Abstract
TAFRO syndrome (thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, and organomegaly) is an atypical manifestation of multicentric Castleman’s disease. Although overproduction of interleukin-6, vascular endothelial growth factor, and other cytokines may partially explain the pathophysiology of this rare syndrome, the precise mechanisms underlying the renal dysfunction associated with the condition remain unclear. Here, we describe a case of a 69-year-old male with TAFRO syndrome. He was treated with immunosuppressive agents and his renal function improved. Tapering of immunosuppressive agents resulted in a deterioration of renal function and an elevation of C-reactive protein. After 20 months of treatment, the patient died from tuberculous peritonitis and gastrointestinal bleeding. An autopsy revealed miliary tuberculosis, mediastinal lymphadenopathy, and gastric ulcers. Renal histopathology showed a membranoproliferative glomerulonephritis-like appearance. Almost all glomeruli showed lobular formations with mesangial proliferation and duplication of glomerular capillary walls on light microscopy. Immunofluorescence showed deposition of C1q and IgM along the glomerular capillary walls. Electron microscopy showed mesangial expansion and widening of the subendothelial space with a large number of electron-dense deposits. The glomerular lesions might be characteristic of TAFRO syndrome, and were regarded as the main cause of the patient’s renal dysfunction.
Keywords: TAFRO syndrome, Renal dysfunction, Hypercytokinemia, Membranoproliferative glomerulonephritis-like lesions
Introduction
Recently, TAFRO syndrome (thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, and organomegaly) has been identified as a unique clinicopathologic variant of multicentric Castleman’s disease in Japan [1–3]. This syndrome is a systemic inflammatory disease characterized by a constellation of symptoms including thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly [1]. Most patients show varying degrees of renal dysfunction [1–3]; however, the mechanisms underlying renal dysfunction in patients with TAFRO syndrome are unclear, because histological examination of the kidneys is rarely reported. Here, we report a case of TAFRO syndrome who showed characteristic glomerular lesions at autopsy. These histological findings may be important for understanding the etiology of renal dysfunction in TAFRO syndrome.
Case report
A 69-year-old Japanese male was admitted to hospital with a fever of unknown origin 6 days before transfer to our hospital. The patient was given antibiotics, but his thrombocytopenia, renal failure, and anasarca worsened. He was transferred to our hospital for further care.
On admission, his blood pressure was 113/79 mmHg, his pulse was 110 beats/min with a regular rhythm, and he had a temperature of 37.1 °C. Physical examination revealed severe bilateral pitting edema of the lower extremities. Superficial lymph nodes were not palpable. Urinalysis showed a small degree of proteinuria (0.25 g/day) with microscopic hematuria (5–9 red blood cells per high power field) and increased urinary α1-microglobulin (Table 1). His white blood cell count was 12,000/µL, his hemoglobin was 11.5 mg/dL, and his platelet count was 2.4 × 104/L. Coagulation tests revealed prolonged activated partial thromboplastin time, fibrin degradation products, and D-dimer elevation. No fragmented erythrocytes were apparent on a peripheral blood smear. ADATMTS13 activity was slightly decreased, but ADAMTS13 inhibitor was undetectable. Serum total protein was 5.0 g/dL, serum albumin was 1.7 g/dL, creatinine was 2.22 mg/dL, and C-reactive protein (CRP) was 18.2 mg/dL. Serum immunoglobulin and complement were within normal ranges. Antinuclear antibodies and proteinase 3 antineutrophil cytoplasmic antibodies (ANCAs) were negative. Myeloperoxidase-ANCA was slightly increased. Serum interleukin-6 (IL-6) and vascular endothelial growth factor were elevated. Interferon gamma release assays were negative. Computed tomography showed bilateral pleural fluid and ascites, but did not show mediastinal or paraaortic lymphadenopathy or hepatosplenomegaly.
Table 1.
Laboratory data on admission
| [Urine tests] | ||
| Protein | 1+ | |
| 0.25 | g/day | |
| Occult blood | 2+ | |
| RBC | 5–9 | /HPF |
| WBC | 1–4 | /HPF |
| α1MG | 23.3 | mg/L |
| NAG | 16.3 | U/L |
| [Blood cell counts] | ||
| WBC | 12,000 | /µL |
| Neutrophil | 87.4 | % |
| Eosinophil | 0.1 | % |
| Lymphocyte | 5.7 | % |
| Monocyte | 6.5 | % |
| RBC | 358 × 104 | /µL |
| Hb | 11.5 | g/dL |
| Ht | 32.8 | % |
| Plt | 2.4 × 104 | /µL |
| [Coagulation tests] | ||
| PT-T | 20.6 | sec |
| PT-INR | 1.75 | |
| APTT | 46.8 | sec |
| FDP | 303.9 | µg/mL |
| D-Dimer | 159.3 | µg/mL |
| ADAMTS13 activity | 26.6 | % |
| ADAMTS13 inhibitor | Negative | |
| [Blood chemistry] | ||
| T.P | 5.0 | g/dL |
| Alb | 1.7 | g/dL |
| T-Bil | 2.3 | mg/dL |
| D-Bil | 1.6 | mg/dL |
| AST | 28 | IU/L |
| ALT | 20 | IU/L |
| ALP | 215 | IU/L |
| γ-GTP | 21 | IU/L |
| LDH | 216 | IU/L |
| CK | 20 | IU/L |
| UN | 79.9 | mg/dL |
| Cr | 2.22 | mg/dL |
| UA | 9.6 | mg/dL |
| Na | 136 | mEq/L |
| K | 4.4 | mEq/L |
| Cl | 107 | mEq/L |
| Ca | 7.8 | mg/dL |
| P | 6.7 | mg/dL |
| Glu | 123 | mg/dL |
| IL-6 | 26.6 | pg/mL |
| VEGF | 183 | pg/mL |
| sIL-2R | 823 | U/mL |
| [Serological tests] | ||
| CRP | 18.2 | mg/dL |
| RF | 11 | IU/mL |
| IgG | 1327 | mg/dL |
| IgA | 398 | mg/dL |
| IgM | 59 | mg/dL |
| C3 | 59 | mg/dL |
| C4 | 18 | mg/dL |
| CH50 | 34 | U/mL |
| ANA | Negative | |
| Anti GBM antibody | Negative | |
| PR3-ANCA | Negative | |
| MPO-ANCA | 11.5 | U/mL |
Considering the patient’s elevated myeloperoxidase–ANCA, steroid pulse therapy (methylprednisolone 500 mg/day for 3 days starting on day 6) was initiated followed by oral prednisolone (20 mg/day). After steroid pulse therapy, the patient’s renal function and CRP level gradually improved, but he required dialysis for uremia from day 21 to day 42. On day 41, bone marrow aspiration was performed because of persistent thrombocytopenia. The marrow aspirates revealed hypocellular bone marrow with mild reticulin fibrosis, but the number of megakaryocytes was not elevated. Based on these findings, the patient was diagnosed with TAFRO syndrome.
Despite two courses of steroid pulse therapy, the patient’s thrombocytopenia did not improve, and a transfusion of platelets was required to treat bleeding diathesis. On day 52, he was treated with cyclosporine A (200 mg/day). His platelet counts gradually increased and he was discharged on day 92.
After discharge, the patient suffered from cholecystitis and cytomegalovirus infection (twice each), which were successfully treated with antibiotics and ganciclovir. Because of the risk of opportunistic infections, cyclosporine A was stopped and prednisolone was reduced. However, tapering prednisolone resulted in deterioration of renal function and CRP elevation.
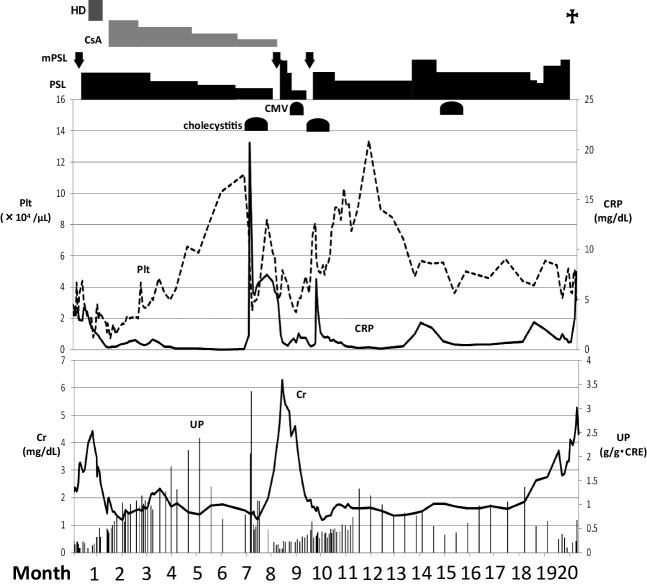
Twenty months after beginning treatment, the patient was again admitted to our hospital with massive ascites, progression of renal dysfunction, and elevated CRP. Paracentesis was performed and the ascites fluid was positive for Mycobacterium tuberculosis. Anti-tubercular drugs (rifampicin, isoniazid, pyrazinamide, and ethambutol) were started, but the patient died of gastrointestinal bleeding 3 weeks after admission (Fig. 1).
Fig. 1.
Clinical course of the TAFRO syndrome case. HD hemodialysis, CsA cyclosporine A, mPSL methylprednisolone, PSL prednisolone, CMV cytomegalovirus infection, Plt platelet, CRP C-reactive protein, Cr creatinine, UP urinary protein
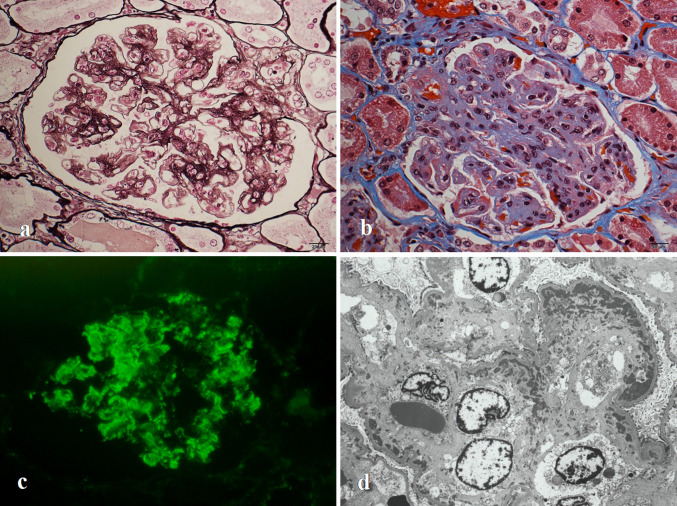
Autopsy revealed pleural effusion (right: 1,200 mL, left: 100 mL), ascites (9,900 mL), mediastinal lymphadenopathy, and ulceration of the stomach. Microscopically, the mediastinal lymph nodes showed partial vascular proliferation and accumulation of plasma cells. Bone marrow analysis revealed hypocellular marrow without reticulin fibrosis. Necrotizing epithelioid granuloma indicative of miliary tuberculosis was observed in both lungs as well as the liver, pleura, and peritoneum. Renal histological findings revealed a membranoproliferative glomerulonephritis-like appearance. Almost all glomeruli showed lobular formations with mesangial proliferation and duplication of glomerular capillary walls on light microscopy (Fig. 2a). Using Masson’s trichrome staining, red-stained material was observed inside several glomerular tufts (Fig. 2b). Immunofluorescence microscopy showed deposition of C1q and IgM along the glomerular capillary walls (Fig. 2c). Electron microscopy showed mesangial expansion and widening of the subendothelial space with a large number of electron-dense deposits (Fig. 2d).
Fig. 2.
a Light microscopy findings using periodic acid methenamine silver staining revealed global mesangial proliferation with duplication of the glomerular capillary walls (original magnification: ×400). b Light microscopy findings using Masson’s trichrome staining revealed lobular formations with red-stained material along several glomerular tufts (original magnification: ×400). c Immunofluorescence microscopy showed globally coarse granular staining of C1q along the glomerular capillary walls (original magnification: ×200). d Electron microscopy revealed proliferation of mesangial cells and widening of the subendothelial space with electron-dense deposits (original magnification: ×3,000)
Discussion
In 2010, Takai et al. [4] reported three patients exhibiting a constellation of symptoms including thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly; they termed this condition TAFRO syndrome. TAFRO syndrome was defined as a novel systemic inflammatory disease at a Japanese consensus meeting in 2012 [1]. Masaki et al. proposed a new classification for diagnostic criteria and disease severity based on 28 cases of TAFRO syndrome [5].
The case described here showed progressive renal dysfunction with substantial thrombocytopenia. However, fragmented erythrocytes, a notable decrease of ADAMTS13 activity and ADAMTS13 inhibitor were not detected. These findings were not consistent with hemolytic-uremic syndrome or thrombotic thrombocytopenic purpura. Multicentric Castleman’s disease and POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome frequently present with ascites, pleural effusion, organomegaly and renal dysfunction [6, 7]. These clinical features resemble TAFRO syndrome (Table 2) [6–8]. The case described here did not display the characteristic multiple lymphadenopathy of multicentric Castleman’s disease nor the polyneuropathy and M proteinemia of POEMS syndrome. Instead, he fulfilled the major criteria (anasarca, thrombocytopenia and systemic inflammation) and two of four minor criteria (reticulin myelofibrosis in bone marrow and progressive renal insufficiency) of TAFRO syndrome. Based on these findings, we diagnosed the patient with TAFRO syndrome.
Table 2.
Clinical characteristics of multicentric Castleman’s disease, POEMS syndrome and TAFRO syndrome
| Multicentric Castleman’s disease | POEMS syndrome | TAFRO syndrome | |
|---|---|---|---|
| Reference (no) | [6] | [7] | [8] |
| Anasarca (pleural effusion/ascites) | 13% | 29–87% | 100% |
| Thrombocytopenia | 17% | 54–88% | 100% |
| Organomegaly | 19% | 45–85% | 88.9% |
| Lymphadenopathy | 100% | 26–74% | 94.4% |
| Renal insufficiency | 19–54% | 6–9% | 55.6% |
| Neuropathy | – | 100% | – |
| Endocrinopathy | – | 67–84% | – |
| Monoclonal gammopathy | – | 100% | – |
| Cutaneous lesions | – | 68–89% | – |
The etiology of TAFRO syndrome remains unclear, but some characteristic findings, such as elevated serum IL-6 and lymph node histopathology, are similar to those seen in patients with multicentric Castleman’s disease [9, 10]. Thus, TAFRO syndrome is classified as an atypical variant of multicentric Castleman’s disease which is associated with hypercytokinemia [11]. In multicentric Castleman’s disease, polyclonal B-cell activation results in hypergammaglobulinemia and autoantibody production. Fruichi et al. reported a case of MPO-ANCA positive rapidly progressive glomerulonephritis associated with Castleman’s disease [12]. We theorize that a similar mechanism might have produced MPO-ANCA titers in the case described here.
Although a standard treatment for TAFRO syndrome has not been established, most patients are treated with steroids, including immunosuppressive drugs such as tocilizumab [13, 14], cyclosporine A [10, 15, 16], and rituximab [17]. These treatments have been reported to be effective in most patients, although some individuals were refractory to treatment and progressed to a fatal outcome [2, 18, 19]. In the case described here, steroid and cyclosporine A treatment were also initially effective, but the patient’s repetitive cholecystitis and cytomegalovirus infection prevented us from starting other immunosuppressive drugs such as tocilizumab or rituximab.
TAFRO syndrome involves varying degrees of renal dysfunction, and some patients may require hemodialysis [2, 13, 14, 20]. Renal dysfunction is one of the characteristic features of TAFRO syndrome; however, its precise mechanism is unclear because histological examination of the kidneys is rarely performed. It has been suggested that renal dysfunction results from intravascular volume depletion because of excessive vascular permeability. Several autopsy cases have been reported, but made little mention of renal lesions [2, 18, 19]. To date, five biopsy cases describing the renal histology of TAFRO syndrome have been reported (Table 3). Two cases (case 1 and case 4) showed thrombotic microangiopathy-like lesions, another two cases (case 3 and case 5) showed a membranoproliferative glomerulonephritis-like appearance, and one case (case 2) showed diffuse endocapillary proliferation with mesangiolysis [20–24]. Immunofluorescence studies were negative for immunoglobulins or complement factors in three cases (cases 2, 3, and 4). One case (case 5) showed partially granular IgM deposition by immunofluorescence microscopy. Electron microscopy showed glomerular endothelial swelling in three cases (cases 2, 3, and 4) and one case (case 5) showed duplication of the glomerular basement membrane with mesangial interposition. For case 1, immunofluorescence and electron microscopy findings were not described. However, Xu et al. reported renal histological findings in a case of multicentric Castleman’s disease with renal involvement, which showed thrombotic microangiopathy-like lesions with features of endothelial swelling and subendothelial space widening with double contour or subendothelial accumulation of protein and debris [25]. Moreover, Nakamoto et al. [26] reported renal histological findings of a case of POEMS syndrome, which showed glomerular enlargement, cellular proliferation, mesangiolysis and swelling of endothelial and mesangial cells. These renal histological findings are also similar to those of TAFRO syndrome.
Table 3.
Renal histological findings in TAFRO syndrome
| Case | Author | Reference (no) | Age/sex | Tissue source | Light microscopy | Immunofluorescence staining | Electron microscopy |
|---|---|---|---|---|---|---|---|
| 1 | José et al. | [20] | 61/female | Biopsy | TMA with mesangial expansion and duplication of GBM | Not described | Not described |
| 2 | Mizuno et al. | [21] | 84/male | Biopsy | Diffuse endocapillary proliferation with mesangiolysis | Negative | Swelling of glomerular endothelial cells |
| Autopsy | Collapsed glomeruli | Not described | Collapsed glomeruli | ||||
| 3 | Tanaka et al. | [22] | 70/male | Biopsy | MPGN-like lesions | Negative | Glomerular endothelial swelling and electron-lucent widening of the subendothelial space |
| 4 | Ozeki et al. | [23] | 51/female | Biopsy | TMA-like lesions | Negative | Glomerular endothelial swelling |
| 5 | Furuto et al. | [24] | 55/female | Biopsy | Glomerular lobulation with duplication of GBM | Partially granular depositions of IgM | Partial duplication of GBM and mesangial interposition |
| Present case | 69/male | Autopsy | MPGN-like lesions | Deposition of C1q and IgM along the glomerular capillary walls | Mesangial expansion and widening of the subendothelial space with a large amount of electron-dense deposits |
TMA thrombotic microangiopathy, MPGN membranoproliferative glomerulonephritis, GBM glomerular basement membrane
In the case described here, renal function improved with steroid treatment and the patient was able to discontinue hemodialysis during his first hospitalization. Although proteinuria and mild renal dysfunction persisted, we could not perform a renal biopsy because of sustained thrombocytopenia. Renal histological findings at autopsy revealed a membranoproliferative glomerulonephritis-like appearance, mesangial proliferation and duplication of the glomerular capillary walls, and deposition of C1q and IgM. However, other immunoglobulins or complement factors were not observed by immunofluorescence microscopy. From these findings, we hypothesized that deposition occurred through leakage into the subendothelial space through injury to the glomerular endothelium. The glomerular lesions, suggesting chronic injury to the glomerular endothelium, might be induced by hypercytokinemia as a potential mechanism of TAFRO syndrome.
From these observations, we deduced that the etiology of renal dysfunction accompanying TAFRO syndrome involves the prerenal factors of hypovolemia and glomerular injury, with thrombotic microangiopathy- or membranoproliferative glomerulonephritis-like lesions caused by endothelial impairment.
In summary, we described an autopsy case of TAFRO syndrome with membranoproliferative glomerulonephritis-like lesions. More case reports with descriptions of the renal histopathology will be necessary to clarify the precise mechanisms underlying the renal dysfunction associated with TAFRO syndrome.
Conflict of interest
The authors have declared no conflicts of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Kawabata H, Takai K, Kojima M, Nakamura N, Aoki S, Nakamura S, Kinoshita T, Masaki Y. Castleman-Kojima disease (TAFRO Syndrome), a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012) J Clin Exp Hematop. 2013;53:57–61. doi: 10.3960/jslrt.53.57. [DOI] [PubMed] [Google Scholar]
- 2.Takai K, Nikkuni K, Motomi A, Nagai K, Igarashi N, Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J Clin Exp Hematop. 2013;53:63–68. doi: 10.3960/jslrt.53.63. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata H, Kadowaki N, Nishikori M, Kitawaki T, Kondo T, Ishikawa T, Yoshifuji H, Yamakawa N, Imura Y, Minori T, Mtsumura Y, Miyachi Y, Matsubara T, Yanagita M, Haga H, Takaori-Kondo A. Clinical features and treatment of multicentric Castleman’s disease: a retrospective study of 21 Japanese patients at a single institute. J Clin Exp Hematop. 2013;53:69–77. doi: 10.3960/jslrt.53.69. [DOI] [PubMed] [Google Scholar]
- 4.Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsho Ketsueki. 2010;51:320–325. [PubMed] [Google Scholar]
- 5.Masaki Y, Kawabata H, Takai K, Kojima M, Tsukamoto N, Ishigaki Y, Kurose N, Ide M, Murakami J, Nara K, Yamamoto H, Ozawa Y, Takahashi H, Miura K, Miyauchi T, Yoshida S, Momoi A, Awano N, Ikushima S, Ohta Y, Furuta N, Fujimoto S, Kawanami H, Sakai T, Kawanami T, Fujita Y, Fukushima T, Nakamura S, Kinoshita T, Aoki S. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103:686–692. doi: 10.1007/s12185-016-1979-1. [DOI] [PubMed] [Google Scholar]
- 6.Szalat R, Munshi NC. Diagnosis of castleman disease. Hematol Oncol Clin N Am. 2018;32:53–64. doi: 10.1016/j.hoc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Kourelis T, Buandi F. POEMS syndrome diagnosis and investigative work-up. Hematol Oncol Clin N Am. 2018;32:119–139. doi: 10.1016/j.hoc.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Igawa T, Sato Y. TAFRO syndrome. Hematol Oncol Clin N Am. 2018;32:107–118. doi: 10.1016/j.hoc.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Iwaki N, Sato Y, Takata K, Kondo E, Ohno K, Takeuchi M, Orita Y, Nakao S, Yoshino T. Atypical hyaline vascular-type Castleman’s disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J Clin Exp Hematop. 2013;53:87–93. doi: 10.3960/jslrt.53.87. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporine A: a case report. J Clin Exp Hematop. 2013;53:95–99. doi: 10.3960/jslrt.53.95. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki N, Fajgenbaum DC, Nabel CS, Gion Y, Kondo E, Kawano M, Masunari T, Yoshida I, Moro H, Nikkuni K, Takai K, Matsue K, Kurosawa M, Hagihara M, Saito A, Okamoto M, Yokota K, Hiraiwa S, Nakamura N, Nakao S, Yoshino T, Sato Y. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220–226. doi: 10.1002/ajh.24242. [DOI] [PubMed] [Google Scholar]
- 12.Furuichi K, Wada T, Shimizzu M, Segawa C, Ohta S, Takasawa K, Kobayashi K, Yokoyama H. Antimyeloperoxidase-antibody-positive rapidly progressive glomerulonephritis associated with Castleman’s disease. Nephrol Dial Transplant. 1998;13:1556–1558. doi: 10.1093/ndt/13.6.1556. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata H, Kotani S, Matsumura Y, Kondo T, Katsurada T, Haga H, Kadowaki N, Takaori-Kondo A. Successful treatment of a patient with multicentric Castleman’s disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody. Intern Med. 2013;52:1503–1507. doi: 10.2169/internalmedicine.52.9482. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara S, Mochinaga H, Nakata H, Ohshima K, Matsumoto M, Uchida M, Mikami Y, Hata H, Okuno Y, Mitsuya H, Nosaka K. Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol. 2016;103:718–723. doi: 10.1007/s12185-016-1978-2. [DOI] [PubMed] [Google Scholar]
- 15.Konishi Y, Takahashi S, Nishi K, Sakamaki T, Mitani S, Kaneko H, Mizutani C, Ukyo N, Hirata H, Tsudo M. Successful treatment of TAFRO syndrome, a variant of multicentric Castleman’s disease, with cyclosporine A: possible pathogenetic contribution of interleukin-2. Tohoku J Exp Med. 2015;236:289–295. doi: 10.1620/tjem.236.289. [DOI] [PubMed] [Google Scholar]
- 16.Yamaga Y, Tokuyama K, Kato T, Yamada R, Murayama M, Ikeda T, Yamakita M, Kunieda T. Successful treatment with cyclosporin A in tocilizumab-resistant TAFRO syndrome. Intern Med. 2016;55:185–190. doi: 10.2169/internalmedicine.55.4710. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa T, Kosugi S, Kito M, Onishi M, Kida T, Nakata S, Take H, Katagiri S. Efficacy of rituximab for a variant type of multicentric Castleman’s disease termed the TAFRO syndrome. Rinsho Ketsueki. 2013;55:350–355. [PubMed] [Google Scholar]
- 18.Masaki Y, Nakajima A, Iwao H, Kurose N, Sato T, Nakamura T, Miki M, Sakai T, Kawanami T, Sawaki T, Fujita Y, Tanaka M, Fukushima T, Okazaki T, Umehara H. Japanese variant of multicentric Castleman’s disease associated with serositis and thrombocytopenia—a report of two cases: Is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop. 2013;53:79–85. doi: 10.3960/jslrt.53.79. [DOI] [PubMed] [Google Scholar]
- 19.Tadokoro A, Kanaji N, Hara T, Matsumoto K, Ishii T, Takagi T, Watanabe N, Kita N, Kawauchi M, Ueno M, Kadowaki N, Bandoh S. An uncharted constellation: TAFRO syndrome. Am J Med. 2016;129:938–941. doi: 10.1016/j.amjmed.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 20.José FF, Kerbauy LN, Perini GF, Blumenschein DI, Pasqualin DC, Malheiros DMAC, Neto GCC, Santos FPS, Piovesan R, Hamerschlak N. A life-threatening case of TAFRO syndrome with dramatic response to tocilizumab, rituximab, and pulse steroids. Medicine. 2017;96(13):e6271. doi: 10.1097/MD.0000000000006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno H, Sekine A, Oguro M, Oshima Y, Kawada M, Sumida K, Yamanouchi M, Hayami N, Suwabe T, Hiramatsu R, Hasegawa E, Hoshino J, Sawa N, Fujii T, Takaichi K, Ohashi K, Ubara Y. Renal histology in a patient with TAFRO syndrome: a case report. Hum Pathol. 2018 doi: 10.1016/j.humpath.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Tsujimoto H, Yamamoto K, Shimoda S, Oka K, Takeoka H. Clinicopathological features of progressive renal involvement in TAFRO syndrome. Medicine. 2017;96(40):e8216. doi: 10.1097/MD.0000000000008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozeki T, Tsuji M, Yamamoto J, Shigematu C, Maruyama S. Thrombotic microangiopathy on kidney biopsy in a patient with TAFRO syndrome. Cen Case Rep. 2018 doi: 10.1007/s13730-018-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuto Y, Hashimoto H, Horiuti H, Shibuya Y. Membranoproliferative glomerulonephritis-like findings for TAFRO syndrome, associated with an anterior mediastinal tumor: a case report. Medicine. 2018;97(24):e11057. doi: 10.1097/MD.0000000000011057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Lv J, Dong Y, Wang S, Su T, Zhou F, Zou W, Zhao M, Zhang H. Renal involvement in a large cohort of Chinese patients with Castleman disease. Nephrol Dial Transplant. 2012;27(supple3):iii119–iii125. doi: 10.1093/ndt/gfr245. [DOI] [PubMed] [Google Scholar]
- 26.Nakamoto Y, Imai H, Yasuda T, Wakui H, Miura AB. A spectrum of clinicopathological features of nephropathy associated with POEMS syndrome. Nephrol Dial Transplant. 1999;14:2370–2378. doi: 10.1093/ndt/14.10.2370. [DOI] [PubMed] [Google Scholar]