Abstract
Rituximab (RTX) is increasingly used for the treatment of refractory nephrotic syndrome due to its inhibitory effect on B cells which extends the period of remission, while lowering the dose of steroids needed for disease management. However, RTX can lead to various side effects, including Crohn’s disease. Herein, we describe a case of a 15-year-old boy with refractory nephrotic syndrome diagnosed at age 9 years who developed Crohn’s disease following RTX treatment. RTX was initiated in this patient at the age of 13 years 6 months due to occurrence of 12 relapses of nephrotic syndrome over a 4-year period, despite treatment using cyclosporine, steroid pulse therapy, and mycophenolate mofetil. The patient received 4 doses of RTX over a 2-year period (dose, 375 mg/m2). Although the treatment was effective in extending the disease-free duration up to 6 months, at the age of 15 years 9 months, the patient developed abdominal pain, associated with frequent watery stools and rapid weight loss. Based on clinical and endoscopic findings, he was diagnosed with Crohn’s disease and treated using infliximab. Remission of Crohn’s disease was achieved with this treatment, with no further relapse of nephrotic syndrome. Infliximab is thought to extend the remission period of nephrotic syndrome. In this case, we propose that Crohn’s disease was caused by an abnormal immune tolerance, secondary to the use of RTX, although the exact underlying mechanism remains to be clarified. Therefore, inflammatory bowel disease should be considered if severe abdominal symptoms with weight loss following RTX administration are observed.
Keywords: Refractory nephrotic syndrome, Rituximab, Crohn’s disease, Infliximab
Introduction
Steroids are the first-line treatment for idiopathic nephrotic syndrome of childhood, with approximately 80% of cases achieving remission [1]. However, 80% of cases eventually relapse [2], with half of these patients experiencing frequent relapses [3]. Steroid-dependent patients who experience frequent relapses require large total doses of steroids for treatment, which increases their susceptibility to developing adverse drug reactions. In this regard, rituximab (RTX) might be useful in preventing relapsing nephrotic syndrome through its inhibition of B-cell humoral immunity and B–T cell interaction, allowing a reduction in the required steroid dose while extending the duration of remission [4]. However, RTX has numerous reported side effects, including infection. We report the case of 15-year-old boy who developed Crohn’s disease, which is thought to be a side effect of RTX, with disease remission obtained by infliximab, and infliximab also extended the duration of remission of nephrotic syndrome.
Case description
The patient was a 15-year-old boy, with an unremarkable family history. The child had developed nephrotic syndrome at the age 9 years, treated using cyclosporine, steroid pulse therapy and mycophenolate mofetil (MMF) for steroid-dependent, frequently relapsing, intractable nephrotic syndrome. However, the patient experienced 12 relapses over a period of 4 years. Moreover, by the age 13 years, he had developed obesity (body mass index, 30.4 kg/m2), fatty liver disease, and osteoporosis, secondary to the prolonged steroid treatment. We initiated RTX treatment at the age of 13 years 6 months, at an initial dose of 375 mg/m2, with a concomitant decrease in the steroid dose. RTX treatment was effective in extending the duration of remission, with relapse occurring, on average, once every 6 months. Over a 2-year period, the patient received four doses of RTX.
The period of remission with RTX was associated with a reduced CD19 expression. Of note, every 3–4 months, the serum level of CD19 expression increased, with this increase being following by a relapse of the nephrotic syndrome. Based on this time-history of relapse, the parents requested that the fourth dose of RTX be administered despite the absence of an increase in CD19 expression at that time. During RTX treatment, other medications had been reduced, as follows: MMF, 2000 mg/day with a trough value of 1.1 µg/ml. Steroid administration was discontinued at the age of 13 years 11 months, namely 5 months after the initial RTX dose.
Current clinical presentation
At the age of approximately 15 years 9 months, the patient experienced abdominal pain, localized in the area of the umbilicus. Over the subsequent month, he began passing watery stools, up to four times daily. We considered these symptoms as being possible side effects of MMF and, therefore, the drug was discontinued. The patient subsequently experienced rapid weight loss of 10 kg over a period of 2 months. As the gastrointestinal symptoms were not improving, the patient was admitted to our hospital.
On admission, vital signs were as follows: pulse, 73 bpm; blood pressure, 113/73 mmHg; and temperature, 36.5 °C. Over the previous 2.5 months, his weight had decreased from 69.3 to 56.8 kg, a loss of around 13 kg. He was passing watery stools four times daily. His abdomen was flat, but he reported lower abdominal pain around the umbilicus. Oral aphtha was present. The laboratory data on admission are reported in Table 1, and were indicative of an inflammatory reaction, with a white blood cell count of 14,100/µl and C-reactive protein level of 8.35 mg/dl. His erythrocyte sedimentation rate was elevated and complement activity was high.
Table 1.
Laboratory data on admission
| Unit | Unit | ||||
|---|---|---|---|---|---|
| TP | 7.1 | g/dl | Complete blood count | ||
| Alb | 3.5 | g/dl | WBC | 141 | × 102/µl |
| T-Bil | 0.7 | mg/dl | RBC | 503 | × 104/µl |
| AST | 13 | U/L | Hb | 13.6 | g/dl |
| ALT | 14 | U/L | HCT | 40.1 | % |
| LDH | 158 | U/L | MCV | 79.7 | fl |
| ALP | 412 | U/L | MCH | 27.0 | pg |
| γ-GT | 32 | U/L | MCHC | 33.9 | % |
| AMY | 48 | U/L | Platelet | 39.0 | × 104/µl |
| BUN | 9 | mg/dl | Seg | 80.0 | % |
| UA | 8.0 | mg/dl | Lym | 12.0 | % |
| Cre | 0.52 | mg/dl | Mono | 6.5 | % |
| Na | 139 | mmol/l | Eos | 1.0 | % |
| K | 3.8 | mmol/l | |||
| T-Cho | 142 | mg/dl | Urine analysis | ||
| HDL | 40 | mg/dl | β2MG | 358 | ng/ml |
| LDL | 82 | mg/dl | β2MG/Cre | 98.9 | µg/g Cre |
| TG | 89 | mg/dl | TP/Cre | 0.06 | g/g Cre |
| CRP | 8.35 | mg/dl | Cre | 362 | mg/dl |
| PCT | 0.12 | ng/ml | Na | 96 | mmol/l |
| ESR30m | 28 | mm | K | 51.6 | mmol/l |
| ESR1h | 74 | mm | Cl | 156 | mmol/l |
| ESR2h | 112 | mm | SG | 1.030 | |
| ANA | 40 < | times | pH | 6.0 | |
| IgG | 1021 | mg/dl | Glucose | Negative | |
| IgA | 404 | mg/dl | Protein | Negative | |
| IgM | 31 | mg/dl | OB | Negative | |
| C3 | 181 | mg/dl | |||
| C4 | 46 | mg/dl | |||
ESR erythrocyte sedimentation rate, ANA antinuclear antibody, SG specific gravity, OB occult blood
The post-admission course is summarized in Fig. 1a. As infectious enteritis was suspected, antibiotics, including ceftriaxone and cefcapene, were administered. However, test results for infectious enteritis were negative (Table 2). Therefore, a contrast-enhanced computed tomography scan of the abdomen was performed on post-admission day 6, revealing circumferential thickening of the intestinal wall, from the ileocecal junction to the ascending colon. A total colonoscopy was performed in the Department of Inflammatory Bowel Disease on day 7. The endoscopy revealed multiple punched-out ulcers and cobblestone-like appearance, mainly evident in the ascending colon, with scattered patches of aphtha observable from the transverse colon to the rectum (Fig. 2). Video capsule endoscopy also revealed numerous erosions in the small intestine (Fig. 3). Based on these findings, Crohn’s disease was suspected and total parenteral nutrition therapy was initiated. As the cause of the water stools was now known, MMF therapy was restarted.
Fig. 1.
a Clinical course from admission, through diagnosis of Crohn’s disease and amelioration of the disease status. The dashed line shows the change in white blood cell count, the solid line the change in C-reactive protein, and the area graph, the change in frequency of watery stools. CF colono fiberscope, VCE video capsule endoscopy, CTRX ceftriaxone, CFPN-PI cefcapene pivoxil, LVFX levofloxacin. b Serum levels of CD19 expression, from the onset of Crohn’s disease to post-remission. The dotted line shows the change in white blood cell count, the solid line the change in C-reactive protein, and the dashed line the increase in serum levels of CD19
Table 2.
Infectious enteritis test
| Fecal norovirus antigen | Negative |
| Fecal rotavirus antigen | Negative |
| Entamoeba histolytica smear | Negative |
| Entamoeba histolytica iodine stain | Negative |
| Fecal culture | Negative |
| Fecal acid-fast bacterium | Negative |
| Tuberculosis PCR | Negative |
| QFT-TB | Negative |
| QFT-TBAg | Negative |
| Serum β-d glucan | < 11 pg/ml |
| Serum HSV-IgG | 34.2 (positive) |
| Serum HSV-IgM | 0.41 (negative) |
| Serum CMV-IgG | < 2.0 (negative) |
| Serum CMV-IgM | 0.36 (negative) |
| Serum CMV PCR | Negative |
Fig. 2.

Colonofiberscopy findings, showing a cobble stone appearance and deep ulceration of the ascending colon and aphtha extending from the transverse colon to the rectum
Fig. 3.
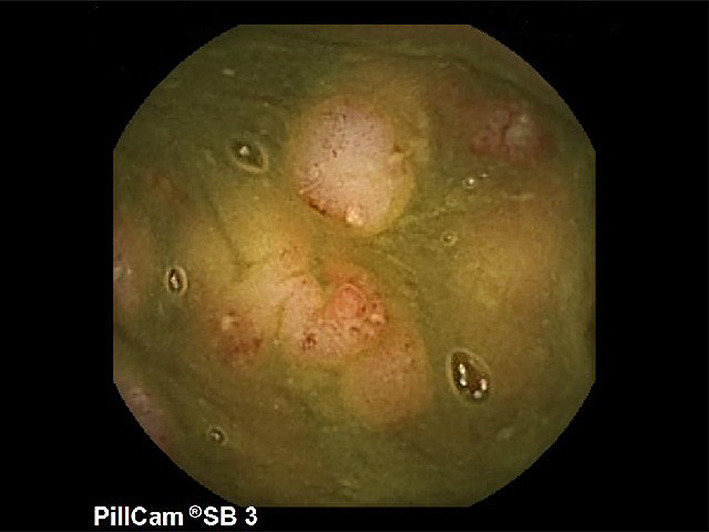
Video capsule endoscopy findings, showing a large number of areas of erosion in the small intestine
The oral aphtha was treated using mesalazine mouthwash. On post-admission day 13, the inflammatory reaction, watery stool and oral aphtha had all improved. Pathological examination revealed chronic colitis with ulceration and severe inflammatory cell infiltration, but with no obvious epithelioid granuloma, cytomegalic inclusion bodies or caseous necrosis observed. The patient was diagnosed as having Crohn’s disease on the basis of the presence of one major and one minor findings, as per the Japanese diagnostic criteria for Crohn’s disease summarized in Table 3, as well as taking into consideration the denial of infectious enteritis, the improvement in symptoms and inflammatory reaction with fasting, the age of presentation which is typical for Crohn’s disease, and the presence of gastrointestinal lesions at typical sites and skip lesions. Infliximab (IFX) treatment was initiated at a dose of 5 mg/kg. After the initial dose, IFX was administered again at 2 and 6 weeks, and subsequently at 8-week intervals. The expression of CD19 from the onset of Crohn’s disease symptoms to disease remission is shown in Fig. 1b. Even after oral intake was restarted, there was no recurrence of the symptoms of Crohn’s disease or indication of an inflammatory reaction.
Table 3.
The Japanese diagnostic criteria for Crohn’s disease [5]
| (1) Major findings |
| Longitudinal ulcer |
| Cobblestone-like appearance |
| Non-caseating epithelioid granuloma |
| (2) Auxiliary findings |
| Irregular shaped or circular ulcer or aphtha over an extensive range of the digestive tract |
| Characteristic anal lesion |
| Characteristic gastroduodenal lesion |
| Certain diagnosis |
| Major findings of A or B |
| Major findings of A and auxiliary findings of A or B |
| All auxiliary findings |
Following treatment of nephrotic syndrome using RTX and treatment of the secondary Crohn’s disease using IFX, the patient has been symptom free of 1 year and 8 months, with no relapse of nephrotic syndrome.
Discussion
Nephrotic syndrome is caused by an abnormal humoral immune response of Th2 or B cells, whereas Crohn’s disease is believed to be caused by a Th1 or Th17 immune reaction, or an autoimmune abnormality [6, 7]. Remission of nephrotic syndrome with the use of RTX has previously been reported in cases when RTX was used to treat patients with refractory nephrotic syndrome who presented with concomitant idiopathic thrombocytopenic purpura (ITP) or Epstein–Barr virus-associated post-transplant lymphoproliferative disorder (PTLD) [8]. The suppression of CD20-positive B cells by RTX has been shown to extend the disease-free period of nephrotic syndrome, as well as to reduce the dosage of steroids needed [9]. Therefore, RTX has been increasingly used for the treatment of nephrotic syndrome However, RTX is associated with numerous side effects, including neutropenia, interstitial pneumonia, gammaglobulinemia, infections (such as pneumocystis pneumonia and hepatitis B), and progressive multifocal leukoencephalopathy (PML) [10], these side effects being caused by immunosuppression associated with B-cell inhibition.
Ardelean et al. [11] reported on the case of a 4-year-old boy with refractory nephrotic syndrome who was treated with RTX and developed ulcerative colitis, with elevated CD3-positive T cells and T-regulatory cells on flow cytometry. He had developed nephrotic syndrome at the age 2 years, treated using cyclosporine, steroid pulse therapy and tacrolimus for steroid-dependent, frequently relapsing, intractable nephrotic syndrome. However, the patient experienced 4 relapses over a period of a year. He developed significant steroid-related toxicity including bilateral cataracts. Two years after his initial presentation, he was treated with a 4-week course of rituximab at a dose of 375 mg/m2 per week. His nephrotic syndrome remitted, and tacrolimus was subsequently weaned.
Six weeks after rituximab therapy, he developed crampy abdominal pain, bloody diarrhea with 20 stools per day, weight loss, buccal mucosa ulcers, and intermittent fevers. He did not have supportive features of Behçet disease. An extensive infectious workup was performed and included blood, stool, and urine cultures, serologies, and Clostridium difficile assays. The studies only yielded torovirus in the stool, detected by electron microscopy. Results of repeat testing remained negative.
Colonoscopy revealed severe, grade IV inflammation with deep ulcers affecting the descending colon to the rectum. Histology of the colonic biopsy specimens revealed focal areas of cryptitis and a mixed inflammatory cell infiltrate in the lamina propria composed of lymphocytes, plasma cells, and few eosinophils. He was diagnosed with ulcerative colitis from repeated bloody diarrhea, colonoscopic findings, and histological findings of the colon. Based on their findings, the authors suggested that the ulcerative colitis may have been caused by cellular immune dysregulation due to the loss of B cells and subsequent activation of cytotoxic T cells. Similarly, Crohn’s disease is believed to be caused by an abnormal immune tolerance, including mutations of the nucleotide-binding oligomerization domain 2 (NOD2) and abnormal automatic bacteriophage [12, 13]. In our case, we propose that inhibition of B–T cell interaction caused by B-cell depletion resulted in the activation of Th1 and Th17 cells, and consequently Crohn’s disease developed due to innate immunity and abnormality of immune tolerance induced.
Of note was the absence of a relapse of nephrotic syndrome after IFX treatment, despite discontinuation of RTX treatment and elevation in CD19 expression. David et al. reported that serum TNF-α levels were elevated in nephrotic syndrome and normalized with remission of the disease. And in his case infliximab controlled the proteinuria of nephrotic syndrome [14]. TNF-α is suggested to cause proteinuria based on a laboratory model. Among them, it is presumed that superoxide produced by TNF-α causes glomerular barrier dysfunction and proteinuria appears [15]. Tabrez et al. reported that TNF-α gene polymorphism may influence susceptibility to idiopathic nephrotic syndrome and might affect steroid response in idiopathic nephrotic syndrome patients [16]. There are reports that nephrotic syndrome was developed as a side reaction of IFX [17], but the authors think of the onset accompanying amyloid nephropathy that complicates Crohn’s disease and rheumatoid arthritis. We plan to gather more cases to investigate the efficacy of RTX as a treatment for refractory nephrotic syndrome. As well, although Crohn’s disease developed as a secondary complication of the use of RTX, the fact remains that RTX is an effective treatment for refractory nephrotic syndrome. We are planning to collect more cases to study the mechanism by which B-cell depletion by RTX develops Crohn’s disease and the possibility that infliximab may extend the duration of remission of nephrotic syndrome.
Conclusion
The suppression of CD20-positive B cells by RTX has been shown to extend the disease-free period of nephrotic syndrome and the dose of steroid therapy needed. However, the immunosuppression associated with B-cell inhibition can cause various side effects, such as infections. As demonstrated in our case, B-cell inhibition by RTX might also be associated with the development of Crohn’s disease in children with nephrotic syndrome. Future studies are needed to clarify the mechanisms by which RTX can lead to Crohn’s disease. Currently, we do know that RTX is effective for the treatment of refractory nephrotic syndrome in children, but when abdominal symptoms accompanied by weight loss are observed after administration of RTX, inflammatory bowel disease is suspected and endoscopic examination should be performed when infectious enteritis is denied.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Koskimies O. Long-term outcome of primary nephrotic syndrome. Arch Dis Child. 1982;5:544–548. doi: 10.1136/adc.57.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarshish P. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8:769–776. doi: 10.1681/ASN.V85769. [DOI] [PubMed] [Google Scholar]
- 3.Arbeitsgemeinschaft für Pädiatrische Nephrologie. Weber HP, Olbing H, et al. Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Engl Med. 1982;306:451–454. doi: 10.1056/NEJM198202253060803. [DOI] [PubMed] [Google Scholar]
- 4.Takei T, Sugiura H, Nitta K. Biological preparation rituximab. J Clin Exp Med. 2011;237:9. [Google Scholar]
- 5.Suzuki Group. The diagnostic criteria and guide to medical care of ulcerative colitis and Crohn’s disease: research study about the refractory inflammatory intestinal disorder. Public welfare labor science research expenses subsidy Policy study business such as refractory disease 2015. 2015.
- 6.Shalhoub J. Pathogenesis of lipoid nephrosis:a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/S0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki K, Chiba T. Cytokine network abnormality of the inflammatory bowel disease. Bioclinica. 2002;17:2833. [Google Scholar]
- 8.Nozu K, Iijima K, Fujisawa M. Rituximab treatment for post-transplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660–1663. doi: 10.1007/s00467-005-2013-7. [DOI] [PubMed] [Google Scholar]
- 9.Iijima K. Rituximab physician-led clinical trial for childhood refractory nephrotic syndrome. Jpn Pharmacol Ther. 2014;42:2. [Google Scholar]
- 10.Takao M. Molecularly targeted therapy and progressive multifocal leukoencephalopathy. Jpn J Clin Med. 2015;73:869–876. [PubMed] [Google Scholar]
- 11.Ardelean DS, Gonska T, Wires S, et al. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010;126:e243–e246. doi: 10.1542/peds.2009-3395. [DOI] [PubMed] [Google Scholar]
- 12.Tomohiro W, Tsutomu C. Immunopathogenesis of Crohn’s disease due to NOD2 mutations. J Clin Exp Med. 2012;241:191–195. [Google Scholar]
- 13.Tomohiro W. An automatic bacteriophage and Crohn’s disease. IBD. 2009;3:216–221. [Google Scholar]
- 14.David R, Ovadia S, Yaakov JA, Robert W, Vivian B. Tumor necrosis factor-α blocking agent as a treatment for nephrotic syndrome. Pediatr Nephrol. 2004;19:1281–1284. doi: 10.1007/s00467-004-1573-2. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy ET, Sharma R, Scharma M, Li JZ, Ge XL, Dileezpan KN. TNFα increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433–438. doi: 10.1681/ASN.V93433. [DOI] [PubMed] [Google Scholar]
- 16.Tabrez J, Suraksha A, Abbas AM, Raj KS, et al. Cytokine gene polymorphism in idiopathic nephrotic syndrome children. Ind J Clin Biochem. 2011;3:296–302. doi: 10.1007/s12291-011-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin G, Luxton G, Harvey JM. Infliximab and nephrotic syndrome. Nephrol Dial Transplant. 2005;20:2824–2826. doi: 10.1093/ndt/gfi180. [DOI] [PubMed] [Google Scholar]



