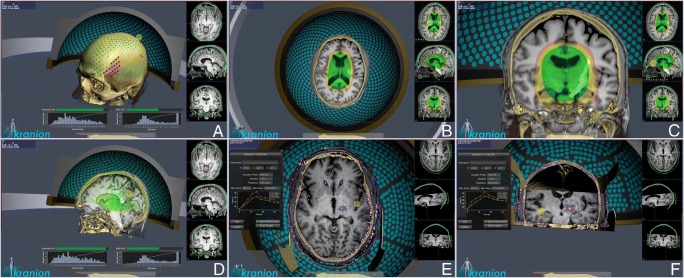
Fig. 2.
Kranion software. Different screenshots of Kranion, a modeling software for transcranial focused ultrasound treatment. (A) Pretreatment CT and MRI scan are fused and used to plan treatment taking into account bone density and structure in order to set the phases of each single transducer insonation. Red dots represent not usable transducers while the green dots refer to the potentially employable. (B, C) Green, yellow, and red areas represent the treatment envelope or the volume in which the maximal energy could be deployed. (D) Each transducer insonation path is displayed converging to the target. (E, F) MRI thermometry allows to plan and monitor FUS treatment; note the specificity of thermal generation in the left panel (in red maximal and in green average temperature are displayed).
Acoustic Cavitation
Ultrasound cavitation consists of the formation and oscillation of gas bubbles originating from pre-existing stabilized gas bodies or nuclei [20]. US is a mechanical wave comprising positive and negative components; the first lead to compression while the second to expansion of gas-filled crevices or bubbles. Under US exposure, the bubbles oscillate depending on positive and negative components of the wave. If the oscillation is continuous and stable the phenomenon is called non-inertial cavitation. When the pressure wave increases, the bubbles oscillate until they explode producing shock waves and high-velocity jets with deleterious effects on the tissue (inertial cavitation) (Fig. 3) [21]. Bubbles can be generated from dissolved gases present in the body or can be injected prior to treatment to enhance the acoustic effect. Microbubbles (MBs) have been in use since the 1960s as ultrasound contrast agents (UCA). They are currently used in different clinical settings, especially for imaging with the contrast-enhanced ultrasound (CEUS) technique, which permits a real-time assessment of contrast enhancement and measurement of vascularity of organs during different dynamic phases, and analysis of tissue perfusion [22]. Recently, MBs have been exploited during neurosurgical procedures to highlight different cerebral and spinal pathologies [23, 24]. However, at least in the brain, their behavior in terms of distribution in time and in different brain areas has never been thoroughly correlated with direct imaging but only inferred by passive cavitation detectors (PCD) [25]. Direct MB imaging obtained in animal models or during neurosurgical procedures might help to better understand this phenomenon.
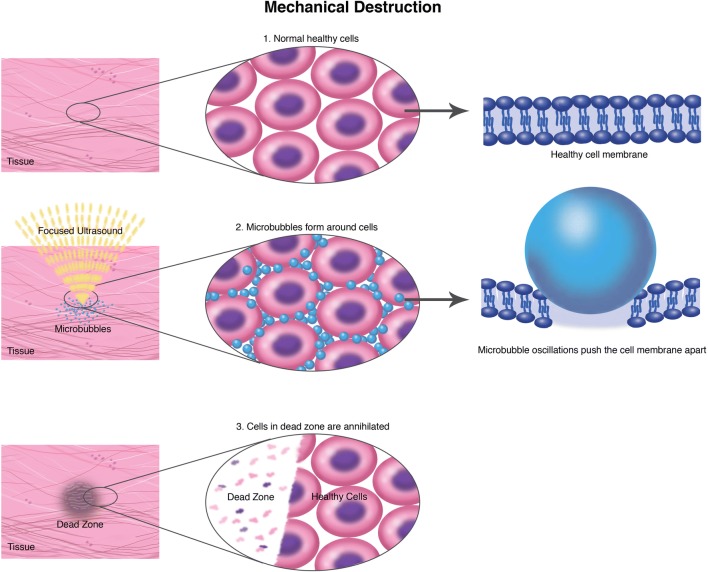
Fig. 3.
Mechanical destruction. Schematic representation of mechanical destruction mechanism and specificity.
Radiation Forces and Acoustic Streaming
US waves when hitting a surface (reflecting or absorbing) transfer a discrete force that produce a little, stable, and unidirectional mechanical stress along the direction of the beam. These are called radiation forces and, when intense enough, can lead to focal tissue displacement thus producing tissue strain [26]. If radiation forces are applied to a liquid medium, the result is called acoustic streaming; this leads to liquid movements that could enhance convection and determine shear forces able to damage the focal tissue [27].
Clinical Applications
The first clinical application of FUS, in 2001, was ablation of breast fibroadenomas mainly because of the accessibility of breast tissue without bone obstruction. The study demonstrated the response to treatment as complete or partial loss of gadolinium uptake on T1-MRI [28]. On the basis of these results, investigation moved to breast cancer, proving the feasibility to ablate the tumor although some uncertainties remain for tumor at the margins [29].
Moreover, FUS acquired CE Mark in Europe in October 2002 and Food and Drug Administration (FDA) approval in the USA in October 2004 for the treatment of uterine fibroids [30]. Numerous studies reported on the efficacy as symptom reduction, improvement in quality of life, and fertility [31–33].
FUS represents also an alternative treatment for pain caused by bone metastases (it acquired CE mark in 2007). The mechanism is related to heat generation by bone US absorption, thus leading to denervation and pain control [34].
Furthermore, it has to be said that FUS is a promising tools for a number of oncological applications, such as: bladder cancer, testicular carcinoma, pancreas tumor, rectum tumor, kidney tumor, ocular tumor, prostate tumor, and liver tumors [35–38].
The application of FUS during neurosurgical procedures started in the 1950s but its efficacy was hampered by the presence of the skull. Actual systems overcome the physical limitations posed by the cranial vault and multiple arrays of phased transducer are able to deliver acoustic energy efficiently. Furthermore, MR guidance coupled with stereotactic frames allows for precise targeting and quasi real-time monitoring of the procedure, providing information on the amount of energy delivered and temperature rise (Fig. 2) [11, 12]. In the field of neurosurgery, the most established clinical application is for essential tremor [39], while many others are under investigation.
Magnetic Resonance–Guided Focused Ultrasound (MRgFUS)—Procedure
In brain applications, FUS is generally performed under MRI guidance (MRgFUS), allowing for targeted deposition of acoustic effects and real-time visualization of treatment (e.g., thermal effect).
MRgFUS planning requires a volumetric CT scan to take into account bone thickness and density, thus correcting the phase aberration that prevent exact focusing (Fig. 2) [40, 41].
The head is shaved to improve the delivery of acoustic energy and the patient is immobilized by a stereotactic head frame. A silicone barrier, fixed to the transducer, is placed around the head to fill the transducer cavity with cooled degassed water in order to allow acoustic coupling and prevent thermal damage to the scalp [41].
Pretreatment volumetric MRI is acquired to identify the target volume and to plan US delivery (depending on the required type of effect (Fig. 2)); calibration and monitoring is performed with MRI thermometry (Fig. 2). During the treatment, different parameters are adjusted such as power, duration of the sonication, number, and phase of the elements within the arrays. The treatment is monitored by using MRI thermometry and PCDs to analyze the acoustic spectrum in order to stop the procedure in case of occurrence of excessive thermal rise or cavitation (Fig. 2).
Focused Ultrasound for Neuro-oncological Applications
Brain tumors, particularly malignant lesions such as glioma, represent a daunting challenge for both physicians and patients. These tumors are characterized by an awful prognosis and a treatment able to control these diseases constitutes a major unmet clinical need. Brain tumors, because of BBB presence, reside in a sort of sanctuary making them inaccessible to most of the existing chemotherapeutic agents. Surgery is insufficient because of their intrinsic invasive behavior and the necessity to respect neurological function, which often prevent radical resection. Radiotherapy alone is not able to control the explosive growth and is itself limited by radiotoxicity.
In this scenario FUS, alone or in combined approaches, could represent a pivotal breakthrough to improve patient outcome. Herein, we illustrate different possible applications of FUS in treating brain tumors (Table 1).
Table 1.
Summary of the most relevant literature in regards of FUS applications in neuro-oncology and neurovascular
| FUS application | Authors | Year | Title | Clinical/preclinical |
|---|---|---|---|---|
| Thermal ablation | Heimburger R | 1985 | Ultrasound augmentation of central nervous system tumor therapy | Clinical |
| Guthkelch A, Carter L, Cassady J, Hynynen K, Iacono R, Johnson P, et al. | 1991 | Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial | Clinical | |
| Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z | 2004 | A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study | Preclinical | |
| Ram Z, Cohen ZR, Harnof S, Tal S, Faibel M, Nass D, et al. | 2006 | Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy | Clinical | |
| Park JW, Jung S, Jung TY, Lee MC | 2006 | Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: a preliminary report | Clinical | |
| Hynynen K, Clement G | 2007 | Clinical applications of focused ultrasound—the brain. International Journal of Hyperthermia | Clinical | |
| McDannold N, Clement GT, Black P, Jolesz F, Hynynen K | 2010 | Transcranial magnetic resonance imaging–guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. | Clinical | |
| Coluccia D, Fandino J, Schwyzer L, O’Gorman R, Remonda L, Anon J, et al. | 2014 | First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound | Clinical | |
| McDannold NJ, Vykhodtseva NI, Hynynen K | 2006 | Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits | Preclinical | |
| Blood–brain barrier disruption and FUS-assisted drug delivery | Mehier-Humbert S, Bettinger T, Yan F, Guy RH | 2005 | Plasma membrane poration induced by ultrasound exposure: implication for drug delivery | Preclinical |
| Kinoshita M, McDannold N, Jolesz FA, Hynynen K | 2006 | Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption | Preclinical | |
| Hynynen K | 2008 | Ultrasound for drug and gene delivery to the brain. Advanced Drug Delivery Reviews | Review | |
| Liu Y, Paliwal S, Bankiewicz KS, Bringas JR, Heart G, Mitragotri S, et al. | 2010 | Ultrasound-enhanced drug transport and distribution in the brain | Preclinical | |
| Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, et al. | 2012 | A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue | Preclinical | |
| Ziadloo A, Xie J, Frenkel V | 2013 | Pulsed focused ultrasound exposures enhance locally administered gene therapy in a murine solid tumor model. | Preclinical | |
| Hsu PH, Wei KC, Huang CY, Wen CJ, Yen TC, Liu CL, et al. | 2013 | Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound | Preclinical | |
| Burgess A, Hynynen K | 2014 | Drug delivery across the blood-brain barrier using focused ultrasound | Review | |
| McDannold N, Vykhodtseva N, Hynynen K | 2006 | Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity | Preclinical | |
| Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K | 2007 | Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound | Preclinical | |
| Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, et al. | 2009 | Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging–guided focused ultrasound | Preclinical | |
| Liu H-L, Hua M-Y, Yang H-W, Huang C-Y, Chu P-C, Wu J-S, et al. | 2010 | Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain | Preclinical | |
| McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS | 2012 | Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques | Preclinical | |
| Wei KC, Chu PC, Wang HY, Huang CY, Chen PY, Tsai HC, et al. | 2013 | Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study | Preclinical | |
| Alonso A, Reinz E, Leuchs B, Kleinschmidt J, Fatar M, Geers B, et al. | 2013 | Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening | Preclinical | |
| Fan C-H, Ting C-Y, Chang Y-C, Wei K-C, Liu H-L, Yeh C-K | 2015 | Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood–brain barrier opening and brain-tumor drug delivery | Preclinical | |
| Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL | 2015 | Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study | Preclinical | |
| Interstitial focused ultrasound | Deng J, Zhang Y, Feng J, Wu F | 2010 | Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses | Preclinical |
| Canney MS, Chavrier F, Tsysar S, Chapelon JY, Lafon C, Carpentier A | 2013 | A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors | Preclinical | |
| N’Djin WA, Burtnyk M, Lipsman N, Bronskill M, Kucharczyk W, Schwartz ML, et al. | 2014 | Active MR-temperature feedback control of dynamic interstitial ultrasound therapy in brain: in vivo experiments and modeling in native and coagulated tissues | Preclinical | |
| FUS immunomodulation | Hu Z, Yang XY, Liu Y, Morse MA, Lyerly HK, Clay TM, et al. | 2005 | Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. | Preclinical |
| Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. | 2007 | Investigation of HIFU-induced anti-tumor immunity in a murine tumor model | Preclinical | |
| Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F | 2009 | Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer | Clinical | |
| Chen PY, Wei KC, Liu HL | 2015 | Neural immune modulation and immunotherapy assisted by focused ultrasound induced blood-brain barrier opening | Review | |
| Cohen-Inbar O, Xu Z, Sheehan JP | 2016 | Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept | Review | |
| Mauri G, Nicosia L, Xu Z, Di Pietro S, Monfardini L, Bonomo G, et al. | 2018 | Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer | Review | |
| Hyperthermia and radiation treatment | Guthkelch A, Carter L, Cassady J, Hynynen K, Iacono R, Johnson P, et al. | 1991 | Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial | Clinical |
| Kampinga HH | 2006 | Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field | Review | |
| Finley DS, Pouliot F, Shuch B, Chin A, Pantuck A, Dekernion JB, et al. | 2011 | Ultrasound-based combination therapy: potential in urologic cancer | Review | |
| Sonoporation | Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. | 2007 | Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial | Clinical |
| D’Souza AL, Tseng JR, Pauly KB, Guccione S, Rosenberg J, Gambhir SS, et al. | 2009 | A strategy for blood biomarker amplification and localization using ultrasound | Preclinical | |
| Liang H, Tang J, Halliwell M | 2010 | Sonoporation, drug delivery, and gene therapy | Review | |
| Collis J, Manasseh R, Liovic P, Tho P, Ooi A, Petkovic-Duran K, et al. | 2010 | Cavitation microstreaming and stress fields created by microbubbles | Preclinical | |
| Escoffre J-M, Novell A, Serriere S, Lecomte T, Bouakaz A | 2013 | Irinotecan delivery by microbubble-assisted ultrasound: in vitro validation and a pilot preclinical study | Preclinical | |
| Sensitization to chemotherapy | Song C, Park H, Lee C, Griffin R | 2005 | Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment | Review |
| Yu T, Li S, Zhao J, Mason TJ | 2006 | Ultrasound: a chemotherapy sensitizer | Preclinical | |
| Muenyi CS, Pinhas AR, Fan TW, Brock GN, Helm CW, States JC | 2012 | Sodium arsenite ± hyperthermia sensitizes p53-expressing human ovarian cancer cells to cisplatin by modulating platinum-DNA damage responses | Preclinical | |
| Lee Y-Y, Cho YJ, Choi J-J, Choi CH, Kim T-J, Kim B-G, et al. | 2012 | The effect of high-intensity focused ultrasound in combination with cisplatin using a xenograft model of cervical cancer | Preclinical | |
| Implantable ultrasound device system and cranial implants as acoustic windows | Beccaria K, Canney M, Goldwirt L, Fernandez C, Adam C, Piquet J, et al. | 2013 | Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits | Preclinical |
| Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, et al. | 2013 | Potential intracranial applications of magnetic resonance–guided focused ultrasound surgery: a review | Review | |
| Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. | 2016 | Clinical trial of blood-brain barrier disruption by pulsed ultrasound | Clinical | |
| Beccaria K, Canney M, Goldwirt L, Fernandez C, Piquet J, Perier M-C, et al. delivery: an experimental study in rabbits | 2016 | Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan | Preclinical | |
| Goldwirt L, Canney M, Horodyckid C, Poupon J, Mourah S, Vignot A, et al. using an implantable ultrasound device | 2016 | Enhanced brain distribution of carboplatin in a primate model after blood–brain barrier disruption | Preclinical | |
| Horodyckid C, Canney M, Vignot A, Boisgard R, Drier A, Huberfeld G, et al. | 2017 | Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model | Preclinical | |
| Gutierrez MI, Penilla EH, Leija L, Vera A, Garay JE, Aguilar G | 2017 | Novel cranial implants of yttria-stabilized zirconia as acoustic windows for ultrasonic brain therapy | Preclinical | |
| Sonodynamic therapy | Tachibana K, Feril LB, Jr., Ikeda-Dantsuji Y | 2008 | Sonodynamic therapy | Review |
| Tang W, Liu Q, Wang X, Wang P, Zhang J, Cao B | 2009 | Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro | Preclinical | |
| Li Y, Zhou Q, Hu Z, Yang B, Li Q, Wang J, et al. | 2015 | 5-Aminolevulinic acid-based sonodynamic therapy induces the apoptosis of osteosarcoma in mice | Preclinical | |
| Hu Z, Fan H, Lv G, Zhou Q, Yang B, Zheng J, et al. | 2015 | 5-Aminolevulinic acid-mediated sonodynamic therapy induces anti-tumor effects in malignant melanoma via p53-miR-34a-Sirt1 axis | Preclinical | |
| Suehiro S, Ohnishi T, Yamashita D, Kohno S, Inoue A, Nishikawa M, et al. | 2018 | Enhancement of antitumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model | Preclinical | |
| Cerebrovascular applications | Anschuetz R, Bernard HR | 1965 | Ultrasonic irradiation and atherosclerosis | Preclinical |
| Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, et al. | 2005 | Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial | Clinical | |
| Eggers J, Konig IR, Koch B, Handler G, Seidel G | 2008 | Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study | Clinical | |
| Medel R, Crowley RW, McKisic MS, Dumont AS, Kassell NF | 2009 | Sonothrombolysis: an emerging modality for the management of stroke | Review | |
| Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr., Xu Z | 2011 | Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model | Preclinical | |
| Wright C, Hynynen K, Goertz D | 2012 | In vitro and in vivo high-intensity focused ultrasound thrombolysis | Preclinical | |
| Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K | 2012 | High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke | Preclinical | |
| Monteith SJ, Harnof S, Medel R, Popp B, Wintermark M, Lopes MB, et al. | 2013 | Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound | Preclinical | |
| Weiss HL, Selvaraj P, Okita K, Matsumoto Y, Voie A, Hoelscher T, et al. | 2013 | Mechanical clot damage from cavitation during sonothrombolysis | Review | |
| Monteith SJ, Kassell NF, Goren O, Harnof S | 2013 | Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage | Review | |
| Ahadi G, Welch CS, Grimm MJ, Fisher DJ, Zadicario E, Ernstrom K, et al. | 2013 | Transcranial sonothrombolysis using high-intensity focused ultrasound: impact of increasing output power on clot fragmentation | Preclinical | |
| Harnof S, Zibly Z, Hananel A, Monteith S, Grinfeld J, Schiff G, et al. | 2014 | Potential of magnetic resonance-guided focused ultrasound for intracranial hemorrhage: an in vivo feasibility study | Preclinical | |
| Ilyas A, Chen CJ, Ding D, Romeo A, Buell TJ, Wang TR, et al. | 2018 | Magnetic resonance-guided, high-intensity focused ultrasound sonolysis: potential applications for stroke | Review |
Thermal Ablation
Thermal therapies are divided into two categories: ablation (> 50 °C) or hyperthermia (ranging from 42 to 45 °C). In thermal ablation, cell necrosis takes place because of an irreversible denaturation of membrane proteins that leads to thermo-coagulative lesions (Fig. 1) [42]. Tumor ablation consists of focal destruction of tumor parenchyma sparing surrounding brain tissue (Figs. 1 and 2). This approach has already been described in 1980s and 1990s as preliminary experiences with focus ultrasound guided by CT or US [9, 43]. Thanks to improvement of MRgFUS, in recent years, numerous studies have addressed brain tumors ablation. Ram et al. described MRgFUS performed through a surgical craniectomy to treat three patients with recurrent glioblastoma, obtaining the following survival: 10, 31, and 33 months. In their experience, MRgFUS produced thermo-coagulative lesions also visible on MRI. Moreover, they reported in one patient the formation of a secondary focal lesion outside the target due to the heating of brain tissue in the sonication path [44]. Park et al. [45] described the effective ablation of an anaplastic astrocytoma without the need for surgical craniectomy. Follow-up imaging at 6 months highlighted the decrease in tumor volume and edema. Few years later, Brigham and Women’s Hospital started to employ ExAblate 3000 (InSightec, Tirat Carmel, Israel) to treat deep-seated brain tumors. The trial was suspended because one patient died after intracranial hemorrhage occurred, allegedly due to US-induced cavitation [46, 47]. These studies failed in obtaining thermal-coagulation in the focus because of technical limitation of the prototype system. Coluccia et al. [48], in 2014, were the first to achieve MRgFUS tumor ablation without neurological deficits or adverse effects, in a patient with recurrent glioblastoma.
Currently, three trials (NCT01698437, NCT00147056, NCT01473485) are ongoing in an effort to demonstrate safety and feasibility of thermal ablation in brain tumors. Unfortunately, definitive results are still not available.
One of the main limitations of the current system is the inability to target skull base or posterior fossa tumors due to bone heating and challenges in focusing US outside of the central regions of the brain (Fig. 2).
US contrast agents could represent an adjunct to MRgFUS, permitting enhanced thermal effect with a lower time-averaged power [49]. Indeed, US contrast agents, such as intravascular microbubbles, can enhance tissue ablation exploiting cavitation, permitting a reduction in time-averaged acoustic power and overcome the limitation of bone heating compared to standard thermal tumor ablation (Fig. 3) [50].
Blood–Brain Barrier Disruption
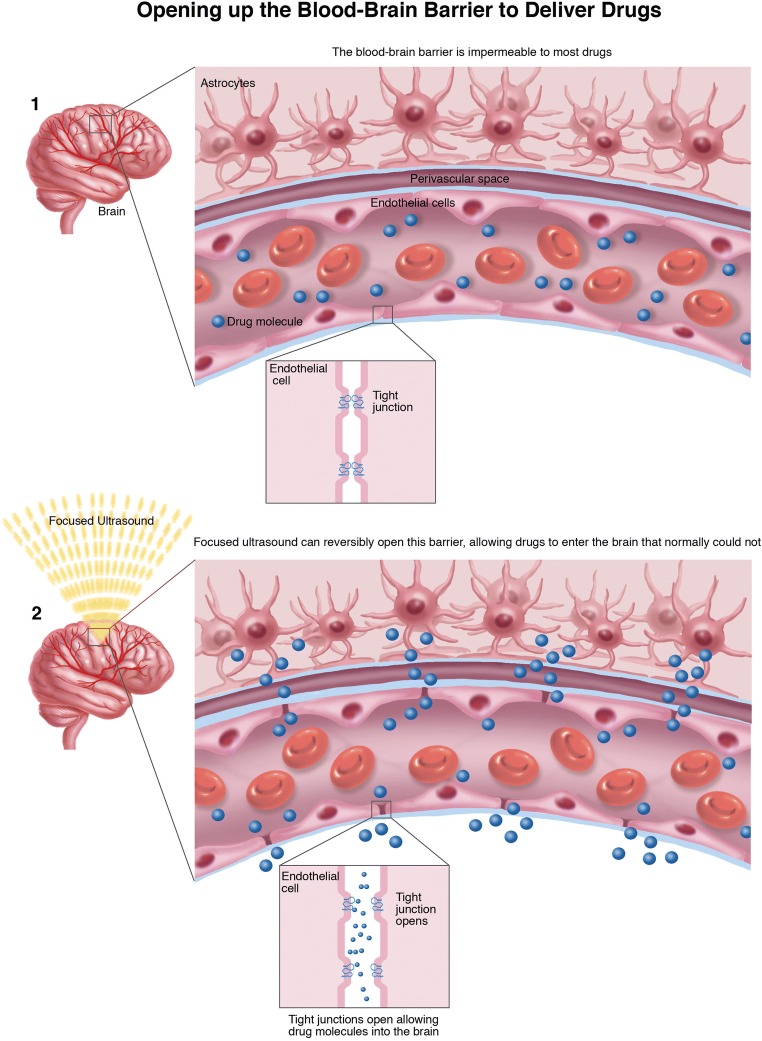
The blood–brain barrier (BBB) represents the principal obstacle to chemotherapy efficacy. It is essential to protect central nervous system from chemical and humoral injuries, but, on the other hand, complicates obtaining therapeutic concentrations of certain drugs without paying a systemic toxicity. FUS permits selective mechanical opening of the BBB allowing for delivery of therapeutic concentrations of chemotherapy without systemic side effects (Fig. 4).
Fig. 4.
BBB disruption. Schematic representation of BBB opening mechanism.
Initial experiences focused on high-intensity US, higher than cavitation threshold, obtaining BBB disruption but also brain tissue damage [51]. The consequent evolution was to employ US contrast agent that is administered before FUS treatment, which is performed with pulsed exposures at low acoustic pressure and low frequencies if compared to standard FUS treatments (~500 kHz vs 1 MHz).
The resulting non-inertial cavitation stress vessels wall and in particular cells tight junction fundamental for BBB integrity; this mechanical stress ultimately leads to BBB disruption (up to 4–6 h) [52]. The use of ultrasound contrast agent is required because microbubbles can lower the acoustic energy required to induce cavitation, thus not injuring surrounding tissue while maximizing BBB opening [53].
Numerous preclinical studies have addressed BBB opening through MRgFUS. The first demonstration employed liposome-encapsulated doxorubicin [54] followed by intravenous methotrexate [55], 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) [56], BCNU-loaded bubbles [57], temozolomide [58], IL-12 [59], DNA for gene therapy [60], and the monoclonal antibody trastuzumab [61]. The safety of this procedure was proved in nonhuman primates, repeatedly disrupting BBB in basal ganglia or central visual field targets [62].
On the basis of these results, an ongoing phase I trial was started (NCT02343991) to evaluate MRgFUS combined to intravenous microbubbles and doxorubicin to treat brain tumor patients. MRgFUS is exploited in order to open BBB, which is confirmed by contrast medium extravasation in MRI. Subsequently, the patient is operated and areas in which BBB was or was not opened are compared for doxorubicin accumulation.
FUS-Assisted Targeted Drug Delivery
The blood–brain barrier is only the first obstacle to drug/compound diffusion in tumor parenchyma and to reaching therapeutic concentration in situ. The second critical structure is the tissue/tumor penetration barrier [63]. This is constituted by electrostatically charged extracellular matrix and glial-lymphatic system and represents a dense structure, which limit the diffusion of therapeutic agents [64]. Preclinical studies demonstrated that FUS in combination with engineered nanoparticles with dense poly(ethylene glycol) coating could overcome these diffusion limitations [65–67].
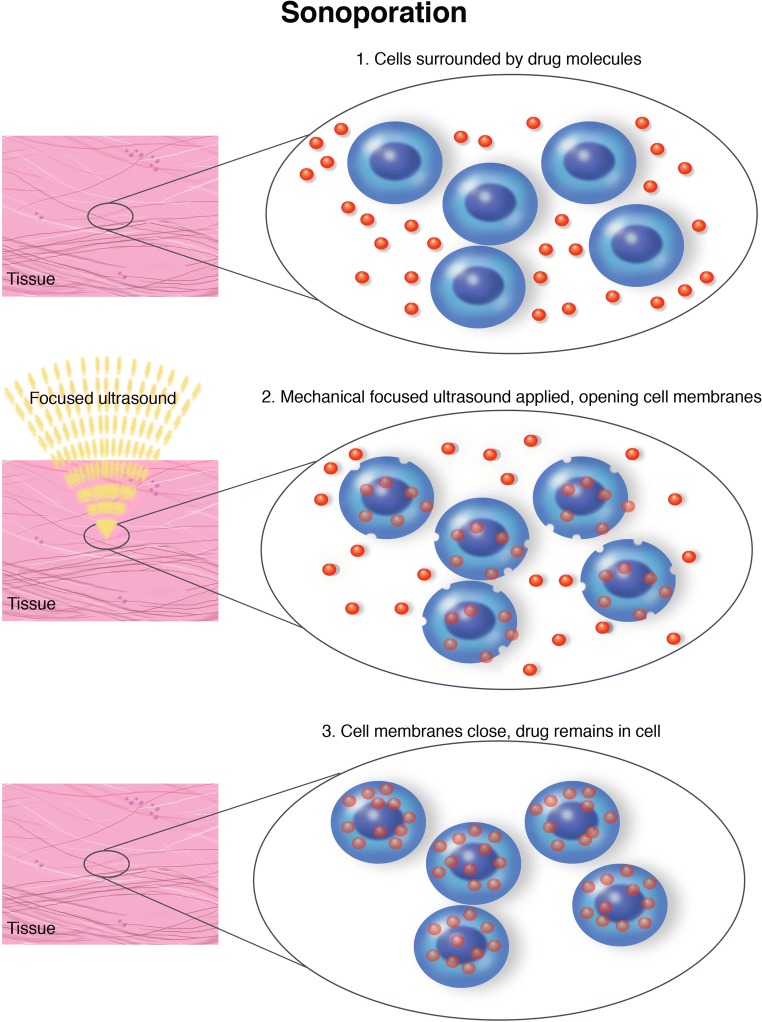
Another option is reversible cell membrane poration with FUS (Fig. 5). This is an in vitro demonstrated technique, allowing for incorporation in tumor cells of heat-activated chemotherapy, gene therapy, nanoparticles, and liposomes avoiding the effects of a gene therapy or systemic drug [68].
Fig. 5.
Sonoporation. Schematic representation of sonoporation mechanism and specificity.
Interstitial Focused Ultrasound
There is no doubt that in neuro-oncological surgery attention is mainly dedicated to extracranial MRgFUS. Nevertheless, interstitial FUS, also known as catheter-based FUS, has been studied by different groups principally in preclinical models [69, 70]. It consists of multi-elemental, cooled catheter with cylindrical elements allowing for precise shaping of the ablative field also in case of large tumors. The same catheter can be used both to perform FUS and tissue biopsies to confirm the target volume. Interstitial FUS exploiting catheter configuration and the more shaped heating overcomes the limitations of lesion location and proximity to blood vessels [71].
FUS Immunomodulation
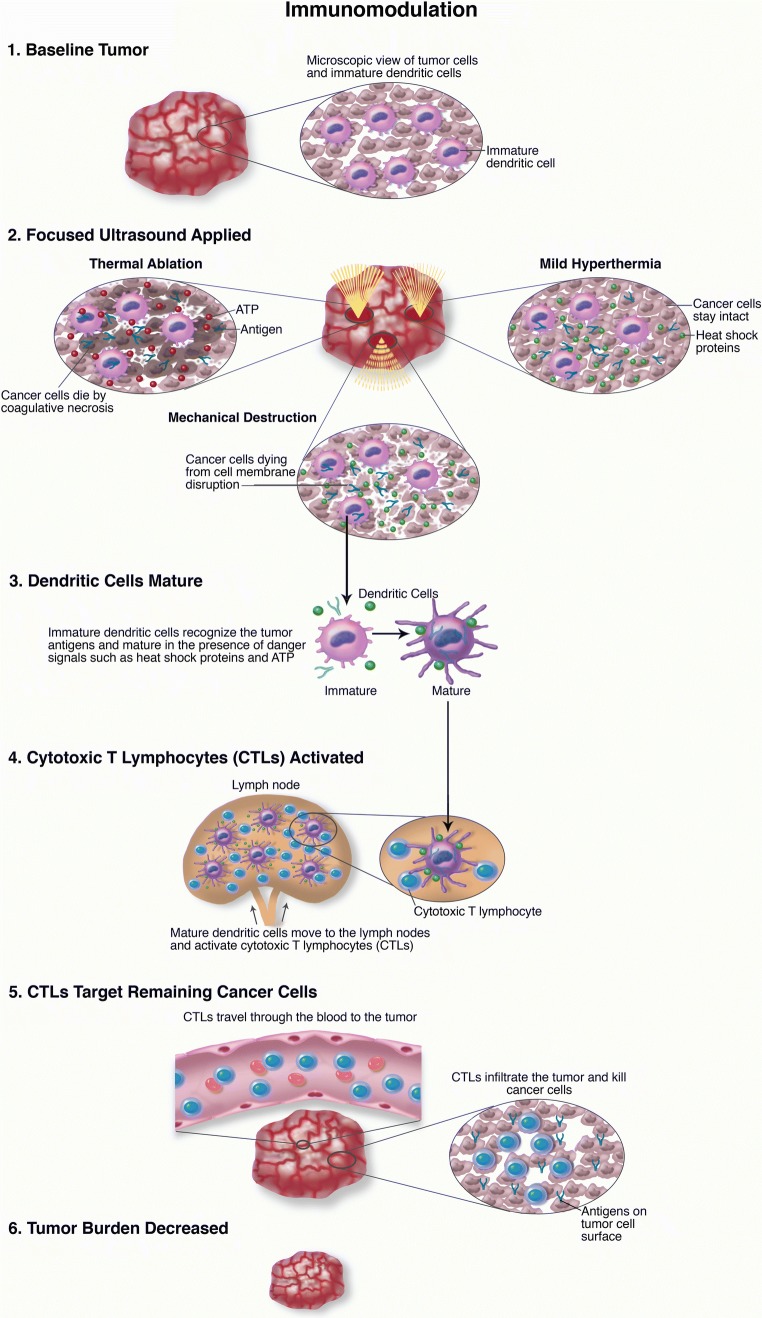
Numerous clinical and preclinical studies have demonstrated the possibility to influence immune response against cancer. It has to be said that all the immunomodulation has been described on other tumors and has not been validated in brain tumors [72, 73]. The mechanism behind immunomodulation is complex and is related to all the FUS effects (Fig. 6). Thermal ablation induces overexpression of heat shock proteins that represent a potent immune response inducer [74]. Mechanical effects, such as cavitation, lead to superior BBB permeability for antigens, immune cells, and pro-inflammatory molecules/ chemokines; notably tumor mechanical disruption also generates a bulk of tumor-related debris and antigens activating dendritic cells (Figs. 3, 4, 5, and 6) [75–77]. Furthermore, FUS can moderate tumor-related immunosuppression, enhance tumor-infiltrating cells population, and facilitate neuroglial cell activation (Fig. 6) [72, 77–79]. It has to be noted that all the mentioned mechanisms create the basis for a synergistic application of FUS and immunotherapy [75].
Fig. 6.
Immunomodulation. Schematic representation of the different mechanisms behind FUS immunomodulation.
Hyperthermia and Radiation Treatment
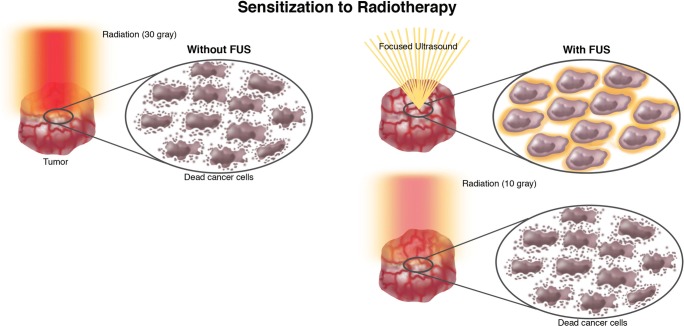
Hyperthermia induces relevant changes in tumor milieu that could enhance tumor responsiveness to radiation treatment: hyperthermia produces an increased blood flow and consequently delivery of oxygen and trophic molecules thus enhancing tumor metabolic activities. This feature lastly leads to an increase of tumor responsiveness to radiation therapy [80]. It is possible to say that FUS induces a sensitization to radiotherapy (Fig. 7) [80]. Sensitized tumors could require a lower dose of radiation to be effective, thus potentially reducing radiation-induced side effects [81]. Furthermore, FUS and radiation are potentially complementary under the biological aspect. Cells in S phase of cell cycle are more sensitive to hyperthermia while are relatively resistant to radiation [43]. The combined use of radiation and FUS is another field in which MRgFUS could have future clinical utility (Fig. 7).
Fig. 7.
Sensitization to radiotherapy. Schematic representation of the potential role of FUS in reducing radiotherapy exposure.
Sonoporation
The plasma membrane acts as a barrier for large molecules such as certain drugs and gene vectors, thus inhibiting therapeutic effect. FUS through mechanical effect, namely stable cavitation, can modulate cell permeability, therefore permitting molecules entrance and interactions [82]. Stable cavitation induces both transient pore opening in membrane and, through acoustic streaming, an increase fluid motions in cell micro-environment [83]. This effect is described as sonoporation and allows enhanced drug/gene penetration and actions (Fig. 5) [84]. Relying on these premises, FUS permits reduction in the dose of a systemic compound while obtaining an unchanged concentration in tumor cells. Notably, thanks to FUS spatial specificity, this technique is particularly indicated for gene therapy [85].
Another possible application of sonoporation if combined with BBB disruption is the amplification of blood-based biomarker. Indeed, the mechanical effects of FUS induce the release of specific molecules and structures (e.g., microvesicles) in the blood stream. This approach could be employed to obtain a liquid biopsy of the tumor through a minimally invasive blood sample [86].
It has to be said that sonoporation in brain tumors has been studied only in preclinical settings; however, results are encouraging and support a future translation in clinical trial [82].
Sensitization to Chemotherapy
Similarly to what happen in sensitization to radiotherapy, FUS could enhance the sensitivity to chemotherapy due to several mechanisms. First of all, local hyperthermia induces local increase in blood flow, permitting a greater delivery of drugs, oxygen, and trophic molecules. This leads to an augmented metabolic activity and as a consequence to a sensitization to chemotherapeutic agents [87, 88]. Second, FUS could revert tumor-acquired resistance to certain drugs, thus regaining a therapeutic efficacy [88]. Furthermore, local hyperthermia is particularly effective in tumor cells because of their reduced ability to scatter heat, in contrast with healthy cells [89]. Finally, FUS offers a non-invasive method to induce an enhanced sensitivity to chemotherapy as it has been demonstrated in preclinical studies supporting the potential application in numerous types of tumor in clinical setting [89, 90].
Implantable Ultrasound Device System and Cranial Implants as Acoustic Windows
The principal limitation to FUS application in brain tumors is actually the thickness of the skull. Indeed, it absorbs up to 90% of US beam, thus requiring multiphased arrays to prevent skull heating and US weakening [91, 92]. Notably, it requires MRI for planning and monitoring, several hours of insonation and stereotaxic frame to fix the head. All these features complicate the repetitive applications of FUS and especially the treatment of superficial tumors, making more feasible handling of a deep-seated single lesion. A solution to this problem is represented by an implantable ultrasound device, namely SonoCloud which permits repeatable, diffuse BBB opening [91]. Several studies demonstrate the safety and feasibility of unfocused low-intensity pulsed US with implantable device in order to obtain repeated BBB disruption in preclinical and animal settings [91, 93–96]. Relying on these results, a trial was started (NCT02253212) to evaluate safety and feasibility of SonoCloud implantation in recurrent GBM patients in order to obtain monthly BBB opening before systemic administration of carboplatin. It has to be said that sonication volume is limited to a ~5-cm3 BBB opening by glial and immune reaction observed in animals. Only interim data are available suggesting that SonoCloud has the potential to enhance chemotherapy distribution in brain tumors.
Trying to overcome the limitations of an implantable transmitter, Gutierrez et al. studied different polycrystalline 8 mol/yttria-stabilized-zirconia (8YSZ) ceramics with different porosity in order to obtain a biocompatible cranial implant. They tested 8YSZ ceramics for their acoustic properties and validated the results exploiting a finite element model mimicking US propagation through 8YSZ windows. They concluded that 8YSZ implants could be used as acoustic window to allow FUS treatment [97].
Sonodynamic Therapy
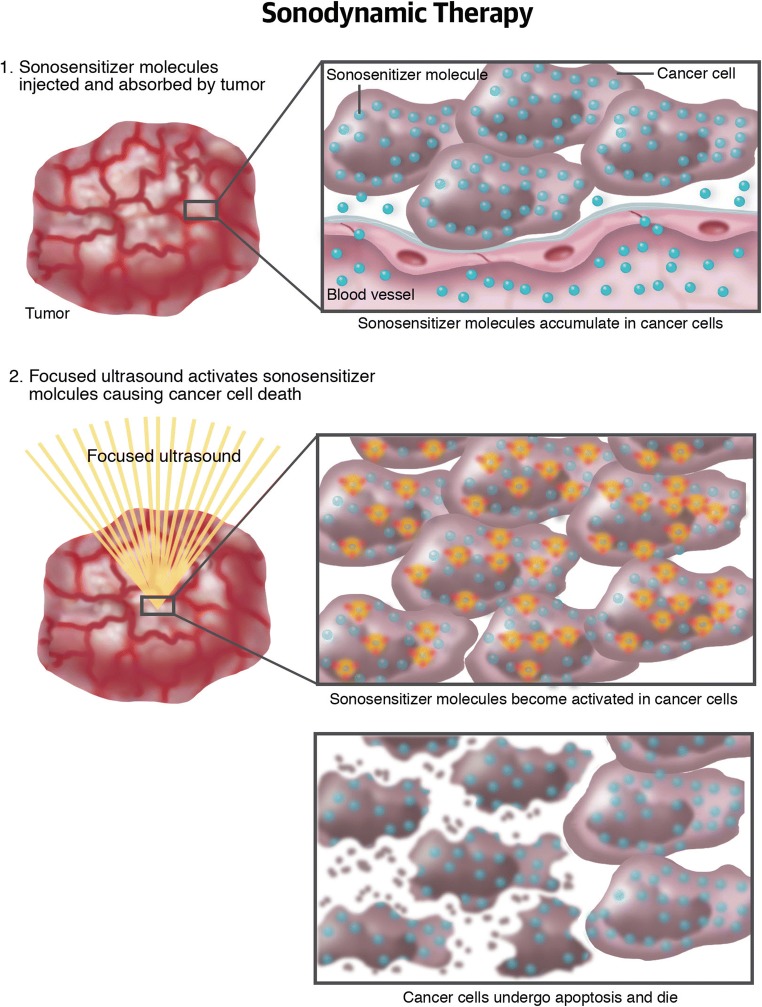
Sonodynamic therapy relies on sonosensitizers compounds such as 5-aminolevulinic acid (5-ALA) and FUS to generate intracellular reactive oxygen species capable to induce damage to DNA and induce apoptosis (Fig. 8). FUS is able to deeply and specifically penetrate brain tissue and to excite sonosensitizer only in the target area. Combining the specificity of 5-ALA accumulation in some types of tumors to the specificity of FUS insonation is possible to obtain necrosis and apoptosis only in the target volume [98]. Numerous studies demonstrated the efficacy in various cancers also comprising malignant glioma [98–100]. Satoshi Suehiro et al. [98] studied the combined application of 5-ALA and FUS demonstrating effective cytotoxicity towards glioma cells thus resulting in prolonged survival of tumor-bearing mice. Growing body of evidences support the superiority of sonodynamic therapy over photodynamic therapy. Indeed, this technique is equally effective in generating reactive oxygen species, does not require craniotomy or surgical intervention, is able to activate chemical agents deeply located in the brain, and permits conformal dosage of energy [98, 101, 102].
Fig. 8.
Sonodynamic therapy. Schematic representation of sonodynamic therapy mechanism and specificity.
Focused Ultrasound Applications for Cerebrovascular Disease
Cerebrovascular disease is often arbitrarily divided into ischemic and hemorrhagic subtypes, although unruptured aneurysms and vascular malformations do not technically belong to either category. “Stroke” as an overarching entity, that encompasses all of the above-mentioned pathologies, is the third cause or mortality and the number one cause of morbidity in the USA and in most developed countries [103, 104].
FUS can have broad applications to the treatment of ischemic and hemorrhagic stroke. Current treatment algorithm for ischemic stroke includes administration of intravenous tissue plasminogen activator (IV-tPA) for patients arriving to clinical attention within 3 h of symptom onset, plus or minus the concomitant use of endovascular mechanical thrombectomy (EMT) for those patients with proximal vessel occlusion [105]. Despite these advances, fewer than 10% and 15% of patients with AIS are eligible for IV-tPA and EMT, respectively [106, 107], mostly due to delayed arrival at centers that can offer treatment, although this may be changing with the recent publication of the DAWN and DEFUSE-3 trials [108, 109].
The heterogeneous nature of hemorrhagic stroke, which includes patients with intraparenchymal hemorrhage (IPH), subarachnoid hemorrhage (SAH), and other less common hemorrhagic patterns (e.g., amyloid angiopathy, moyamoya disease) makes generalizations more difficult; however, in most cases, medical and surgical intervention result in marginal improvement in patient outcomes [110]. These poor statistics of eligibility for intervention and poor outcome with most current therapies demonstrate a clinical need for further innovation to expand stroke therapy to patients.
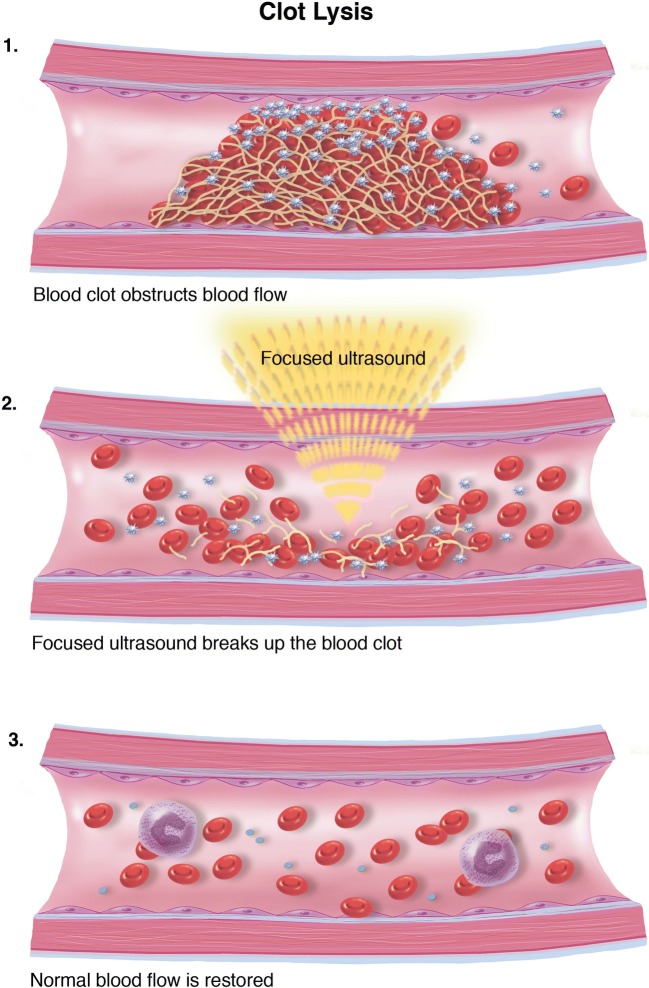
FUS has been suggested as an alternate treatment paradigm for patients with both ischemic and hemorrhagic stroke. Intuitively, one could imagine using ultrasound to break up clots, both intravascularly and intraparenchymally. Sonothrombolysis refers to ultrasound-mediated thrombolysis using adjuvant therapy (microbubbles being the most common), while sonolysis refers to the use of ultrasound without the use of adjuncts (Fig. 9). Specifically, transcranial sonolysis has been proposed for clot lysis in patients with large vessel occlusion who are not candidates (or have failed) for mechanical thrombectomy and for clot hemolysis in patients with spontaneous intraparenchymal hemorrhage [111–114] (Table 1).
Fig. 9.
Clot lysis. Schematic representation of clot lysis mechanism.
Initial Studies on Use of Sonolysis for Cerebrovascular Disease and Potential Applications
The concept of sonolysis was proposed in 1965 by Anschuetz and Bernard [115], but it took the introduction of transcranial Doppler ultrasonography (TCD-US) [116] to allow for the use of this application for intracranial pathologies [117].
Several preclinical studies have investigated the use of FUS for sonothrombolysis. Maxwell et al. [118] investigated the use of FUS in an animal model and noted that of 10 cases, 7 showed Doppler ultrasonographic evidence of improved flow after FUS thrombolysis. Similar results were obtained by Wright et al. in a separate extracranial model, with only minimal risk of hemorrhage associated with the use of FUS [119]. Burgess et al. performed in vivo studies of intracranial ELVO thrombolysis using MRgHIFU. These studies reproduced the extracranial findings, notably that FUS is safe (with only minimal chance of intracerebral hemorrhage and no cases of arterial injury) and that with higher FUS ranges, robust recanalization can be achieved [111]. Two studies have demonstrated the feasibility of transcranial MRgHIFU in ICH. Monteith et al. [120] demonstrated a > 95% liquefaction rate of ICH with FUS sonolysis permitting minimally invasive clot aspiration and complete lysis of 4-ml clots without additional brain injury. Harnof et al. [121] confirmed the safety and efficacy of the use of sonolysis for ICH in an independent model.
In 2000, Alexandrov et al. [122] reported on the improved recanalization rates of AIS patients treated with IV-tPA and TCD-US sonothrombolysis. Subsequent randomized clinical trials demonstrated efficacy of low-intensity, high-frequency (> 300 kHz) ultrasound sonothrombolysis [123–125].
Ultrasound-assisted thrombolysis has several inherent limitations. These include the need for administration of IV-tPA, attenuation of the signal by the skull, operator dependence, and the need for prolonged application over several hours to obtain the necessary therapeutic response. Further, ultrasound-assisted thrombolysis results in a minimal thermal signature, making it less than ideal for lysis of intraparenchymal clots [126].
Magnetic resonance–guided FUS (MRgFUS) sonolysis overcomes several of these disadvantages (Figs. 2 and 9), although clinical results with this technique are scarce and many studies are underway to develop this into a clinically relevant therapy. As with any therapy, this modality has several shortcomings. These challenges include the issue of thermal damage to brain parenchyma due to heating of the skull, and the inability of this modality to lyse clots within 2 cm of the calvaria due to signal attenuation, which may limit its application to select patients.
Cerebral Cavernous Malformations and Use of Photoacoustic Sensitizers
As with oncological applications, FUS may be used to target vascular malformations. The most likely immediate clinical application of this technology is likely to be for treatment of low-flow vascular malformations, such as cerebral cavernous malformations. The fragile nature of vascular malformations may result in pause for most practitioners in using sonodynamic therapies for this class of disease, but with improvements in technology, this class of malformations may be amenable to ultrasound treatment. Addition of sonosensitizers, such as 5-aminolevulinic acid (5-ALA), may allow for more targeted administration of ultrasound to specific domains of vascular malformations, akin to targeting performed in radiosurgical treatment of these lesions.
Future FUS Application and Limitations
Actually FUS is at the dawn of its application for neuro-oncological and cerebrovascular disease. It is not certain whether FUS will have differential effects on different tumor pathologies. In brain tumors any kind of declination for tumor subtype is premature and not sustained by existing literature, although the most investigated, because of its poor prognosis, is glioblastoma.
Indeed, technical and preclinical researches should go along with clinical research: for example FUS, when combined with sensitizers might specifically and differentially target more vascular tumors. Sonodynamic therapy has been so far applied in glioma surgery; however, theoretically, any tumor uptaking a sono-active drug, such as cerebral metastasis or meningioma, could be treated by sonodynamic therapy [127]. Also sonodynamic therapy could be used to tackle deep-seated benign lesions such as cavernoma, reducing surgical morbidity.
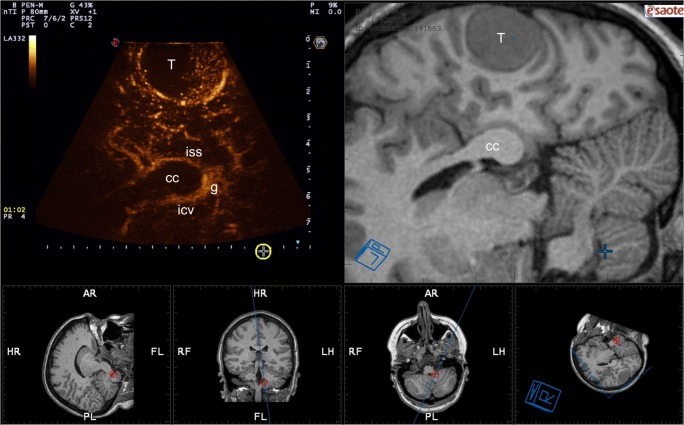
FUS can be used, alone or in combination with other particles, to deliver drugs, neurotrophic factors, or gene therapy, making it a suitable adjunct to tackle intrinsic brain tumors or repair damages caused by stroke, tumor invasion, or surgical morbidity. Particularly interesting is the use of FUS in combination with MBs. MBs can be used to open the BBB in order to enhance drug delivery; they can vehicle therapeutic agents themselves or they might be used to perform vascular ablation enhancing FUS mechanical effects. Different type of tumors or diseased brain areas can therefore become the target of such treatments. However, distribution and localization of MBs in different areas of the normal human brain and in pathological conditions and their concentration over time are not yet fully known: FUS treatments are therefore limited because of this lack of data. Recently, MBs have been thoroughly studied during neurosurgical procedures to highlight different pathologies. Contrast-enhanced ultrasound (CEUS) performed during neurosurgical procedures is an ideal method for creating a chart to dynamically localize MBs in different brain areas and in different brain pathologies, allowing to optimize MB-enhanced FUS treatments [22–24, 128, 129] (Fig. 10).
Fig. 10.
MB distribution. Intra-operative navigated contrast-enhanced ultrasound in a case of right parasagittal meningioma in the venous phase. MBs are mainly confined in posterior venous circulation. Note the different enhancement of brain parenchyma and corpus callosum (T = tumor, cc = corpus callosum, icv = internal cerebral veins, iss = inferior sagittal sinus, g = galen vein).
Patients suffering for brain tumors, both intra- and extra-axial, and cerebrovascular diseases often undergo surgery to relieve mass effect and intracranial hypertension. The possibility to implant US transducer [91] or ultrasound transparent cranial windows [97] will facilitate any kind of FUS treatment, overcoming the obstacle posed by the presence of the skull. The possibility that such windows allows for US imaging would also open new scenarios on MB direct imaging for BBB opening and drug delivery in case of malignant tumors or neurotrophic factors in case of stroke. It will facilitate follow-up and drug delivery in an outpatient setting in glioma patients, and it will be particularly envisaged for recurrent glioblastoma. US-guided histotripsy could also benefit by such a development, being possibly applied for the treatment of recurrent glioma or meningioma.
The risks associated with the use of FUS are mainly related to heating caused by the FUS treatment, rapid cell death causing release of toxic metabolites, and cavitation’s control, such as in opening of the BBB, which may result in cerebral edema in the setting of disease in the brain. The issue of heat generation and dissipation can be addressed by titrating ultrasound timing, delivery and pulsing protocols, and with the future development of three-dimensional thermometry. However, areas close to the bone, especially the skull base, pose a particular threat, as bone heating might injure cranial nerves in their passage into bony structures. The more selective opening of BBB made possible by FUS could remedy the issue of unselective BBB opening; in the case of MB-enhanced treatment with FUS, a thorough understanding of MB dynamics is necessary (Fig. 10). Nonetheless, these risks remain a challenge associated with the use of FUS for brain oncological and vascular indications.
Conclusion
In this review, we tried to summarize the different biological mechanisms offered by focused ultrasound pertinent to neuro-oncological and vascular diseases: with the promising results associated with the use of FUS for essential tremor [130], although first tested in brain tumors, more groups are likely to begin clinical studies for other disease states, as the number of ongoing preclinical researches and clinical application is steadily increasing.
FUS allows to deliver its effect within different areas of the brain: the system used for thermal ablation allows precise targeting within a stereotactic frame with MR guidance, featuring real-time thermometry and cavitation feedback using high frequencies; however, in this regard, the treatment envelope is still limited by the skull geometry, making it suitable for centrally locate lesions. The same system envisages the use of low frequency helmet that allows to reach brain areas closer to the skull. This system, together with other devices such as those as large aperture single transducers coupled with navigation system or ultrasound transparent skull replacements implantable transducers, coupled with microbubbles or other sensitizers, will exploit new mechanism of action greatly expanding the treatment envelope.
Actually only a little knowledge derived from FUS technical and preclinical research has been translated into clinical practice. The development of FUS technology, along with real-time monitoring and feedback, will increase the safety margins of this technic, permitting to further push the treatment envelope beyond its current status and all the biological effects explored in preclinical research that we illustrated in this review should be explored in a clinical settings, positively impacting the care of neuro-oncological and vascular patients.
Electronic Supplementary Material
(PDF 2466 kb)
Acknowledgments:
The authors would like to thank the Focused Ultrasound Foundation for the support provided for both manuscript and figures. The authors also would like to acknowledge John Snell, PhD, for having developed and shared the images for fig. n. 2 from Kranion software (open source interactive transcranial focused ultrasound visualization system software).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Previous Presentation
This paper has never been presented.
References
- 1.Curie J, Curie P. Development by pressure of polar electricity in hemihedral crystals with inclined faces. Bull soc min de France. 1880;3:90. [Google Scholar]
- 2.Constantin C, Paul L. Production of submarine signals and the location of suemarine orjects. Google Patents 1923.
- 3.Hill C, Bamber J. Methodology for clinical investigation. Physical Princip Med Ultrason 2004:255–302.
- 4.Gersten J, Kawashima E. Recent advances in fundamental aspects of ultrasound and muscle. Br J Phys Med. 1955;18(5):106–109. [PubMed] [Google Scholar]
- 5.James JA, Dalton G, Bullen M, Freundlich H, Hopkins J. The ultrasonic treatment of Meniere’s disease. J Laryngol Otol. 1960;74(10):730–757. doi: 10.1017/S0022215100057182. [DOI] [PubMed] [Google Scholar]
- 6.Smith A, Fisher G, Macleod I, Preshaw R, Stavney L, Gordon D. The effect of ultrasound on the gastric mucosa and its secretion of acid. Br J Surg. 1966;53(8):720–725. doi: 10.1002/bjs.1800530819. [DOI] [PubMed] [Google Scholar]
- 7.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(2):179. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry W, Meyers R. Ultrasonic method of modifying brain structures. Stereotact Funct Neurosurg. 1962;22(3–5):315–327. doi: 10.1159/000104377. [DOI] [PubMed] [Google Scholar]
- 9.Heimburger R. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med. 1985;78:469–476. [PubMed] [Google Scholar]
- 10.Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52(1):100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 11.Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J (Sudbury, Mass) 2002;8:S100–S112. doi: 10.1097/00130404-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Jolesz FA, Hynynen K, McDannold N, Tempany C. MR imaging–controlled focused ultrasound ablation: a noninvasive image-guided surgery. Magn Reson Imaging Clin. 2005;13(3):545–560. doi: 10.1016/j.mric.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol. 2001;27(3):301–333. doi: 10.1016/S0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 14.Hynynen K. Demonstration of enhanced temperature elevation due to nonlinear propagation of focussed ultrasound in dog’s thigh in vivo. Ultrasound Med Biol. 1987;13(2):85–91. doi: 10.1016/0301-5629(87)90078-0. [DOI] [PubMed] [Google Scholar]
- 15.ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 16.Ghanouni P, Pauly KB, Elias WJ, et al. Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. Am J Roentgenol. 2015;205(1):150–159. doi: 10.2214/AJR.14.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259(1):39–56. doi: 10.1148/radiol.11100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel V, Etherington A, Greene M, et al. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006;13(4):469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 21.Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci 2011:201015771. [DOI] [PMC free article] [PubMed]
- 22.Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version) Ultraschall Med. 2018;39(02):e2–e44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 23.Prada F, Perin A, Martegani A, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery. 2014;74(5):542–552. doi: 10.1227/NEU.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 24.Prada F, Vitale V, Del Bene M, et al. Contrast-enhanced MR imaging versus contrast-enhanced US: a comparison in glioblastoma surgery by using intraoperative fusion imaging. Radiology. 2017;285(1):242–249. doi: 10.1148/radiol.2017161206. [DOI] [PubMed] [Google Scholar]
- 25.Tung Y-S, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice. Phys Med Biol. 2010;55(20):6141. doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock HA, Smith LH, Cuesta J, et al. Investigations into pulsed high-intensity focused ultrasound–enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med Biol. 2009;35(10):1722–1736. doi: 10.1016/j.ultrasmedbio.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frenkel V, Gurka R, Liberzon A, Shavit U, Kimmel E. Preliminary investigations of ultrasound induced acoustic streaming using particle image velocimetry. Ultrasonics. 2001;39(3):153–156. doi: 10.1016/S0041-624X(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 28.Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–185. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 29.Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance–guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85(1):22–29. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 31.Fennessy FM, Kong CY, Tempany CM, Swan JS. Quality-of-life assessment of fibroid treatment options and outcomes. Radiology. 2011;259(3):785–792. doi: 10.1148/radiol.11100704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 33.Zaher S, Lyons D, Regan L. Uncomplicated term vaginal delivery following magnetic resonance-guided focused ultrasound surgery for uterine fibroids. Biomed Imaging Intervention J. 2010;6(2):e28. doi: 10.2349/biij.6.2.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—preliminary clinical experience. Ann Oncol. 2006;18(1):163–167. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 35.Blana A, Walter B, Rogenhofer S, Wieland WF. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology. 2004;63(2):297–300. doi: 10.1016/j.urology.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Illing R, Kennedy J, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie T, Kennedy J. High intensity focused ultrasound in the treatment of abdominal and gynaecological diseases. Int J Hyperthermia. 2007;23(2):173–182. doi: 10.1080/02656730601150514. [DOI] [PubMed] [Google Scholar]
- 38.Uchida T, Ohori M, Egawa S. Minimally invasive therapy for bladder and prostate cancer. Gan to kagaku ryoho Cancer Chemother. 2001;28(8):1094–8. [PubMed] [Google Scholar]
- 39.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 40.Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—a primate study. Eur J Radiol. 2006;59(2):149–156. doi: 10.1016/j.ejrad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Medel R, Monteith SJ, Elias WJ, et al. Magnetic resonance–guided focused ultrasound surgery: part 2: a review of current and future applications. Neurosurgery. 2012;71(4):755–763. doi: 10.1227/NEU.0b013e3182672ac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepock JR. Measurement of protein stability and protein denaturation in cells using differential scanning calorimetry. Methods. 2005;35(2):117–125. doi: 10.1016/j.ymeth.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Guthkelch A, Carter L, Cassady J, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–284. doi: 10.1007/BF00177540. [DOI] [PubMed] [Google Scholar]
- 44.Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound forbrain tumortherapy. Neurosurgery. 2006;59(5):949–956. doi: 10.1227/01.NEU.0000254439.02736.D8. [DOI] [PubMed] [Google Scholar]
- 45.Park JW, Jung S, Jung TY, Lee MC, editors. Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: a preliminary report. AIP Conference Proceedings; 2006: AIP.
- 46.Hynynen K, Clement G. Clinical applications of focused ultrasound—the brain. Int J Hyperthermia. 2007;23(2):193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 47.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging–guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2:17. doi: 10.1186/2050-5736-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z. A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study. Urol Res. 2004;32(1):14–19. doi: 10.1007/s00240-003-0362-x. [DOI] [PubMed] [Google Scholar]
- 50.McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241(1):95–106. doi: 10.1148/radiol.2411051170. [DOI] [PubMed] [Google Scholar]
- 51.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60(10):1209–1217. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess A, Hynynen K. Drug delivery across the blood–brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11(5):711–721. doi: 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 54.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 55.Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging–guided focused ultrasound. J Ultrasound Med. 2009;28(7):871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 56.Liu H-L, Hua M-Y, Yang H-W, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci 2010:201003388. [DOI] [PMC free article] [PubMed]
- 57.Fan C-H, Ting C-Y, Chang Y-C, Wei K-C, Liu H-L, Yeh C-K. Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood–brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015;15:89–101. doi: 10.1016/j.actbio.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8(3):e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med. 2015;13:93. doi: 10.1186/s12967-015-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso A, Reinz E, Leuchs B, et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol Ther Nucleic Acids. 2013;2:e73. doi: 10.1038/mtna.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. 2014;4:126. doi: 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Paliwal S, Bankiewicz KS, et al. Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech. 2010;11(3):1005–1017. doi: 10.1208/s12249-010-9458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nance EA, Woodworth GF, Sailor KA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149):149ra19. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziadloo A, Xie J, Frenkel V. Pulsed focused ultrasound exposures enhance locally administered gene therapy in a murine solid tumor model. J Acoust Soc Am. 2013;133(3):1827–1834. doi: 10.1121/1.4789390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: implication for drug delivery. J Control Release. 2005;104(1):213–222. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Canney MS, Chavrier F, Tsysar S, Chapelon JY, Lafon C, Carpentier A. A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors. J Acoust Soc Am. 2013;134(2):1647–1655. doi: 10.1121/1.4812883. [DOI] [PubMed] [Google Scholar]
- 70.N’Djin WA, Burtnyk M, Lipsman N, et al. Active MR-temperature feedback control of dynamic interstitial ultrasound therapy in brain: in vivo experiments and modeling in native and coagulated tissues. Med Phys. 2014;41(9):093301. doi: 10.1118/1.4892923. [DOI] [PubMed] [Google Scholar]
- 71.Christian E, Yu C, Apuzzo ML. Focused ultrasound: relevant history and prospects for the addition of mechanical energy to the neurosurgical armamentarium. World Neurosurg. 2014;82(3–4):354–365. doi: 10.1016/j.wneu.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Cohen-Inbar O, Xu Z, Sheehan JP. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J Ther Ultrasound. 2016;4:2. doi: 10.1186/s40349-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mauri G, Nicosia L, Xu Z, et al. Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol. 2018;91(1083):20170641. doi: 10.1259/bjr.20170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Z, Yang XY, Liu Y, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun. 2005;335(1):124–131. doi: 10.1016/j.bbrc.2005.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen PY, Wei KC, Liu HL. Neural immune modulation and immunotherapy assisted by focused ultrasound induced blood-brain barrier opening. Hum Vaccin Immunother. 2015;11(11):2682–2687. doi: 10.1080/21645515.2015.1071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng J, Zhang Y, Feng J, Wu F. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound Med Biol. 2010;36(3):441–448. doi: 10.1016/j.ultrasmedbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu PH, Wei KC, Huang CY, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8(2):e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145(3):286–293. doi: 10.1016/j.surg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Finley DS, Pouliot F, Shuch B, et al. Ultrasound-based combination therapy: potential in urologic cancer. Expert Rev Anticancer Ther. 2011;11(1):107–113. doi: 10.1586/era.10.174. [DOI] [PubMed] [Google Scholar]
- 81.Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia. 2006;22(3):191–196. doi: 10.1080/02656730500532028. [DOI] [PubMed] [Google Scholar]
- 82.Escoffre J-M, Novell A, Serriere S, Lecomte T, Bouakaz A. Irinotecan delivery by microbubble-assisted ultrasound: in vitro validation and a pilot preclinical study. Mol Pharm. 2013;10(7):2667–2675. doi: 10.1021/mp400081b. [DOI] [PubMed] [Google Scholar]
- 83.Collis J, Manasseh R, Liovic P, et al. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 2010;50(2):273–279. doi: 10.1016/j.ultras.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Liang H, Tang J, Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc Inst Mech Eng H J Eng Med. 2010;224(2):343–361. doi: 10.1243/09544119JEIM565. [DOI] [PubMed] [Google Scholar]
- 85.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet (London, England) 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 86.D’Souza AL, Tseng JR, Pauly KB, et al. A strategy for blood biomarker amplification and localization using ultrasound. Proc Natl Acad Sci U S A. 2009;106(40):17152–17157. doi: 10.1073/pnas.0903437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song C, Park H, Lee C, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 2005;21(8):761–767. doi: 10.1080/02656730500204487. [DOI] [PubMed] [Google Scholar]
- 88.Yu T, Li S, Zhao J, Mason TJ. Ultrasound: a chemotherapy sensitizer. Technol Cancer Res Treat. 2006;5(1):51–60. doi: 10.1177/153303460600500107. [DOI] [PubMed] [Google Scholar]
- 89.Muenyi CS, Pinhas AR, Fan TW, Brock GN, Helm CW, States JC. Sodium arsenite ± hyperthermia sensitizes p53-expressing human ovarian cancer cells to cisplatin by modulating platinum-DNA damage responses. Toxicol Sci. 2012;127(1):139–149. doi: 10.1093/toxsci/kfs085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee Y-Y, Cho YJ, Choi J-J, et al. The effect of high-intensity focused ultrasound in combination with cisplatin using a xenograft model of cervical cancer. Anticancer Res. 2012;32(12):5285–5289. [PubMed] [Google Scholar]
- 91.Carpentier A, Canney M, Vignot A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 92.Monteith S, Sheehan J, Medel R, et al. Potential intracranial applications of magnetic resonance–guided focused ultrasound surgery: a review. J Neurosurg. 2013;118(2):215–221. doi: 10.3171/2012.10.JNS12449. [DOI] [PubMed] [Google Scholar]
- 93.Beccaria K, Canney M, Goldwirt L, et al. Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J Neurosurg. 2013;119(4):887–898. doi: 10.3171/2013.5.JNS122374. [DOI] [PubMed] [Google Scholar]
- 94.Beccaria K, Canney M, Goldwirt L, et al. Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan delivery: an experimental study in rabbits. J Neurosurg. 2016;124(6):1602–1610. doi: 10.3171/2015.4.JNS142893. [DOI] [PubMed] [Google Scholar]
- 95.Goldwirt L, Canney M, Horodyckid C, et al. Enhanced brain distribution of carboplatin in a primate model after blood–brain barrier disruption using an implantable ultrasound device. Cancer Chemother Pharmacol. 2016;77(1):211–216. doi: 10.1007/s00280-015-2930-5. [DOI] [PubMed] [Google Scholar]
- 96.Horodyckid C, Canney M, Vignot A, et al. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg. 2017;126(4):1351–1361. doi: 10.3171/2016.3.JNS151635. [DOI] [PubMed] [Google Scholar]
- 97.Gutierrez MI, Penilla EH, Leija L, Vera A, Garay JE, Aguilar G. Novel cranial implants of yttria-stabilized zirconia as acoustic windows for ultrasonic brain therapy. Adv Healthc Mater. 2017;6(21):1700214. doi: 10.1002/adhm.201700214. [DOI] [PubMed] [Google Scholar]
- 98.Suehiro S, Ohnishi T, Yamashita D, et al. Enhancement of antitumor activity by using 5-ALA–mediated sonodynamic therapy to induce apoptosis in malignant gliomas: significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J Neurosurg 2018:1–13. [DOI] [PubMed]
- 99.Hu Z, Fan H, Lv G, et al. 5-Aminolevulinic acid-mediated sonodynamic therapy induces anti-tumor effects in malignant melanoma via p53-miR-34a-Sirt1 axis. J Dermatol Sci. 2015;79(2):155–162. doi: 10.1016/j.jdermsci.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Zhou Q, Hu Z, et al. 5-Aminolevulinic acid-based sonodynamic therapy induces the apoptosis of osteosarcoma in mice. PLoS One. 2015;10(7):e0132074. doi: 10.1371/journal.pone.0132074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tachibana K, Feril LB, Jr, Ikeda-Dantsuji Y. Sonodynamic therapy. Ultrasonics. 2008;48(4):253–259. doi: 10.1016/j.ultras.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 102.Tang W, Liu Q, Wang X, Wang P, Zhang J, Cao B. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics. 2009;49(8):786–793. doi: 10.1016/j.ultras.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Heron M. Deaths: leading causes for 2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2016;65(5):1–96. [PubMed]
- 104.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 105.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 106.Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy: a population-based study. Stroke. 2016;47(5):1377–1380. doi: 10.1161/STROKEAHA.116.013165. [DOI] [PubMed] [Google Scholar]
- 107.Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543–549. doi: 10.1161/CIRCOUTCOMES.111.000095. [DOI] [PubMed] [Google Scholar]
- 108.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 110.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 111.Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS One. 2012;7(8):e42311. doi: 10.1371/journal.pone.0042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ilyas A, Chen CJ, Ding D, et al. Magnetic resonance-guided, high-intensity focused ultrasound sonolysis: potential applications for stroke. Neurosurg Focus. 2018;44(2):E12. doi: 10.3171/2017.11.FOCUS17608. [DOI] [PubMed] [Google Scholar]
- 113.Monteith SJ, Kassell NF, Goren O, Harnof S. Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage. Neurosurg Focus. 2013;34(5):E14. doi: 10.3171/2013.2.FOCUS1313. [DOI] [PubMed] [Google Scholar]
- 114.Weiss HL, Selvaraj P, Okita K, et al. Mechanical clot damage from cavitation during sonothrombolysis. J Acoust Soc Am. 2013;133(5):3159–3175. doi: 10.1121/1.4795774. [DOI] [PubMed] [Google Scholar]
- 115.Anschuetz R, Bernard HR. Ultrasonic irradiation and atherosclerosis. Surgery. 1965;57(4):549–553. [PubMed] [Google Scholar]
- 116.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 117.Medel R, Crowley RW, McKisic MS, Dumont AS, Kassell NF. Sonothrombolysis: an emerging modality for the management of stroke. Neurosurgery. 2009;65(5):979–993. doi: 10.1227/01.NEU.0000350226.30382.98. [DOI] [PubMed] [Google Scholar]
- 118.Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011;22(3):369–377. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wright C, Hynynen K, Goertz D. In vitro and in vivo high-intensity focused ultrasound thrombolysis. Invest Radiol. 2012;47(4):217–225. doi: 10.1097/RLI.0b013e31823cc75c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Monteith SJ, Harnof S, Medel R, et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J Neurosurg. 2013;118(5):1035–1045. doi: 10.3171/2012.12.JNS121095. [DOI] [PubMed] [Google Scholar]
- 121.Harnof S, Zibly Z, Hananel A, et al. Potential of magnetic resonance-guided focused ultrasound for intracranial hemorrhage: an in vivo feasibility study. J Stroke Cerebrovasc Dis. 2014;23(6):1585–1591. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 122.Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial doppler monitoring. Stroke. 2000;31(3):610–614. doi: 10.1161/01.STR.31.3.610. [DOI] [PubMed] [Google Scholar]
- 123.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 124.Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36(7):1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 125.Eggers J, Konig IR, Koch B, Handler G, Seidel G. Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39(5):1470–1475. doi: 10.1161/STROKEAHA.107.503870. [DOI] [PubMed] [Google Scholar]
- 126.Ahadi G, Welch CS, Grimm MJ, Fisher DJ, Zadicario E, Ernstrom K, et al. Transcranial sonothrombolysis using high-intensity focused ultrasound: impact of increasing output power on clot fragmentation. J Ther Ultrasound. 2013;1:22. doi: 10.1186/2050-5736-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamp MA, Fischer I, Buhner J, et al. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget. 2016;7(41):66776–66789. doi: 10.18632/oncotarget.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prada F, Bene MD, Fornaro R, Vetrano IG, Martegani A, Aiani L, et al. Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg Focus. 2016;40(3):E7. doi: 10.3171/2015.11.FOCUS15573. [DOI] [PubMed] [Google Scholar]
- 129.Prada F, Mattei L, Del Bene M, et al. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int. 2014;2014:9. doi: 10.1155/2014/484261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang TR, Bond AE, Dallapiazza RF, et al. Transcranial magnetic resonance imaging-guided focused ultrasound thalamotomy for tremor: technical note. Neurosurg Focus. 2018;44(2):E3. doi: 10.3171/2017.10.FOCUS17609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2466 kb)