Abstract
Over the last years, deep brain stimulation has seen many technological innovations. New electrode designs allowing to direct the current flow not only in the vertical but also in the horizontal plane are the most recent. We summarize the concept of “directional deep brain stimulation” with its opportunities and challenges and the available study data and discuss the use of imaging techniques to assist programming deep brain stimulation devices.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0667-7) contains supplementary material, which is available to authorized users.
Key Words: Deep brain stimulation, directional DBS, VTA modeling
Introduction
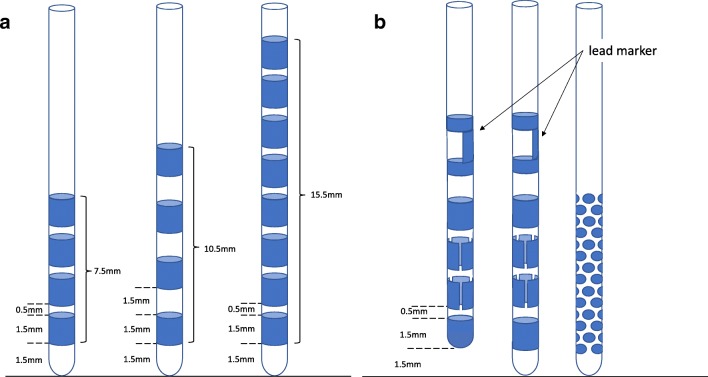
Deep brain stimulation (DBS) is an effective, long-term treatment for movement disorders like essential tremor [1–4], Parkinson’s disease [5, 6], or dystonia [7–9]. Efficacy and safety of DBS depend on the spatial restriction of the stimulation field to a functional target responsible for the beneficial effect of DBS. Stimulation-induced side effects emerge predominantly, if the volume of tissue activated (VTA) expands into adjacent, eloquent structures like the cortico-spinal tract, the medial lemniscus, or the ventral, limbic part of the subthalamic nucleus [10, 11]. Until 2015, CE-marked DBS leads had 4 to 8 cylindric contacts and variable interspacing spanning a vertical distance of 7.5 to 15.5 mm from the tip of the electrode (see Fig. 1A). With these leads and the corresponding internal pulse generators, it was possible to adjust the VTA vertically by polarity selection of the 4 to 8 contacts. The cylindric electrodes, however, always create a radial current diffusion in the horizontal plane of the lead and accurate positioning of the lead in x and y directions is critical to avoid adverse effects caused by current spread outside of the target region. Because the anatomical shape of most target areas like the subthalamic nucleus, the nucleus ventralis intermedius, and the globus pallidus internus is not spheric, there are practical limitations as to how a complete coverage of the intended target area can be achieved by a single lead with cylindric contacts [12–14].
Fig. 1.
Overview of different electrode design schemes. (A) Scheme of the most common DBS electrodes with 4–8 cylindric contacts at variable interspacings. (B) Schematic drawing of 2 currently available “directional DBS electrodes” with 8 contacts from which 6 “segmented” contacts span about 120°, which replace the 2 cylindric contacts in the middle, and a 40-contact lead design, used so far only within studies. The 2 commercially available directional DBS electrodes use either an “active tip” contact to allow more “downwards” current flow or a cylindric contact as the most distal. Both have a lead marker on top, which allows to control the rotational orientation via fluoroscopy
Concept of Directional DBS
In recent years, several new electrode designs have been proposed allowing to shape the electrical field perpendicular to the lead (see Fig. 2). Industry and clinicians hoped that “directional DBS” (dDBS) would reduce the risk of stimulation-induced adverse effects and optimize the clinical benefit of DBS, but this hypothetical concept could only be tested after first technical solutions became available for clinical use. Prototypes varied from electrodes with up to 40 small circular contacts of about 0.8 mm, which were evenly distributed over the last 5 to 6 mm of the electrode, and more simple models which split up the conventional ring contacts in 3 to 4 segments spanning 90° or about 120° [15] (see Fig. 1B). In 2014, two studies were published, which corroborated the principal hypothesis of dDBS, that current steering in the horizontal plane could modify the current threshold for beneficial and adverse effects, depending on whether current was injected towards or away from the underlying anatomical structures. Both studies used a similar acute, intraoperative design by evaluating current thresholds for stimulation induced clinical effects (e.g., rigidity reduction, dysarthria, or muscle contraction) comparing omnidirectional stimulation (simulated ring mode) against dDBS. This testing was performed intraoperatively by temporarily implanting a directional lead into the subthalamic nucleus, which was later exchanged for a conventional DBS electrode, because no dDBS system had regulatory approval at that time. The electrodes tested in these intraoperative studies had either 32 small circular contacts or segmented ring contacts [16, 17] and both designs resulted in comparable clinical steering effects. The electrode design with multiple small circular contacts proposed by the company Sapiens did not reach market level due to imponderabilities in the manufacturing process and control of stimulation by the associated pulse generator. In this context, it is important to note that lead and pulse generator form a functional unit and the way that stimulation is technically achieved (e.g., current vs voltage control or multiple independent current control) will have an impact on how reliably a computer-simulated field shape for particular polarity settings will be reflected in the “real” implant situation, where tissue properties interact with the technical capabilities of the system in an unpredictable way.
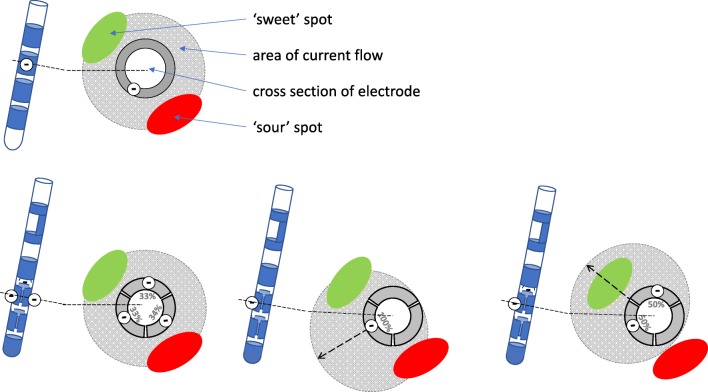
Fig. 2.
Concept of directional DBS. If the spot evoking the best therapeutic effect (“sweet” spot, green) lies equidistant to, but in a different direction from the DBS electrode than the spot causing limiting adverse effects (“sour” spot, red), a current flow from a cylindric contact strong enough to cover the “sweet” spot will also cause side effects by current spread to the “sour” spot (upper row). Instead of using all 3 segmented contacts of a directional electrode in the same location, one can steer the current flow away from the “sour” spot by activating only 1 or 2 of the segmented contacts which are oriented towards the “sweet” spot
Variations of the segmented lead design tested by Pollo and colleagues have meanwhile been approved for clinical use in Europe and lately in the USA. Both commercial leads (Boston Scientific Cartesia and Abbott St. Jude Infinity) are based on the classic design of a quadrupolar DBS lead, but the two middle electrode levels are segmented into 3 contacts each spanning approx. 120° of the circumference. If all segments are activated together, a ring electrode is simulated and a corresponding spherical VTA is generated (omnidirectional stimulation). By activating only 1 or 2 segments as cathode, the VTA can be shaped in the horizontal plane and current will be injected in a preferential angular direction.
First Clinical Experience with Directional DBS
In September 2015, the CartesiaTM lead (Boston Scientific, Valencia, CA) obtained CE mark, quickly followed by the Infinity directional lead of Abbott St. Jude Medical. First studies of the chronically implanted electrodes were conducted to corroborate the findings of intraoperative studies using a similar design of an acute monopolar review. We were able to document and quantify the effect of steering the VTA in steps of 60° around the lead on effect and adverse effect thresholds [18]. In comparison to omnidirectional stimulation (ring mode), directional DBS expanded or diminished the therapeutic window (TW) in relation to the angle of maximal current injection. Larger effects were seen for electrodes which had a suboptimal stimulation result in classical ring configuration, which indicates that dDBS could potentially compensate for small inaccuracies of lead placement, whereas little could be gained in comparison to a perfectly placed ring electrode [18]. Meanwhile, additional studies, of which one was blinded, have confirmed this result and shown that dDBS may also result in a better motor function [19, 20].
Segmented contacts have a smaller surface and therefore produce higher charge density with the same amplitude of current. This could be the reason why most studies have observed lower current thresholds for beneficial or adverse effects of dDBS as compared to those of omnidirectional stimulation. For most medical therapies, the dose is directly related to the “toxicity” of the treatment. Hence, one could hypothetically anticipate that the reduced current injection with dDBS may result in a better tolerated therapy. Rebelo et al. showed in their study of dDBS in essential tremor patients first evidence that dDBS might also be more energy efficient [20] compared to conventional DBS. Whether the lower charge injection will also result into increased battery longevity is difficult to answer from a technical perspective and is open to longitudinal observations, because the increased tissue impedance of smaller contacts will require a higher voltage for constant current stimulation, which will at least partially counterbalance savings from reduced amplitude settings.
A number of pressing questions related to dDBS cannot be answered by the “acute” proof of concept studies outlined above: [1] What is the incremental clinical benefit of dDBS as compared to optimized placement of a conventional lead?, [2] What is the number of patients needed to treat with dDBS to generate one better outcome and who should be selected for dDBS?, [3] Should dLeads be placed by the neurosurgeon in a similar location as compared to conventional leads?, [4] Is dDBS “efficient” given the more complex adjustment period with a manifold of programming options compared to conventional DBS?
Some of these questions may be answered by a small but blinded multicenter study comparing the chronic use of dDBS and ring-mode DBS. The study has completed recruitment and first results can be expected beginning of next year [21].
Challenges of dDBS
The availability of dDBS may be viewed as an invitation to lay less care in the accurate stereotactic placement of the lead, since one has more programming options to compensate for a small deviation from the optimal target, and could encourage less experienced teams to start DBS implants. In our opinion, the opposite is true: First, the distance in z-axis optimally covered by stimulation is reduced from the height of 4 classical ring contacts spanning about 7.5 mm to the distance of two segmented levels, which is only 3.5 mm. Therefore, precisely anchoring the lead at the optimal depth is more challenging with dDBS hardware. Second, the additional rotational degree of freedom turned out to be difficult to control by the neurosurgeon intraoperatively. The anterior marker of the lead at the burhole level does not necessarily reflect the orientation at the target level, because the flexible lead may be twisted along the way. On the other hand, the currently available radiopaque marker immediately proximal to the electrode level does not allow to determine the exact rotation on planar fluoroscopy. Several radiological methods have now been proposed to estimate the orientation of the segmented electrodes based on postoperative imaging (see below). Nevertheless, all these methods have limitations and a better intraoperative control not only of implant depth but also of the rotational angle would be desirable.
While the concept of dDBS is plausible from biophysical theory, its clinical value in a real life clinical setting has to be proven. The potential advantage of finetuning stimulation settings to an optimal benefit without stimulation-induced adverse effects is offset by an increased programming burden: Testing all contacts individually in a monopolar review would take at least twice the time of a standard 4-contact lead [22]. It is also questionable, how many Parkinson’s disease patients might tolerate this prolonged procedure in the medication off state. Furthermore, clinical differences between 2 adjacent segmented contacts might be too subtle to become obvious in a standard neurological examination. Combined with other options of advanced programming (multiple independent current control, reduced pulse width, multiple frequency settings, anodic stimulation, or bipolar settings), the number of testable combinations becomes infinite and no longer manageable in clinical practice. Practice guidelines are needed to educate programmers in the use of dDBS, but they are not available yet.
According to our own clinical experience, it is not always necessary to test all 8 contacts and programming time may be saved by a standardized programming approach and/or the use of additional imaging information. As a first step, we evaluate the efficacy threshold (e.g., tremor or rigidity reduction) and adverse effect threshold on all 4 contact levels in ring mode. Thereafter, we focus the monopolar review on the segmented electrode level with the best TW in ring mode. On this level, we evaluate again effect and side effect thresholds for each of the 3 segmented contacts. In most patients, this algorithm results in a contact configuration, which can be used to initiate stimulation. Subsequent finetuning can be achieved by any of the abovementioned advanced programming options, if the outcome is not satisfactory.
Another simplified monopolar review option requires connection of the segmented lead to a pulse generator capable of multiple independent current control. These systems allow to move the electrical field continuously along or perpendicular to the electrode. Hence, one can use a cursor-like option in the programmer software to steer a fixed stimulation current, which is usually sufficient to provide clinical benefit (e.g., 1.0–2 mA), but below the adverse effect threshold, in different directions along and around the electrode and evaluate changes in the type and magnitude of clinical response. When the vertical level with the best response has been found and segmented electrodes are involved, one repeats the same approach in dDBS mode by moving the current vector in the horizontal plane 360° clockwise or counterclockwise until the optimal direction has been determined by clinical comparison. For this resulting electrode configuration only, the side effect threshold is evaluated to ensure a reasonable TW for future programming adjustments.
The initial settings obtained by either method need to be adjusted subsequently depending on the clinical course and the predominant symptoms of a patient. More complex clinical problems such as gait or speech problems may need multiple further adjustments. There are different opinions of how fine graded adjustments of dDBS need to be in order to be clinically meaningful. We usually test increments of at least 10–20%, but other centers favor even smaller changes of 1–5%.
Outlook
MR imaging resolution as well as image fusion software and computational models predicting the volume of tissue activated has made tremendous progress in recent years. Especially, the fusion of a high-resolution postoperative CT, to visualize the electrode without distortion, with the preoperative MRI, used to visualize the target structure for stereotactic planning, can help to predict contacts with good clinical response. For these image-based programming options, one needs an alignment between the electrode and the patient-specific MRI space. For dDBS, the angular orientation of the segmented contacts needs to be determined. We assume that this orientation determined from intraoperative or postoperative imaging remains stable postoperatively, but this is currently not corroborated by any real life data.
Different radiological methods have recently been developed to determine the orientation of a dDBS lead in the chronic implanted state either by rotational fluoroscopy or by CT [23–25]. Using rotational fluoroscopy (e.g., a rotational angiography setup) several weeks to month after implantation, we have seen deviations from the intended orientation of segmented contacts, which were as large as 30°, especially in cases with brain shift due to intraoperative CSF leakage. Therefore, it still has to be shown, whether an electrode stays in its fixed rotational position or still turns within the first weeks after implantation and under which circumstances this may occur.
Conclusion
dDBS has been rapidly adopted in many DBS centers due to its theoretical advantages and may soon become the new standard electrode design for de novo implants. Our clinical experience with dDBS over the past 2 ½ years has been generally positive, which is reflected by approximately 2/3 of our de novo implants with dLeads, which are programmed in directional mode. This program choice was made either based on improved clinical efficacy or in order to diminish stimulation-induced adverse effects. Since we do not feel comfortable in predicting the future need for directional programming at the time of electrode implantation, we are currently using segmented electrodes for most of our implantations. Nevertheless, evidence for a clinical superiority of dDBS over conventional DBS is still lacking and will need to be generated from well-designed clinical studies in order to justify the incremental costs and programming efforts associated with this novel technology.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Electronic supplementary material
(PDF 1225 kb)
References
- 1.Blomstedt P, Hariz GM, Hariz MI, Koskinen LO. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg. 2007;21(5):504–9. doi: 10.1080/02688690701552278. [DOI] [PubMed] [Google Scholar]
- 2.Borretzen MN, Bjerknes S, Saehle T, Skjelland M, Skogseid IM, Toft M, et al. Long-term follow-up of thalamic deep brain stimulation for essential tremor - patient satisfaction and mortality. BMC Neurol. 2014;14:120. doi: 10.1186/1471-2377-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61(11):1601–4. doi: 10.1212/01.WNL.0000096012.07360.1C. [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long-term results of thalamic deep brain stimulation for essential tremor. J Neurosurg. 2010;112(6):1271–6. doi: 10.3171/2009.10.JNS09371. [DOI] [PubMed] [Google Scholar]
- 5.Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord. 2014;20(4):376–81. doi: 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Toft M, Lilleeng B, Ramm-Pettersen J, Skogseid IM, Gundersen V, Gerdts R, et al. Long-term efficacy and mortality in Parkinson’s disease patients treated with subthalamic stimulation. Mov Disord. 2011;26(10):1931–4. doi: 10.1002/mds.23817. [DOI] [PubMed] [Google Scholar]
- 7.Bruggemann N, Kuhn A, Schneider SA, Kamm C, Wolters A, Krause P, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. 2015;84(9):895–903. doi: 10.1212/WNL.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FitzGerald JJ, Rosendal F, de Pennington N, Joint C, Forrow B, Fletcher C, et al. Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. J Neurol Neurosurg Psychiatry. 2014;85(12):1371–6. doi: 10.1136/jnnp-2013-306833. [DOI] [PubMed] [Google Scholar]
- 9.Meoni S, Fraix V, Castrioto A, Benabid AL, Seigneuret E, Vercueil L, et al. Pallidal deep brain stimulation for dystonia: a long term study. J Neurol Neurosurg Psychiatry. 2017;88(11):960–7. doi: 10.1136/jnnp-2016-315504. [DOI] [PubMed] [Google Scholar]
- 10.Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord. 2016;25:1–9. doi: 10.1016/j.parkreldis.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry. 2009;80(6):659–66. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn A, Neumann WJ, Degen K, Schneider GH, Kuhn AA. Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. 2017. 10.1002/hbm.23594. [DOI] [PMC free article] [PubMed]
- 13.Neumann WJ, Horn A, Ewert S, Huebl J, Brucke C, Slentz C, et al. A localized pallidal physiomarker in cervical dystonia. Ann Neurol. 2017;82(6):912–24. doi: 10.1002/ana.25095. [DOI] [PubMed] [Google Scholar]
- 14.Wodarg F, Herzog J, Reese R, Falk D, Pinsker MO, Steigerwald F, et al. Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord. 2012;27(7):874–9. doi: 10.1002/mds.25006. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DN, Osting B, Vorwerk J, Dorval AD, Butson CR. Optimized programming algorithm for cylindrical and directional deep brain stimulation electrodes. J Neural Eng. 2018;15(2):026005. doi: 10.1088/1741-2552/aaa14b. [DOI] [PubMed] [Google Scholar]
- 16.Contarino MF, Bour LJ, Verhagen R, Lourens MA, de Bie RM, van den Munckhof P, et al. Directional steering: A novel approach to deep brain stimulation. Neurology. 2014;83(13):1163–9. doi: 10.1212/WNL.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 17.Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137(Pt 7):2015–26. doi: 10.1093/brain/awu102. [DOI] [PubMed] [Google Scholar]
- 18.Steigerwald F, Muller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord. 2016;31(8):1240–3. doi: 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dembek TA, Reker P, Visser-Vandewalle V, Wirths J, Treuer H, Klehr M, et al. Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord. 2017;32(10):1380–8. doi: 10.1002/mds.27093. [DOI] [PubMed] [Google Scholar]
- 20.Rebelo P, Green AL, Aziz TZ, Kent A, Schafer D, Venkatesan L, et al. Thalamic Directional Deep Brain Stimulation for tremor: Spend less, get more. Brain Stimul. 2018. [DOI] [PubMed]
- 21.Volkmann J, Chabardes S, Steinke GK, Carcieri S, Van Dyck N. (P2020) DIRECT DBS: A prospective, multi-center clinical trial with blinding for a directional DBS lead. Movement Disord. 2016;31(S2):666. [Google Scholar]
- 22.Ten Brinke TR, Odekerken VJJ, Dijk JM, van den Munckhof P, Schuurman PR, de Bie RMA. Directional Deep Brain Stimulation: First experiences in centers across the globe. Brain Stimul. 2018;11(4):949–50. doi: 10.1016/j.brs.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Reinacher PC, Kruger MT, Coenen VA, Shah M, Roelz R, Jenkner C, et al. Determining the Orientation of Directional Deep Brain Stimulation Electrodes Using 3D Rotational Fluoroscopy. AJNR Am J Neuroradiol. 2017;38(6):1111–6. doi: 10.3174/ajnr.A5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitz A, Hoevels M, Hellerbach A, Gierich A, Luyken K, Dembek TA, et al. Determining the orientation angle of directional leads for deep brain stimulation using computed tomography and digital x-ray imaging: A phantom study. Med Phys. 2017;44(9):4463–73. doi: 10.1002/mp.12424. [DOI] [PubMed] [Google Scholar]
- 25.Hunsche S, Neudorfer C, Majdoub FE, Maarouf M, Sauner D. Determining the Rotational Orientation of Directional Deep Brain Stimulation Leads Employing Flat-Panel Computed Tomography. Oper Neurosurg (Hagerstown). 2018. 10.1093/ons/opy163 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)