ABSTRACT
Visual objects can be discriminated by static spatial features such as luminance or dynamic features such as relative movement. Flies track a solid dark vertical bar moving on a bright background, a behavioral reaction so strong that for a rigidly tethered fly, the steering trajectory is phase advanced relative to the moving bar, apparently in anticipation of its future position. By contrast, flickering bars that generate no coherent motion or have a surface texture that moves in the direction opposite to the bar generate steering responses that lag behind the stimulus. It remains unclear how the spatial properties of a bar influence behavioral response dynamics. Here, we show that a dark bar defined by its luminance contrast to the uniform background drives a co-directional steering response that is phase advanced relative to the response to a textured bar defined only by its motion relative to a stationary textured background. The textured bar drives an initial contra-directional turn and phase-locked tracking. The qualitatively distinct response dynamics could indicate parallel visual processing of a luminance versus motion-defined object. Calcium imaging shows that T4/T5 motion-detecting neurons are more responsive to a solid dark bar than a motion-defined bar. Genetically blocking T4/T5 neurons eliminates the phase-advanced co-directional response to the luminance-defined bar, leaving the orientation response largely intact. We conclude that T4/T5 neurons mediate a co-directional optomotor response to a luminance-defined bar, thereby driving phase-advanced wing kinematics, whereas separate unknown visual pathways elicit the contra-directional orientation response.
KEY WORDS: Feature detection, Motion vision, Visual behavior, Tethered flight, Fly flight
Summary: Flies orient toward the vertical edges of landscape objects. Luminance contrast and relative motion drive different components of object-tracking behavior, segregated by motion-detection circuitry.
INTRODUCTION
Owing to robust behavioral reactions to salient objects by flying flies (Reichardt and Wenking, 1969; Wehner, 1972), early work posited the existence of two sub-systems: one that processed object velocity and one that encoded the static position of an object (Reichardt and Poggio, 1975). This framework was drawn from experiments using a solid black bar on a white surround, which provides a highly salient spatial discrimination cue, luminance (Koffka, 1935; Livingstone and Hubel, 1988). Accordingly, numerous studies have used a luminance-defined bar to elicit robust bar tracking and frontal fixation under open-loop or virtual closed-loop feedback conditions (Bahl et al., 2013; Duistermars et al., 2007; Heisenberg and Wolf, 1979; Horn and Wehner, 1975; Maimon et al., 2008; Reiser and Dickinson, 2010; Wehrhahn and Hausen, 1980).
The experimental utility of a luminance-defined object, such as a dark bar on a white background, is limited by the large parameter space it occupies. The moving bar can be distinguished by a number of parameters including static luminance, spatial disparity against the background or relative movement, each parameter supplying different visual information (Cavanagh and Mather, 1989; Livingstone and Hubel, 1988). Perhaps because of potential interaction effects among these stimulus parameters, a dark bar elicits a peculiar behavioral phenomenon by a tethered flying fly – when it is revolved around the animal at constant velocity, the steering wing kinematics and yaw torque responses are phase advanced to the bar. Thus, at the moment that the bar crosses visual midline, the steering effort is oriented in front of the moving bar, seemingly in anticipation of it (Bausenwein et al., 1986; Geiger, 1981; Reiser and Dickinson, 2010).
Flies also readily track and frontally fixate a bar with a texture that matches the background so that luminance and spatial composition disparities are eliminated and the bar can only be discriminated by its relative motion (Fenk et al., 2014; Reichardt and Poggio, 1979; Reichardt et al., 1989; Theobald et al., 2008, 2010). The advantages of using such a stimulus include its reduced parameter space and ability to directly discriminate optomotor steering responses to background motion from steering reactions to the object (Fox and Frye, 2014; Fox et al., 2014; Kimmerle et al., 2000; Reichardt et al., 1989). Visual stimuli like these, however, do not elicit phase-advanced steering responses like the dark bar.
The first-order motion-detecting neurons in Drosophila, small-field columnar T4 (ON) and T5 (OFF) neurons, have spatial receptive fields roughly 15 deg in diameter (Takemura et al., 2013, 2017) (3 ommatidia≈15 deg visual angle) (Gonzalez-Bellido et al., 2011). Individual T4/T5 neurons are directionally tuned, and thereby could also serve the velocity component of bar-tracking behavior (Reichardt and Poggio, 1975). Yet, how the T4/T5 motion detectors participate in bar-tracking behavior is not fully understood; two studies suggest that motion detectors are largely dispensable for dark bar tracking (Bahl et al., 2013; Bausenwein et al., 1986), whereas a third shows that fixating a motion-defined bar is degraded by silencing these neurons (Fenk et al., 2014). Here, we systematically compared the behavioral reactions to a luminance-defined bar and a motion-defined bar over parameters including speed, object size, travel distance and contrast. We tested the hypothesis that the T4/T5 motion-detecting neurons account for behavioral differences elicited by the two types of object stimuli.
We show that any bar distinguished from the background by its mean luminance (e.g. ‘dark bar’) elicits a co-directional optomotor response that starts as the bar enters the rear visual field. Thus, by the time the dark bar reaches the animal's visual midline, its steering effort is phase advanced, appearing to predict the bar's position. However, the same flies respond to the appearance of a motion-defined bar with an initial counter-directional turn, followed by a co-directional steering response. Thus, as the motion-defined bar reaches the animal's visual midline, the steering effort is phase locked to the bar. We show that T4/T5 neurons known to mediate co-directional optomotor responses (Bahl et al., 2013, 2015; Maisak et al., 2013) respond more strongly to a luminance-defined dark bar than to a motion-defined bar. We also show that silencing T4/T5 synaptic output blocks the co-directional steering component, but not the initial counter-directional orientation component. Our results support a conceptual framework for the superposition of motion-dependent and motion-independent processes, that a luminance-defined bar elicits a robust directional ‘velocity’ optomotor response via T4/T5 motion detectors, whereas the textured motion-defined bar elicits an additional non-directional ‘position’ orientation response via unknown pathways.
MATERIALS AND METHODS
Fly stocks
All experiments were performed on 3–7 day old female Drosophila melanogaster reared on a 12 h light/12 h dark cycle at 25°C. A previously established wild-type strain (DL) was used for experiments investigating wild-type behavior. The following stocks were used for calcium imaging and inactivation experiments: w1118; 20XUAS-GCaMP6m in VK00005 (#42750), T4/T5-Split Gal4 (SS00324) [R59E0_AD (attP40); R42F06_DBD(attP2)] (Strother et al., 2017), w+(DL);;pJFRC49-10XUAS-IVS-eGFPKir2.1 (von Reyn et al., 2014) and w1118; BPp65ADZp (attP40); BPZpGdbd (attP2) (Hampel et al., 2015).
Fly preparation and visual display
Flies were cold anesthetized at 4°C and tethered to tungsten pins using UV-activated glue. Flies were then allowed to recover for 1–2 h in a covered acrylic container with a small weighing boat filled with water to maintain humidity under a red desk lamp to provide heat. Allowing flies to recover from the tethering procedure in these conditions generally improved their flight performance.
Flight behavioral experiments in a rigid tether flight simulator were performed as reported previously (Aptekar et al., 2012). Briefly, a visual flight simulator composed of 32×96 pixel cylindrical green LEDs was used to deliver visual stimuli (Reiser and Dickinson, 2008). The arena covered ±165 deg along the azimuth and ±47 deg in elevation. Flies were positioned in the center of the arena and illuminated with an 880 nm infrared LED casting a shadow on an optical wing beat analyzer (JFI Electronics Laboratory, University of Chicago, Chicago, IL, USA) that measured right and left wing beat amplitude (WBA). The difference in the left and right WBA (ΔWBA) is proportional to the fly's steering effort in the yaw axis (Fig. 1A).
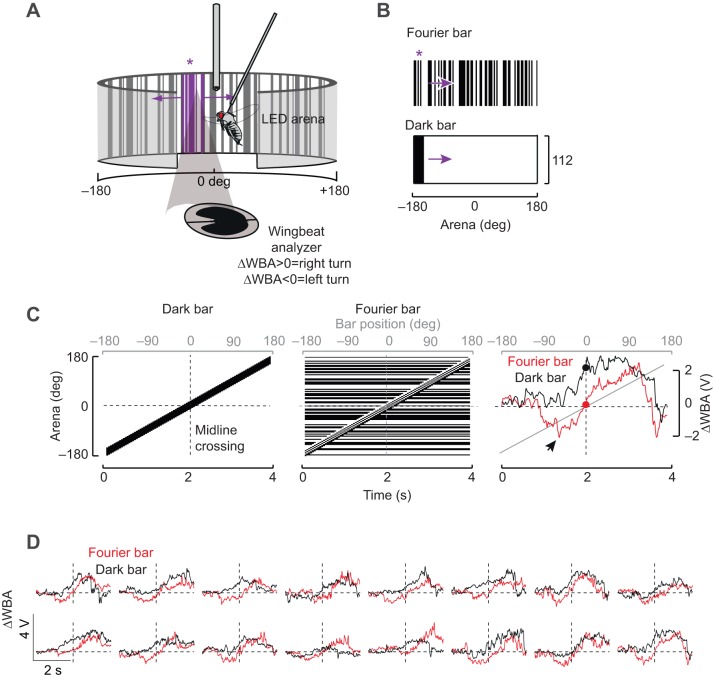
Fig. 1.
Behavioral responses to a luminance-defined dark bar and a motion-defined Fourier bar are distinct in tethered Drosophila melanogaster. (A) Schematic diagram of the visual flight simulator. A fly is tethered to a tungsten pin and surrounded by an arena of LEDs. An infrared light source above the fly casts a shadow on a photodiode that records the wing beat amplitude (WBA). (B) Fourier bar and dark bar stimuli. Purple asterisk indicates the location of the motion-defined Fourier bar (see A). (C) Left and middle: space–time plots for dark and Fourier bars shown for a full rotation around the 360 deg visual azimuth. Dashed vertical line indicates the midline crossing when the bar is directly in front of the fly. Right: ΔWBA responses of a single fly to rotation of a Fourier (red) and dark (black) bar. Red and black dots indicate the ΔWBA when the figure is at the midline. Arrowhead indicates a small saccade-like event. (D) Plots similar to those in C showing single response traces from 16 flies. The right-hand panel is the same as the right panel in C.
Open-loop rigid tether experiments
Each behavioral experiment consisted of randomized blocks of open-loop and closed-loop trials. Open-loop trials in which the responses to the visual patterns were tested were followed by periods of closed-loop control to ensure that the fly was engaged in the experiment. Flies that failed to frontally fixate a dark bar were not used for experiments. Unless indicated in the figures, open-loop test trials and closed-loop rest trials were 4 s each. Each experimental condition was replicated 6–12 times depending on the experiment length. None of the experiments exceeded 30 min. For the T4/T5 silencing experiment, each fly was dissected under a stereo microscope with a fluorescent light source to confirm the expression of Kir2.1 tagged with GFP.
Closed-loop magnetic tether experiments
The magnetic tether arena in which a fly can revolve freely in the yaw plane has been described previously (Duistermars and Frye, 2008). Briefly, the display consists of an array of 96×16 LEDs, each subtending 3.75 deg on the eye, that wrap around the fly, subtending 360 deg horizontally and 54.8 deg vertically (Fig. 1A). Flies were suspended between two magnets, allowing free rotation along the horizontal (yaw) axis. We illuminated the fly from below with an array of eight 880 nm LEDs. We video-recorded the angular position of the fly within the arena at 160 frames s−1 with an infrared-sensitive camera placed directly below the fly (A602f, Basler, Ahrendburg, Germany).
The experimental and data analysis protocol in the magnetic tether has been described previously (Mongeau and Frye, 2017). Briefly, flies were suspended within the magnetic pivot and given a few minutes to acclimate. For each fly, we recorded a trial in which the whole panorama rotated, which elicited smooth optomotor pursuit. Flies that could not robustly track the wide-field panorama were not used further. We rotated either a motion-defined ‘Fourier’ bar on a randomly generated background of ON and OFF pixels or a dark bar over a uniformly lit background of ON pixels. We presented all stimuli at three different angular speeds, and randomized the order, direction, speed and initial position of each stimulus to minimize habituation. We presented each stimulus for a period of 25 s, defining the duration of an individual trial. Between each trial, we presented a fixed visual landscape for 25 s for the fly to rest. For Fig. 2D, we detected events where the bar crossed the visual midline and flies did not generate saccades within a 600 ms time window in order to isolate the smooth movement component of the response. As previously observed, on some occasions, flies generated no saccades upon appearance of the bar in the field of view (Mongeau and Frye, 2017). Saccades were detected using previously described algorithms (Mongeau and Frye, 2017).
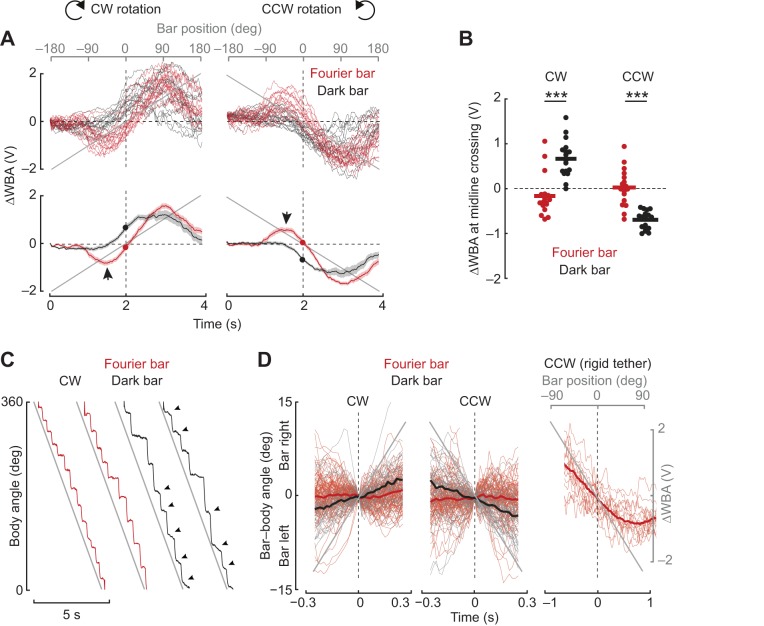
Fig. 2.
The dark bar elicits a co-directional steering response, whereas the Fourier bar elicits a contra-directional orientation response. (A) Top: steering responses of 17 flies to clockwise (CW) and counter-clockwise (CCW) rotation of a Fourier bar (red) and a dark bar (black). Each trace is the mean response to multiple stimulus trials for a single fly. Bottom: population average responses to CW and CCW rotation of the Fourier bar (red) and the dark bar (black). Shaded regions indicate s.e.m., n=17. Arrows indicate the counter-directional turn toward the bar. Filled circles indicate the steering responses as the Fourier bar (red) and the dark bar (black) cross the visual midline. (B) Comparison of the ΔWBA value when the bar reaches the midline ‘zero crossing’ of the arena. Asterisks denote results of a paired t-test: P=1.20×10−7 for CW and P=6.25×10−7 for CCW. (C) Body angle recorded for a magnetically tethered fly in response to a bar revolving at 75 deg s−1. Arrowheads indicate smooth body movements between saccades. (D) The angle between the fly's longitudinal body axis and the azimuthal position of the bar 300 ms segments before and after the bar crossed the fly's visual midline. n=26 flies for the Fourier bar and n=10 flies for the dark bar. Data are pooled from experiments in which the bars rotated at different speeds; thus, the gray line is merely indicative of the direction of bar movement, not its actual position. Mean body angle slope coefficients for the dark and Fourier bar are different from each other at the 99.9% confidence level for both CW and CCW rotation (linear regression, P<0.001, d.f.=29). For comparison, the right panel indicates single response trajectories from 17 flies in the rigid tether 1 s before and after the Fourier bar crosses the fly's visual midline.
Figure-specific visual stimuli
Throughout, figures show a dark bar and a Fourier bar, each extending 30 deg in width and 94 deg in height composed of bright and dark pixels (Fig. 1B). Bright ON pixels had the highest luminance values that LEDs could produce, whereas the dark pixels were OFF. The Fourier bar was generated by a custom-written MATLAB script and composed of randomly distributed bright and dark columns on the LED display. The pattern was high-pass filtered to ensure that no ON or OFF pattern element exceeded 6 pixels in width. At least 12 different random Fourier patterns were used, picked at random for each trial to prevent pattern-specific behavioral artifacts. Patterns were rotated at 90 deg s−1 in the clockwise (CW) and counter-clockwise (CCW) directions.
Fig. 3 shows the dark bar and Fourier bar described above rotated around the fly at varying speeds (Fig. 3A), (B) three bar widths rotated around the fly at 90 deg s−1 (Fig. 3B) and a 30 deg Fourier and dark bar started at eight unique locations on the left side of the fly and moved to the midline (Fig. 3C).
Fig. 3.
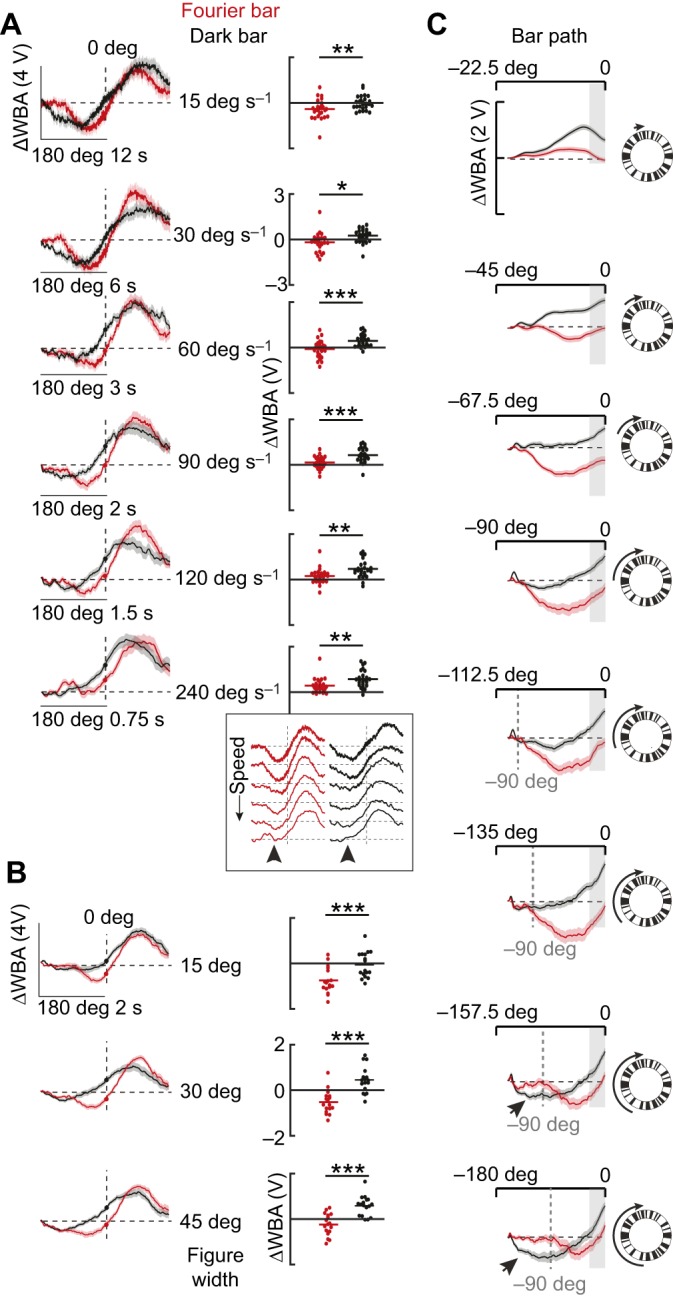
Qualitative differences between Fourier and dark bar responses persist across bar velocity, size and trajectory. (A) Left: average steering responses to a 30 deg Fourier bar (red) and a dark bar (black) revolved CW at varying speed as indicated. Shaded regions indicate s.e.m., n=26. Right: dot plots indicate ΔWBA at the midline crossing. Horizontal lines indicate mean values. Paired t-test (*P<0.05, **P<0.01, ***P<0.001). Inset: mean traces from A re-plotted to highlight the speed-dependent loss of the orientation response (arrowheads). (B) Average steering responses to bars of varying widths, as indicated, revolved CW. Shaded regions indicate s.e.m., n=17. Paired t-test (P=5.32×10−6 for 15 deg, P=1.34×10−5 for 30 deg and P=1.61×10−5 for 45 deg). (C) Average steering responses to 30 deg bars revolved starting from eight different locations along the visual azimuth and ending on the visual midline as indicated with cartoons and arrows. Shaded regions indicate s.e.m., n=37.
Fig. 4 shows a Fourier and a dark object extending 30 deg in width and 30 deg in height rotated 90 deg s−1 around the fly.
Fig. 4.
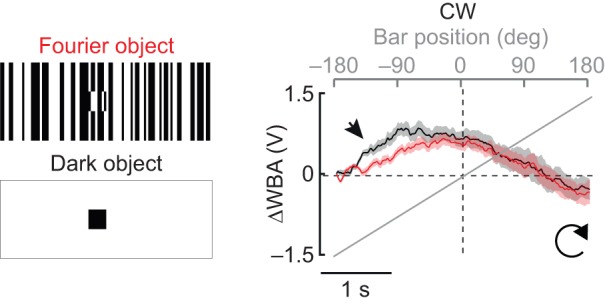
A small Fourier object and a small dark object elicit similar steering responses. Average steering responses to a 30 deg by 30 deg Fourier (red) and dark (black) object revolved CW around the fly. Shaded regions indicate s.e.m., n=16. Arrowhead indicates where the steering responses differ.
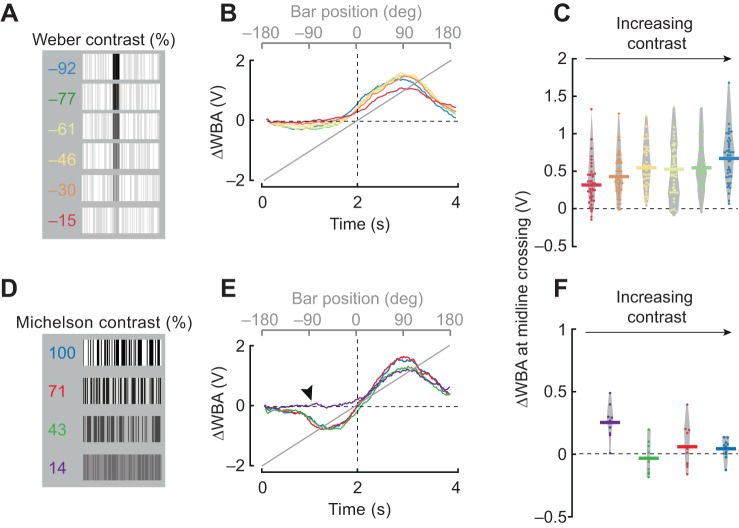
Fig. 5 shows the Weber contrast defined by (IBar−IBackground)/IBackground where the bar and the background are each defined by two luminance values (ON and OFF), and the average luminance is calculated by taking the mean. Weber contrast is generally used to define the contrast of objects that have different luminance values from background. By contrast, Michelson contrast is useful to characterize visual patterns that do not contain any luminance discontinuities, such as the Fourier bar stimuli used here or for periodic grating patterns used in vision experiments (O'Carroll and Wiederman, 2014). Michelson contrast is defined by (Imax−Imin)/(Imax+Imin). Note that whereas Weber contrast values can range between −1 and infinity, Michelson contrast varies between 0 and 1. Therefore, Michelson values are shown as percentages whereas Weber contrast values have arbitrary units. We generated 12 unique patterns for each condition and randomized which pattern was used for each trial. Both bar types were rotated at 90 deg s−1.
Fig. 5.
Qualitative differences between the Fourier and dark bar persist across different contrast conditions. (A) Luminance-defined bar at varying contrasts (indicated) (see Materials and Methods). (B) Mean steering responses to CW rotation of 30 deg bars. Color code is the same as in A. n=42. (C) ΔWBA values at the zero-crossing; each dot represents an individual fly. Horizontal lines represent mean values and the gray envelope encloses all data points. (D) Fourier-type bars with varying Michelson contrast as indicated. (E) Mean steering responses to 30 deg Fourier bars. Black arrowhead indicates the absence of the usual contra-directional steering response to a low-contrast Fourier bar approaching the visual midline. (F) Data presentation of mid-line crossing values from E, plotted similarly to C. n=11.
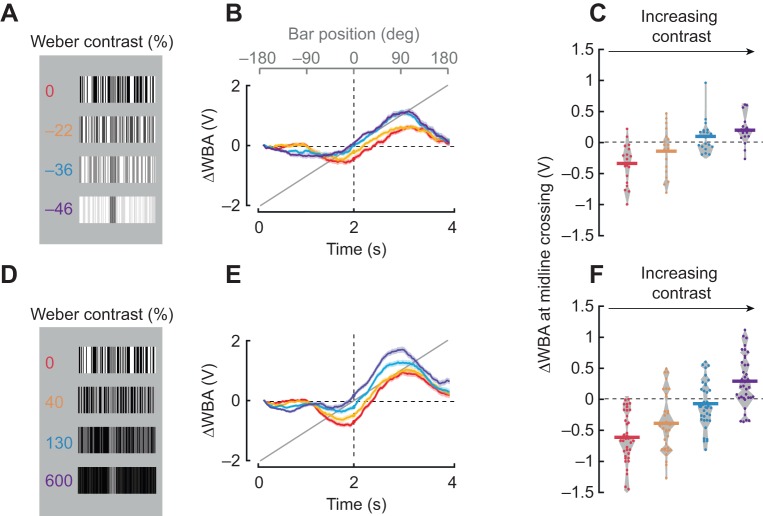
Fig. 6 shows patterns similar to those used in Fig. 5. For Fig. 6A, the ON and OFF values for the bar were kept constant, while one of the two luminance values for the background was systematically varied.
Fig. 6.
Varying luminance contrast between the bar and the background elicits phase lags to bar revolution. (A) A single Fourier-type bar of intermediate contrast tested against four different backgrounds; the luminance contrast of the bar as indicated. For the first bar, the mean luminance per unit area of the bar was matched to the mean luminance of the background (red). For subsequent bars, the background luminance was systematically increased. (B) Average steering responses to CW rotation of 30 deg bars, color coded for stimuli in A. Shaded regions indicate s.e.m., n=19. (C) ΔWBA values at the zero-crossing; each dot represents an individual fly. Horizontal lines represent mean values and the gray envelope encloses all data points. (D) The same bar used in A (red), superimposed upon the background patterns of decreasing mean luminance. (E) Average steering responses to CW rotation of 30 deg bars. Shaded regions indicate s.e.m., n=37. (F) ΔWBA values at the zero-crossing; each dot represents an individual fly. Horizontal lines represent mean values and the gray envelope encloses all data points.
Fig. 7 shows a visual display composed of 470 nm LEDs extending 216 deg along the azimuth and 72 deg in elevation, which was used for calcium imaging experiments. Patterns used were the same as those in Fig. 2A.
Fig. 7.
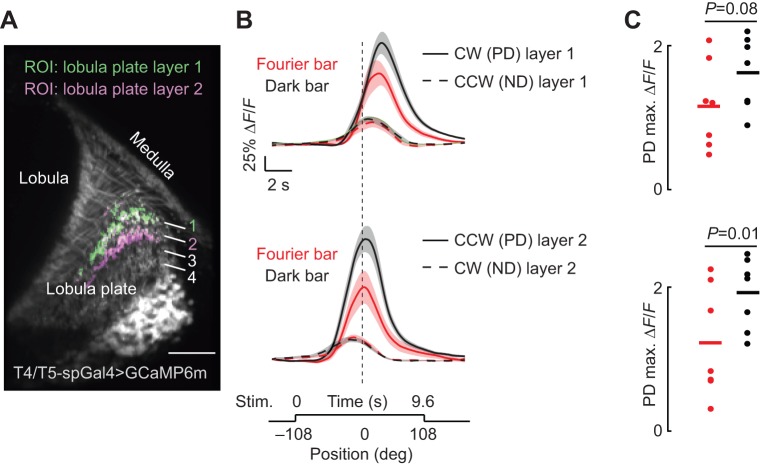
The dark bar elicits stronger preferred directional responses from T4/T5 cells than the Fourier bar. (A) Single two-photon excitation image of T4/T5 cells expressing GCaMP6m. The region of interest (ROI) was restricted to either layer 1 or layer 2 of the lobula plate as indicated. (B) Average calcium responses (ΔF/F, change in fluorescence) of T4/T5 neurons to a CW or CCW rotating Fourier (red) and dark bar (black). Each panel compares preferred direction (PD) and null direction (ND) responses in each layer as indicated. ND traces are time inverted for visual comparison with PD responses. Shaded regions indicate s.e.m., n=7. (C) Maximum calcium responses from PD data in B. Each dot represents data from an individual fly; horizontal line indicates mean responses. Paired t-test P-values comparing peak PD responses as indicated.
Data analysis
All custom-written software is available by contacting the corresponding author. Behavioral data from the visual display and the wing beat analyzer were collected with a Digidata 1440A digitizer (Molecular Devices, San Jose, CA, USA) sampled at 1 kHz. Data were processed using custom-written MATLAB scripts (MathWorks, Natick, MA, USA). Data from each trial were extracted and ΔWBA was calculated by subtracting left WBA from right WBA. The signal was then low-pass filtered at a cut-off frequency of 25 Hz using a zero-phase second-order Butterworth filter. The first 100 ms of each trial were removed and the first data point of the remaining signal was subtracted from the entire trial to set the initial ΔWBA to zero. ΔWBA at midline crossing was measured by taking the average ΔWBA values occurring between 50 ms prior to and following the time at which the bar crossed the midline of the visual display. No statistical tests were conducted to predetermine the sample size. Paired t-tests were used for all experiments in which each fly was subjected to multiple visual stimuli. Wilcoxon rank-sum tests were used to test significance when different populations of flies were presented with the same visual stimuli. The visual crossing was defined by the center of the bar at the fly's visual azimuthal midline.
Functional imaging
Calcium imaging was done as described previously (Keleş and Frye, 2017). Briefly, a fly was anesthetized at 4°C and placed into a chemically etched metal shim attached to a custom 3D-printed holder. Holder design was based on Weir and Dickinson (2015) and details can be found at http://ptweir.github.io/flyHolder/. The head capsule and the thorax were glued to the metal shim using UV-curable glue (www.esslinger.com). Legs and antennae were immobilized using beeswax applied with a heated metal probe (Waxelectric-1, Renfert). The head capsule was then bathed in saline (103 mmol l−1 NaCl, 3 mmol l−1 KCl, 1.5 mmol l−1 CaCl2, 4 mmol l−1 MgCl2, 26 mmol l−1 NaHCO3, 1 mmol l−1 NaH2PO4, 10 mmol l−1 trehalose, 10 mmol l−1 glucose, 5 mmol l−1 TES and 2 mmol l−1 sucrose). A small window on the right rear head capsule was opened using sharp forceps (Dumont, #5SF). Muscles and fat covering the optic lobe were cleared before placing the fly under the 2-photon microscope (3i: Intelligent Imaging Innovations, Denver, CO, USA). T4/T5 neurons expressing GCaMP6m were imaged at 920 nm using a Ti:Sapphire Laser (Chameleon Vision, Coherent). Imaging sessions were not longer than 30 min in any of the experiments. Images were acquired at 10 frames s−1. Three separate recordings were made, each consisting of a random block design of all visual stimuli. A new random block of trials was repeated three times in total.
For calcium imaging experiments, an arena composed of 48, 8×8 pixel LED panels, 470 nm (Adafruit) was used. The panels were assembled into a curved display that extended 216 deg along the azimuth and ±32.2 deg in elevation. Each pixel extended 2.2 deg on the retina at the equatorial axis. However, the size of each pixel on the retina varied somewhat as a result of the difference between curvature of the eye and the screen. To prevent spurious excitation of the imaging photomultiplier tubes, three layers of blue filter (Rosco no. 59 Indigo) were placed over the LED display.
Acquired images were motion corrected using a custom-written MATLAB algorithm (Akin and Zipursky, 2016). Regions of interest (ROI) were selected by setting a threshold for those pixels over the lobula plate that fluoresced at values equal to or greater than two times the standard deviation multiplied by the mean of the entire image. Multiplying the standard deviation of each pixel by the mean of each pixel for the entire time series removes inactive pixels. This ROI thresholding method provides a good estimate of active pixels that are not part of the background. Threshold-passed pixels were then averaged to produce a single time series trace for the entire image. Typical ROI encompassed active axon terminals of many T4 and T5 neurons in lobula plate layers 1 and 2. Repetitions of the same experiment were averaged for each fly and a single average trace per experiment was generated.
RESULTS
A luminance-defined dark bar and motion-defined Fourier bar elicit qualitatively distinct steering responses
To compare behavioral responses to a bar that is defined only by its relative motion with those to a bar that has a static luminance positional cue, we used an electronic visual flight simulator (Fig. 1A). A bar containing indistinct boundaries with the background visual scene has previously been termed a ‘Fourier’ bar (Chubb and Sperling, 1988) and is camouflaged until it moves. By contrast, a dark bar on a uniform bright background contains static luminance cues that reveal its borders and position anywhere in the arena (Fig. 1B). When revolved continuously around the arena, the two stimuli elicit qualitatively distinct steering responses by individual flies. For CW rotation, the dark bar tends to elicit a co-directional orientation response in individual trials (ΔWBA>0, Fig. 1C,D), whereas the Fourier bar tends to elicit an initial contra-directional turn followed by a co-directional steering response (Fig. 1D).
Qualitative differences between the dark and Fourier bar responses were observed for trials averaged for individual flies (Fig. 2A, top) or for trials averaged across the population (Fig. 2A, bottom). In particular, the Fourier bar caused an initial counter-directional turn toward the bar, triggered when the bar was 90 deg from the visual midline, which was entirely absent for a dark bar (Fig. 2A, arrowheads). The most striking difference between the dark bar and Fourier bar responses was the steering effort (ΔWBA) as the bar crossed the visual midline (Fig. 2A, filled circles). The population ΔWBA at midline crossing of the Fourier bar was not significantly different from zero (CW, mean±s.e.m. −0.16±0.11 V, n=17, P=0.18, t-test), indicating that the flies were steering straight when the bar crossed the visual midline. By contrast, for a dark bar presented to the same animals, the mean ΔWBA response at midline crossing was 0.66±0.10 V (P=6.49×10−7, t-test) indicating that flies were, in a sense, steering ‘ahead’ of the rotating CW bar. Comparison of the mean ΔWBA at midline crossing for each animal for both directions showed significant differences between the Fourier and dark bar (Fig. 2B). Under these experimental conditions, we did not observe systematic asymmetry in either the time course or amplitude of steering responses to CCW and CW bar rotation (Fig. 2A,B). Thus, for brevity we present only CW responses in the remaining figures.
Individual trials often showed rapid fluctuations in ΔWBA, so-called ‘wing hitches’ (Heide and Götz, 1996) analogous to ‘torque spikes’ (Heisenberg and Wolf, 1979) that represent the rapid saccadic turns that flies use to re-orient during flight (Bender and Dickinson, 2006). We used the magnetic tether system to directly observe how bar type influences body saccades. Revolving a Fourier bar around an LED display elicited robust tracking in the form of rapid body saccades interspersed with segments of straight flight in which body angle was largely invariant (Fig. 2C, red traces, note steady inter-saccade body angle). By contrast, for the same individual fly, a dark bar set against a uniform background elicited noticeable body movement between saccades in the direction of the moving bar (Fig. 2C, arrowheads). The smooth optomotor-like steering response was also observed in the fly's body angle for instances in which the dark bar happened to cross the visual midline (Fig. 2D, black lines, P<0.001, d.f.=29, slope coefficients were significant at 99% confidence interval for both CW and CCW). The same flies did not follow the Fourier bar when it crossed visual midline (Fig. 2D, red lines). Note that although the Fourier bar failed to elicit smooth steering responses when it crossed the visual midline on the magnetic tether (Fig. 2D, center), the same stimulus elicited strong steering kinematics by a rigidly tethered fly (Fig. 2D, right).
The weak co-directional optomotor response elicited by a dark bar between saccades was not sufficiently strong on the magnetic tether in closed-loop to drive phase-advanced steering reactions (Fig. 2D), whereas the same stimulus on the rigid tether in open-loop seemed to drive the optomotor response continuously as the bar approached and crossed the visual midline (Fig. 2D). Consequently, bar-tracking behavior on the magnetic tether lags the bar, by direct contrast to the rigid tether. These results support the hypothesis that, for open-loop conditions on the rigid tether and closed-loop conditions on the magnetic tether, the dark bar and Fourier bar stimuli elicit qualitatively different tracking behaviors.
Qualitative differences due to bar type persist across bar speed, size and path length
To what extent do response differences elicited by a dark and Fourier bar persist over parameters such as speed, size and trajectory of the figure? We first tested the effect of varying bar velocity over a 16-fold range. At slower bar velocities (15 and 30 deg s−1), both bar types elicited initial contra-directional orientation responses (Fig. 3A), which dissipated with increased bar speed (Fig. 3A, inset). At all speeds, however, ΔWBA at visual midline crossing was larger for the dark bar than for the Fourier bar, which indicates that the qualitatively larger phase-advance responses evoked by the dark bar are independent of speed (Fig. 3A, dot plots). It is noteworthy that the initial counter-directional orientation response was strongly speed dependent, whereas the co-directional tracking response was not (Fig. 3A, inset).
To explore whether responses are affected by the size of the bar, we tested three different bar widths (15, 30 and 45 deg; Fig. 3B). For all three bar widths, the dark bar elicited a phase-advanced steering effort and significantly larger ΔWBA at midline crossing (Fig. 3B, right).
All experiments to this point used a bar moving through the full 360 deg azimuth of the arena. To test whether a long path length is required to elicit qualitatively different behavioral responses to the two bar types, we started the stimulus at different locations along the visual azimuth, all ending at the visual midline (Fig. 3C). The most notable result of this experiment is that the Fourier and dark bar elicited different response trajectories for all path lengths. The contra-directional Fourier bar orientation response was absent for the shortest trajectory, but was present in response to all other path lengths. We observed small-amplitude contra-directional steering responses to the dark bar when it was started at either −157.5 or −180 deg (Fig. 3C, black arrowhead). This was also observed for slow-moving or wide dark bars (Fig. 3A,B), as has been shown in previous studies (Bausenwein et al., 1986; Geiger, 1981; Reiser and Dickinson, 2010). Note, however, that for all path lengths, the average ΔWBA value at midline crossing was larger for the dark bar than for the Fourier bar (Fig. 3C, shaded region). Furthermore, particularly for the dark bar, flies started their counter-directional steering earlier as the stimulus path shifted into the rear field of view. However, the counter-directional orientation response to the Fourier bar consistently started at −90 deg regardless of the path length (Fig. 3C, dashed gray lines).
Whereas D. melanogaster readily approach vertical edges such as landscape features, they steer to avoid small contrasting objects which may represent approaching threats (Maimon et al., 2008). We tested small-object aversion using a Fourier object and dark object. As expected, for a CW-moving object, flies steered towards the right, away from the object (Fig. 4). The steering response amplitude peaked and decreased as the object traversed the visual midline, unlike the continual steering response increase observed for bar movement under identical conditions (Fig. 2). The average initial steering response amplitude was slightly larger for the dark object (Fig. 4, arrowhead), buy the steering trajectories for the two object types converged before visual midline. Thus, the response phase was not influenced by the visual properties of the object and aversion dynamics were largely independent of the spatial form of the small object.
The strength of the ‘dark bar’ co-directional steering effort depends on the luminance contrast between the bar and background
Next, we explored how contrast cues influence the behavioral response to each bar type. We first used a textured dark bar-like stimulus, one for which the bar was darker than the background for a fixed low-contrast background (Fig. 5A). Flies responded with strong co-directional steering responses to bars at all Weber contrast values (Fig. 5B). The steering response at midline crossing was greater than zero for all contrasts tested (P=1.53×10−8 for −0.15, P=1.57×10−12 for −0.30, P=8.77×10−15 for −0.46, P=4.36×10−13 for −0.6, P=5.73×10−15 for −0.77 and P=7.30×10−17, t-test with Bonferroni correction). The steering amplitude at the midline crossing trended with increasing contrast (Fig. 5B,C). Thus, if the bar is defined by its luminance contrast, then the response is qualitatively similar to that for a solid dark bar.
An object that is defined only by its relative motion, with the same mean luminance as the background, cannot be characterized by the Weber contrast, but rather by Michelson contrast (see Materials and Methods). The average steering responses to 100%, 71% and 43% contrast Fourier bars were indistinguishable from one another (Fig. 5E). These all displayed the characteristic early counter-directional orientation response followed by co-directional optomotor tracking response (Fig. 5E), and steering responses at visual midline were essentially zero for each contrast (Fig. 5F). However, the 14% Michelson contrast value delineated the two distinct phases of bar-tracking behavior; the counter-directional orientation response was absent at this contrast level yet the co-directional tracking response was intact (Fig. 5E, arrowhead). This suggests that the detection threshold for the counter-directional orientation phase is distinctly different from that for the co-directional optomotor tracking phase of the bar response.
The results presented in Figs 5 and 3 suggest that any bar discriminated by its static luminance elicits phase-advanced co-directional steering responses (Fig. 5A–C), whereas any bar that is discriminated only by its relative motion elicits phase-locked tracking (Fig. 5D–F). Are there conditions that cause a phase-delayed steering response? We designed Fourier bars with varying Weber contrast, either net negative (bar darker than background) or net positive (bar brighter than background), by varying the background pattern luminance and keeping the bar at fixed medium contrast and luminance values. The initial condition bar for this series is visible when stationary, similar to prior dark bar stimuli, but produces the same mean luminance per unit area as the background, similar to prior Fourier bar stimuli (Fig. 6A,D, 0% contrast conditions). These two bars are distinguished not by the first moment of the luminance distribution (mean) but rather by the second moment (standard deviation). Remarkably, these two bars both produced strong contra-directional orientation responses that were strongly phase delayed at the midline crossing (Fig. 6B,E, red traces). Systematically changing the background luminance created ‘dark’ and ‘bright’ bars compared with the background (Fig. 6A,D). Increasing the luminance contrast between the figure and the background, either negatively (darker bar, Fig. 6A–C) or positively (brighter bar, Fig. 6D–F) progressively diminished the contra-directional responses (Fig. 6B,E) and led to phase-shifted responses similar to those for the dark bar (Fig. 6C,F). These results indicate that the balance between counter-directional orientation and co-directional tracking is modulated by the Weber luminance contrast between the figure and the background as well as the Michelson contrast of the background itself. The brighter (or darker) the bar is than the background, the larger its luminance contrast, the stronger the co-directional tracking and the weaker the counter-directional orientation responses.
T4/T5 is more responsive to the dark bar, and mediates the co-directional optomotor response
In flies, the first stage of visual processing in which directionally selective motion detection arises is within the ‘T4’ and ‘T5’ columnar neurons that innervate the third optic ganglion, the lobula plate. T4 and T5 provide retinotopic small-field local motion signals to postsynaptic multi-columnar wide-field integrating neurons believed to mediate optomotor behaviors (Bahl et al., 2013; Buchner et al., 1984; Maisak et al., 2013). Both bar types elicit co-directional tracking responses, but only the Fourier bar elicits robust counter-directional orientation responses. Therefore, we reasoned that if the co-directional optomotor response component driven by a luminance-defined bar (Fig. 2A,B) is mediated by the motion vision pathway, then the T4/T5 system should be significantly more sensitive to a dark bar than to a Fourier bar.
To test this hypothesis, we expressed a genetically encoded calcium indicator, GCaMP6m (Akerboom et al., 2012; Chen et al., 2013), in T4/T5 cells (Fig. 7A) and imaged the cellular activity using a 2-photon excitation microscope. T4 and T5 each refer to a class of columnar medulla neurons comprising four subtypes within each retinotopic column (a, b, c, d), classified according to their directional preference (backward, forward, up and down, respectively) and innervation in the layers of the lobula plate (1, 2, 3 and 4, respectively). Under stimulus conditions similar to those for the behavioral paradigm (see Materials and Methods), we selected ROI from each of two T4/T5 subtypes, depending upon their innervation layer in the lobula plate. Front-to-back selective T4/T5 neurons (CW ipsilateral to the recording site) innervate layer 1, and back-to-front selective T4/T5 neurons (CCW on eye ipsilateral to the recording site) innervate layer 2 (Fig. 7A–C).
We found that T4/T5 responses in both layers showed stronger calcium responses to a dark bar than to a similarly sized Fourier bar moving in the same direction at the same speed (Fig. 7B). T4/T5 responses to preferred CW (ipsilateral front-to-back) responses in layer 1 showed a trending but statistically insignificant difference in maximum amplitude across the two bar types (Fig. 7C). However, the onset delay and peak magnitude of responses in layer 2 to preferred CCW back-to-front motion were different across bar type. It is this last condition, back-to-front movement toward the visual midline, that generates the counter-directional orientation response which distinguishes Fourier bar from dark bar behavioral responses (Fig. 2A,B). We explored whether null-direction responses in T4/T5 evoked by the Fourier or dark bar are different, which if true would support a role for T4/T5 in the divergent behavioral response to these two bar types. However, null-direction responses to the Fourier and dark bar were identical within both layer 1 and layer 2 (Fig. 7B).
Why does the solid dark bar containing only two edges stimulate columnar motion detectors more strongly than a bar containing multiple edges? There are at least two possibilities. First, the higher spatial frequency content of the Fourier bar may be beyond the optimum detection limit of the underlying motion detectors. The algorithm for motion vision is a matter of strong debate in the literature, but our own rudimentary correlation-type detector model (Theobald et al., 2008) showed stronger output to the dark bar than to the Fourier bar (data not shown). Alternatively, the additional edges comprising the Fourier bar may be expected to evoke stronger responses, but ensemble activity of T4/T5 neurons is reduced through lateral inhibition (Fisher et al., 2015). In either case, our data would support the hypothesis that T4/T5 neurons contribute to the co-directional optomotor steering responses to bar motion in tethered flight, which are significantly stronger for the dark bar than for the Fourier bar.
To explore the functional role of T4/T5 cells in bar-tracking behavior, we expressed an inwardly rectifying potassium channel, Kir2.1 (Baines et al., 2001). For both bar types and stimulus directions, the amplitude of motion-elicited steering responses was diminished in T4/T5-silenced flies by comparison with that in enhancerless Gal4 controls (Fig. 8A,B). Closer inspection shows that the asymmetry in steering responses that occur across the visual midline was diminished in T4/T5-silenced flies (Fig. 8A,B, blue traces), consistent with prior results (Bahl et al., 2013).
Fig. 8.
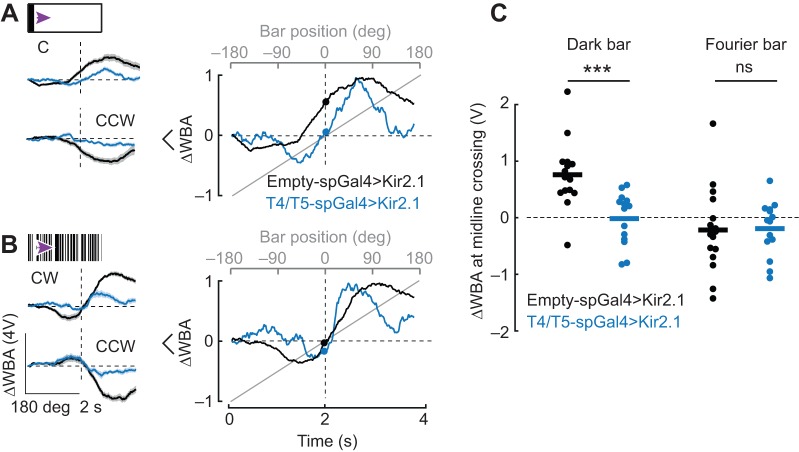
Genetically silencing T4/T5 eliminates the phase-advanced behavioral response to the dark bar. (A) Left: average steering responses to a 30 deg dark bar rotating CW or CCW in control flies (black) and T4/T5-silenced flies (blue). Shaded regions represent s.e.m. Right: average traces from the left panel normalized to their own maximum to facilitate comparison. (B) Left: average steering responses to a 30 deg rotating Fourier bar in CW and CCW directions. Shaded regions represent s.e.m. Right: average traces from left panel normalized to their own maximum to facilitate comparison. (C) ΔWBA values at bar midline crossing; each dot represents a single fly; horizontal line indicates mean. n=16 enhancerless control, n=14 experimental genotype. Wilcoxon rank sum test to compare control and T4/T5-silenced flies: P=3.2×10−4 for dark bar response at midline crossing comparing control and T4/T5-silenced flies; P=0.66 for Fourier bar. ***P<0.001.
To visualize the response dynamics to the two bar types, we normalized the amplitude of the population mean traces. Flies with silenced T4/T5 neurons showed strong contra-directional responses as the dark bar reached −90 deg, whereas control flies lacked strong contra-directional responses, instead showing normal co-directional tracking responses and phase-advanced steering as the bar crossed the visual midline (Fig. 8A, right, filled dots indicate mean ΔWBA at zero crossing). Control flies showed normal Fourier bar responses, whereas T4/T5 driving Kir2.1 resulted in qualitatively similar compound orientation/tracking responses but with more rapid temporal transition in steering direction as the bar crossed the midline (Fig. 8B, right).
When we compared the steering phase via ΔWBA values at the point that the bar traversed the visual midline, remarkably, T4/T5 silencing significantly affected steering responses for a dark bar by reducing the phase advance, but had no effect on the zero crossing of the Fourier bar responses. These results support the hypothesis that the counter-directional orientation response is processed by a separate visual pathway from the co-directional tracking response, and that only the latter is supported by activity by T4/T5 motion detectors.
DISCUSSION
The two bar types produce unequivocally distinct behavioral responses, which can be broadly summarized as a co-directional optomotor response robustly evoked by the dark bar and a counter-directional orientation response robustly evoked by the Fourier bar (Figs 1–6). Two lines of evidence suggest that the dark bar optomotor response is driven by T4/T5 activity: (1) the magnitude of T4/T5 responses was smaller for the Fourier bar than for the dark bar (Fig. 7) and, more compellingly, (2) blocking T4/T5 activity reduced co-directional optomotor responses but preserved counter-directional orientation responses, effectively removing the behavioral differences evoked by the two bar types (Fig. 8). However, the differences in T4/T5 calcium responses to the two bar types are predominantly quantitative (i.e. changes in peak amplitude) rather than qualitative (i.e. changes in dynamics or sign). CCW back-to-front bar motion toward midline generated the strongest qualitative behavioral differences for the two bar types (Fig. 2A), and it is this regime in which T4/T5 responses are more divergent for the two bar types (Fig. 7B). It seems unlikely that the counter-directional orientation response could be supported by T4/T5 activity for two reasons: (1) behavioral orientation responses were preserved under T4/T5 hyperpolarization (Fig. 6) and (2) T4/T5 responses to null direction motion did not differ for the two bar types (Fig. 7B). We posit that as the Fourier bar approaches visual midline, T4/T5 response onset is delayed and peak amplitude is reduced, while at the same time whatever mechanism elicits the counter-directional orientation response is operating maximally.
A dark bar powerfully engages the velocity subsystem, which is dependent on T4/T5 signals and drives co-directional optomotor responses to the bar
Whether walking, flying tethered or in free-flight, a fly will approach a contrasting vertical edge, post or bar, presumably reflecting an orientation response to natural landscape features such as plant stalks (Bulthoff et al., 1982; Frye et al., 2003; van Breugel and Dickinson, 2012). Classical work posited that edge or bar orientation is facilitated by two subsystems: an optomotor response driven by the speed and direction of motion and an orientation response toward the stationary position of a flickering object (Kimmerle et al., 2000; Pick, 1974, 1976; Poggio and Reichardt, 1976; Wehrhahn, 1981; Wehrhahn and Hausen, 1980). The first neurons within the visual processing hierarchy that respond to both flicker and directional motion in flies are T4 and T5 columnar neurons of the medulla (Strother et al., 2017). For a tethered fly walking on an air-supported ball, genetically silencing T4/T5 neurons causes a complete loss of directional wide-field optomotor responses but largely intact orientation toward a dark bar (Bahl et al., 2013). That study supported the classical framework that bar-tracking behavior relies on both motion vision and motion-independent position information, and concluded that the former is supplied by T4/T5 motion-detecting neurons whereas that the latter must be supplied by separate neural pathways. Our results during flight largely confirm these results in flying animals in the sense that silencing T4/T5 neurons suppresses co-directional optomotor responses to a moving bar, but counter-directional orientation responses remain largely intact. As a result, for the luminance-defined dark bar, the normal phase-shifted ΔWBA at zero-crossing is absent in T4/T5-silenced animals (Fig. 8). In other words, silencing T4/T5 has a stronger influence on the co-directional optomotor response than on the counter-directional orientation response.
A recent study of tethered flying flies found that silencing T4/T5 neurons left animals unable to fixate a motion-defined Fourier bar (Fenk et al., 2014). This study contradicted Bahl and colleagues (2013) by concluding that T4/T5 neurons are required for bar-tracking behavior. A systems identification approach showed that the frontally tuned motion-dependent subsystem is fast and co-directional, whereas the peripherally tuned position-dependent subsystem is slow and counter-directional (Aptekar et al., 2012). We would suggest that the experimental conditions of the two studies target the underlying subsystems differently in that high-gain bar fixation in flight may be more reliant on the fast motion responses of T4/T5 neurons than comparatively low gain orientation by a walking fly or a flying fly exposed to relatively slow visual dynamics. In support of this conclusion, neither a dark bar nor a Fourier bar elicited contra-directional orientation responses at high speed, yet both elicited co-directional tracking responses at all speeds (Fig. 3). Silencing T4/T5 neurons leaves orientation responses largely intact for relatively slow bar movement and, importantly, the residual response persists in a similar manner for both the dark bar and Fourier bar.
It is not just the azimuthal tuning and temporal dynamics of the two underlying subsystems that are separable – so too are the relative sensitivities to the visual properties of the object. Switching from a luminance-defined dark bar to a motion-defined Fourier bar moving under identical dynamical conditions changes the balance of co-directional optomotor and counter-directional orientation responses (Figs 1 and 2). The orientation response is more sensitive to low contrast than is the co-directional optomotor response (Fig. 5E). Finally, the luminance-defined dark bar more strongly drives T4/T5 neurons than does the motion-defined Fourier bar (Fig. 7), yet the latter strongly stimulates counter-directional steering responses triggered in the visual periphery (Figs 2, 3, 5 and 6). Thus, any experimenter using a luminance-defined bar should be aware how strongly this stimulus drives the optomotor motion pathway by contrast to the positional orientation pathway.
The interplay between dark bar directional optomotor responses and Fourier bar orientation responses is recapitulated by flies on a magnetic tether. Flies often generate counter-directional body saccades as a Fourier object enters the field of view, followed by bouts of co-directional saccades during pursuit (Mongeau and Frye, 2017). By contrast, in the magnetic tether a dark bar elicited more smooth movement between saccades, which is consistent with the phase-advanced steering response recorded in the rigid tether (Fig. 2C,D). The azimuthal distribution of bar location on the retina for flies in the magnetic tether peaks at roughly ±45 deg, which is in close agreement with the azimuthal domain of the orientation steering response to a Fourier bar on the rigid tether (Fig. 2A). However, saccades are triggered not by the static position of the bar per se but rather by the temporal integral of bar displacement, indicating that movement cues generated by the bar, rather than static position cues, play a key role in eliciting and modulating the dynamics of bar-tracking saccades (Aptekar et al., 2012; Mongeau and Frye, 2017). For this reason, our lab has referred to elementary motion and figure motion subsystems rather than velocity and position (Aptekar et al., 2012).
A Fourier bar strongly engages a positional orientation subsystem independently from T4/T5 activity and drives a counter-directional response
If the co-directional optomotor component of bar tracking is supported by T4/T5 motion detectors, what is the underlying mechanism for counter-directional orientation responses? Our results here suggest that steering responses to both bar types begin in the visual periphery (Figs 1C, 2A and 6B,E). An orientation response toward a laterally displaced stimulus is in agreement with findings of motion-evoked but non-directional behavioral responses to stimuli presented to the fly's lateral or rear fields of view. For example, in a similar fly visual flight simulator setup, presentation with a 90 deg grating projected onto either rear quadrant of the fly's visual field evoked turns oriented toward the stimulus regardless of the direction of grating motion (Tammero, 2004). Freely walking flies halt their forward movement in response to a fly-like object approaching from the rear, but do not do so when the object approaches at the same speed from the front (Zabala et al., 2012). The spatial tuning of flight steering responses to bar displacements that contain no coherent motion cues peaks in the visual periphery (Aptekar et al., 2012), and tracking saccades are triggered by bar motion in the visual periphery (Mongeau and Frye, 2017).
We would posit that for a dark bar, the co-directional optomotor response outweighs the counter-directional orientation response. For the Fourier bar, the two responses are more balanced. Taken together, these results suggest that the visual circuitry that mediates contra-directional orientation responses or saccades toward Fourier bar-like features should include neurons with receptive fields centered on the lateral field of view, be responsive to moving features but without directional selectivity, and be sensitive to spatial features that may not strongly excite directional motion detectors. Neurons of the fly lobula have been shown to display some or all of these properties (Aptekar et al., 2015; Keleş and Frye, 2017; Wu et al., 2016). Yet, to date there is no direct evidence that these known cell circuits are required for the behaviors shown here. Neurons within a set of unpaired higher-order brain structures called the central complex that encode heading orientation in the fly (Seelig and Jayaraman, 2015) have been found to be dispensable for bar tracking in flight; instead, they are required for orientation to a sun-like stimulus (Giraldo et al., 2018). Therefore, to date, the visual pathways that mediate motion-independent object orientation responses in flies are unknown.
Is frontal bar fixation an epiphenomenon of the optomotor response?
For a rigidly tethered fly, if the optomotor response is spatially non-uniform with maximum sensitivity at the frontal midline (Tammero, 2004; Aptekar et al., 2012), and under experimental conditions in which the steering effort closes a feedback loop to the rotational velocity of the bar, then either a contra-directional orientation response toward the bar or a co-directional optomotor response in the direction of the bar would move the bar toward the visual midline. Indeed, animals under virtual closed-loop feedback conditions (i.e. steering output experimentally coupled to reafferent visual input) show highly robust fixation of a bar on the visual midline, even under conditions in which a motion-defined Fourier bar moves opposite or independently from movements of the visual background (Fenk et al., 2014; Fox et al., 2014). By contrast, flies that are tethered on a magnetic pivot execute smooth or saccadic body movements to close the visual feedback loop directly (i.e. no experimental coupling). Under these conditions flies execute saccades to follow a revolving Fourier bar, but do not fixate it on the visual midline, nor do they show significant steering responses to the bar crossing their visual midline (Fig. 2D; and see Mongeau and Frye, 2017). Instead, flies on the magnetic tether behave more like animals in free flight, maintaining straight flight headings between body saccades but smoothly compensating for experimental rotation of the visual panorama (Mongeau and Frye, 2017; Mronz and Lehmann, 2008; Stowers et al., 2017).
We postulate that animals on the rigid tether use the bar to close the smooth optomotor feedback loop, resulting in frontal azimuthal fixation under experimentally imposed (virtual) closed-loop feedback conditions. We do not yet understand why animals on the magnetic tether do not. One possibility is that the efference copy of an optomotor steering command (Kim et al., 2015) does not match the reafference experienced by the experimental feedback signal. Or perhaps the lack of natural inertial dynamics and associated mechanosensory feedback generated by body rotation somehow compromises normal optomotor equilibrium control in a rigidly tethered fly (Bartussek and Lehmann, 2016; Mureli et al., 2017). Notwithstanding the fascinating unresolved issues involving object fixation behavior, some features of bar tracking and orientation under naturalistic conditions are readily observable from the open-loop responses of rigidly tethered animals. For example, the counter-directional orientation response, peaking at roughly 45 deg from midline (Fig. 2) is in good agreement with the spatial domain over which saccades are elicited in the magnetic tether (Aptekar et al., 2012; Mongeau and Frye, 2017). Our findings that bar orientation responses depend upon the spatial properties of the bar itself (Fig. 2), bar speed (Fig. 3), contrast conditions (Figs 5 and 6) and activity of directional motion detectors (Fig. 8) provides a conceptual framework for designing or interpreting state-of-the-art in vivo calcium imaging experiments on rigidly fixed flying animals (e.g. Schnell et al., 2014; Weir and Dickinson, 2015), as well as future behavioral experiments aimed at understanding the neural mechanisms for feature detection and flight navigation.
Acknowledgements
We thank Michael Reiser, Gerry Rubin and Gwyneth Card for fly stocks, members of the Frye lab for carefully reading the manuscript, Ben Hardcastle for fabricating the fly holder for calcium imaging, and Delaine Quaresma and Nina Fukuma for laboratory assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.F.K., M.A.F.; Methodology: M.F.K., J.M.; Software: M.F.K., J.M.; Validation: M.F.K.; Formal analysis: M.F.K., J.M.; Investigation: M.F.K., J.M.; Resources: M.F.K.; Data curation: M.F.K.; Writing - original draft: M.F.K., M.A.F.; Writing - review & editing: M.F.K., J.M., M.A.F.; Visualization: M.F.K., J.M.; Supervision: M.A.F.; Project administration: M.A.F.; Funding acquisition: M.A.F.
Funding
This work was supported by National Institutes of Health grant EY026031. Deposited in PMC for release after 12 months.
References
- Akerboom J., Chen T.-W., Wardill T. J., Tian L., Marvin J. S., Mutlu S., Calderon N. C., Esposti F., Borghuis B. G., Sun X. R. et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819-13840. 10.1523/JNEUROSCI.2601-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin O. and Zipursky S. L. (2016). Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. eLife 5, 961-972. 10.7554/eLife.20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptekar J. W., Shoemaker P. A. and Frye M. A. (2012). Figure tracking by flies is supported by parallel visual streams. Curr. Biol. 22, 482-487. 10.1016/j.cub.2012.01.044 [DOI] [PubMed] [Google Scholar]
- Aptekar J. W., Keleş M. F., Lu P. M., Zolotova N. M. and Frye M. A. (2015). Neurons forming optic glomeruli compute figure-ground discriminations in Drosophila. J. Neurosci. 35, 7587-7599. 10.1523/JNEUROSCI.0652-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl A., Ammer G., Schilling T. and Borst A. (2013). Object tracking in motion-blind flies. Nat. Neurosci. 16, 730-738. 10.1038/nn.3386 [DOI] [PubMed] [Google Scholar]
- Bahl A., Serbe E., Meier M., Ammer G. and Borst A. (2015). Neural mechanisms for Drosophila contrast vision. Neuron 88, 1240-1252. 10.1016/j.neuron.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Baines R. A., Uhler J. P., Thompson A., Sweeney S. T. and Bate M. (2001). Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523-1531. 10.1523/JNEUROSCI.21-05-01523.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartussek J. and Lehmann F. O. (2016). Proprioceptive feedback determines visuomotor gain in Drosophila. R. Soc. Open Sci. 10.1098/rsos.150562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausenwein B., Wolf R. and Heisenberg M. (1986). Genetic dissection of optomotor behavior in drosophila melanogaster studies on wild-type and the mutant optomotor-blind H31. J. Neurogenet. 3, 87-109. 10.3109/01677068609106897 [DOI] [PubMed] [Google Scholar]
- Bender J. A. and Dickinson M. H. (2006). Visual stimulation of saccades in magnetically tethered Drosophila. J. Exp. Biol. 209, 3170-3182. 10.1242/jeb.02369 [DOI] [PubMed] [Google Scholar]
- Buchner E., Buchner S. and Bülthoff I. (1984). Deoxyglucose mapping of nervous activity induced in Drosophila brain by visual movement - I. Wildtype. J. Comp. Physiol. A 155, 471-483. 10.1007/BF00611912 [DOI] [Google Scholar]
- Bulthoff H., Gotz K. G. and Herre M. (1982). Recurrent inversion of visual orientation in the walking fly,Drosophila melanogaster. J. Comp. Physiol. A 148, 471-481. [Google Scholar]
- Cavanagh P. and Mather G. (1989). Motion: the long and short of it. Spat. Vis. 4, 103-129. 10.1163/156856889X00077 [DOI] [PubMed] [Google Scholar]
- Chen T.-W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V. et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295-300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb C. and Sperling G. (1988). Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. J. Opt. Soc. Am. A 5, 1986 10.1364/JOSAA.5.001986 [DOI] [PubMed] [Google Scholar]
- Duistermars B. J. and Frye M. A. (2008). Crossmodal visual input for odor tracking during fly flight. Curr. Biol. 18, 270-275. 10.1016/j.cub.2008.01.027 [DOI] [PubMed] [Google Scholar]
- Duistermars B. J., Reiser M. B., Zhu Y. and Frye M. A. (2007). Dynamic properties of large-field and small-field optomotor flight responses in Drosophila. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 787-799. 10.1007/s00359-007-0233-y [DOI] [PubMed] [Google Scholar]
- Fenk L. M., Poehlmann A. and Straw A. D. (2014). Asymmetric processing of visual motion for simultaneous object and background responses. Curr. Biol. 24, 2913-2919. 10.1016/j.cub.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Fisher Y. E., Silies M. and Clandinin T. R. (2015). Orientation selectivity sharpens motion detection in Drosophila. Neuron 88, 390-402. 10.1016/j.neuron.2015.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. L. and Frye M. A. (2014). Figure-ground discrimination behavior in Drosophila. II. Visual influences on head movement behavior. J. Exp. Biol. 217, 570-579. 10.1242/jeb.080192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. L., Aptekar J. W., Zolotova N. M., Shoemaker P. A. and Frye M. A. (2014). Figure-ground discrimination behavior in Drosophila. I. Spatial organization of wing-steering responses. J. Exp. Biol. 217, 558-569. 10.1242/jeb.097220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M. A., Tarsitano M. and Dickinson M. H. (2003). Odor localization requires visual feedback during free flight in Drosophila melanogaster. J. Exp. Biol. 206, 843-855. 10.1242/jeb.00175 [DOI] [PubMed] [Google Scholar]
- Geiger G. (1981). Is there a motion-independent position computation of an object in the visual system of the housefly? Biol. Cybern. 40, 71-75. 10.1007/BF00326682 [DOI] [Google Scholar]
- Giraldo Y. M., Leitch K. J., Ros I. G., Warren T. L., Weir P. T. and Dickinson M. H. (2018). Sun navigation requires compass neurons in Drosophila. Curr. Biol. 28, 2845-2852.e4. 10.1016/j.cub.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bellido P. T., Wardill T. J. and Juusola M. (2011). Compound eyes and retinal information processing in miniature dipteran species match their specific ecological demands. Proc. Natl. Acad. Sci. USA 108, 4224-4229. 10.1073/pnas.1014438108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel S., Franconville R., Simpson J. H. and Seeds A. M. (2015). A neural command circuit for grooming movement control. eLife 4, e08758 10.7554/eLife.08758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide G. and Götz K. G. (1996). Optomotor control of course and altitude in Drosophila melanogaster is correlated with distinct activities of at least three pairs of flight steering muscles. J. Exp. Biol. 199, 1711-1726. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. and Wolf R. (1979). On the fine structure of yaw torque in visual flight orientation of Drosophila melanogaster. J. Comp. Physiol 140, 69-80. [Google Scholar]
- Horn E. and Wehner R. (1975). The mechanism of visual pattern fixation in the walking fly,Drosophila melanogaster. J. Comp. Physiol. 101, 39-56. 10.1007/BF00660118 [DOI] [Google Scholar]
- Keleş M. F. and Frye M. A. (2017). Object-detecting neurons in Drosophila. Curr. Biol. 27, 680-687. 10.1016/j.cub.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. J., Fitzgerald J. K. and Maimon G. (2015). Cellular evidence for efference copy in Drosophila visuomotor processing. Nat. Neurosci. 18, 1247-1255. 10.1038/nn.4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerle B., Eickermann J. and Egelhaaf M. (2000). Object fixation by the blowfly during tethered flight in a simulated three-dimensional environment. J. Exp. Biol. 203, 1723-1732. [DOI] [PubMed] [Google Scholar]
- Koffka K. (1935). Principles of Gestalt Psychology. Mimesis International. [Google Scholar]
- Livingstone M. and Hubel D. (1988). Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240, 740-749. 10.1126/science.3283936 [DOI] [PubMed] [Google Scholar]
- Maimon G., Straw A. D. and Dickinson M. H. (2008). A simple vision-based algorithm for decision making in flying Drosophila. Curr. Biol. 18, 464-470. 10.1016/j.cub.2008.02.054 [DOI] [PubMed] [Google Scholar]
- Maisak M. S., Haag J., Ammer G., Serbe E., Meier M., Leonhardt A., Schilling T., Bahl A., Rubin G. M., Nern A. et al. (2013). A directional tuning map of Drosophila elementary motion detectors. Nature 500, 212-216. 10.1038/nature12320 [DOI] [PubMed] [Google Scholar]
- Mongeau J.-M. and Frye M. A. (2017). Drosophila spatiotemporally integrates visual signals to control saccades. Curr. Biol. 27, 2901-2914. 10.1016/j.cub.2017.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mronz M. and Lehmann F.-O. (2008). The free-flight response of Drosophila to motion of the visual environment. J. Exp. Biol. 211, 2026-2045. 10.1242/jeb.008268 [DOI] [PubMed] [Google Scholar]
- Mureli S., Thanigaivelan I., Schaffer M. L. and Fox J. L. (2017). Cross-modal influence of mechanosensory input on gaze responses to visual motion in Drosophila. J. Exp. Biol. 220, 2218-2227. 10.1242/jeb.146282 [DOI] [PubMed] [Google Scholar]
- O'Carroll D. C. and Wiederman S. D. (2014). Contrast sensitivity and the detection of moving patterns and features. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369, 20130043 10.1098/rstb.2013.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick B. (1974). Visual flicker induces orientation behaviour in the fly Musca. Z. Naturforsch 29, 310-312. 10.1515/znc-1974-5-629 [DOI] [Google Scholar]
- Pick B. (1976). Visual pattern discrimination as an element of the fly's orientation behaviour. Biol. Cybern. 23, 171-180. 10.1007/BF00344749 [DOI] [Google Scholar]
- Poggio T. and Reichardt W. (1976). Visual control of orientation behaviour in the fly: Part II. Towards the underlying neural interactions. Q. Rev. Biophys. 9, 377 10.1017/S0033583500002535 [DOI] [PubMed] [Google Scholar]
- Reichardt W. and Poggio T. (1975). A theory of the pattern induced flight orientation of the fly Musca Domestica II. Biol. Cybern. 18, 69-80. [DOI] [PubMed] [Google Scholar]
- Reichardt W. and Poggio T. (1979). Figure-ground discrimination by relative movement in the visual system of the fly. Biol. Cybern. 35, 81-100. 10.1007/BF00337434 [DOI] [Google Scholar]
- Reichardt W. and Wenking H. (1969). Optical detection and fixation of objects by fixed flying flies. Naturwissenschaften 56, 424 10.1007/BF00593644 [DOI] [PubMed] [Google Scholar]
- Reichardt W., Egelhaaf M. and Guo A.-K. (1989). Processing of figure and background motion in the visual system of the fly. Biol. Cybern. 61, 327-345. 10.1007/BF00200799 [DOI] [Google Scholar]
- Reiser M. B. and Dickinson M. H. (2008). A modular display system for insect behavioral neuroscience. J. Neurosci. Methods 167, 127-139. 10.1016/j.jneumeth.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Reiser M. B. and Dickinson M. H. (2010). Drosophila fly straight by fixating objects in the face of expanding optic flow. J. Exp. Biol. 213, 1771-1781. 10.1242/jeb.035147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell B., Weir P. T., Roth E., Fairhall A. L. and Dickinson M. H. (2014). Cellular mechanisms for integral feedback in visually guided behavior. Proc. Natl. Acad. Sci. USA 111, 5700-5705. 10.1073/pnas.1400698111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. D. and Jayaraman V. (2015). Neural dynamics for landmark orientation and angular path integration. Nature 521, 186-191. 10.1038/nature14446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers J. R., Hofbauer M., Bastien R., Griessner J., Higgins P., Farooqui S., Fischer R. M., Nowikovsky K., Haubensak W., Couzin I. D. et al. (2017). Virtual reality for freely moving animals. Nat. Methods 14, 995-1002. 10.1038/nmeth.4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother J. A., Wu S.-T., Wong A. M., Nern A., Rogers E. M., Le J. Q., Rubin G. M. and Reiser M. B. (2017). The emergence of directional selectivity in the visual motion pathway of Drosophila. Neuron 94, 168-182.e10. 10.1016/j.neuron.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Takemura S.-Y., Bharioke A., Lu Z., Nern A., Vitaladevuni S., Rivlin P. K., Katz W. T., Olbris D. J., Plaza S. M., Winston P. et al. (2013). A visual motion detection circuit suggested by Drosophila connectomics. Nature 500, 175-181. 10.1038/nature12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S.-Y., Nern A., Chklovskii D. B., Scheffer L. K., Rubin G. M. and Meinertzhagen I. A. (2017). The comprehensive connectome of a neural substrate for ‘ON’ motion detection in Drosophila. eLife 6, e24394 10.7554/eLife.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammero L. F. (2004). Spatial organization of visuomotor reflexes in Drosophila. J. Exp. Biol. 207, 113-122. 10.1242/jeb.00724 [DOI] [PubMed] [Google Scholar]
- Theobald J. C., Duistermars B. J., Ringach D. L. and Frye M. A. (2008). Flies see second-order motion. Curr. Biol. 18, R464-R465. 10.1016/j.cub.2008.03.050 [DOI] [PubMed] [Google Scholar]
- Theobald J. C., Shoemaker P. A., Ringach D. L. and Frye M. A. (2010). Theta motion processing in fruit flies. Front. Behav. Neurosci. 4, 1-10. 10.3389/fnbeh.2010.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel F. and Dickinson M. H. (2012). The visual control of landing and obstacle avoidance in the fruit fly Drosophila melanogaster. J. Exp. Biol. 215, 1783-1798. 10.1242/jeb.066498 [DOI] [PubMed] [Google Scholar]
- von Reyn C. R., Breads P., Peek M. Y., Zheng G. Z., Williamson W. R., Yee A. L., Leonardo A. and Card G. M. (2014). A spike-timing mechanism for action selection. Nat. Neurosci. 17, 962-970. 10.1038/nn.3741 [DOI] [PubMed] [Google Scholar]
- Wehner R. (1972). Spontaneous pattern preferences of Drosophila melanogaster to black areas in various parts of the visual field. J. Insect Physiol. 18, 1531-1543. 10.1016/0022-1910(72)90232-6 [DOI] [PubMed] [Google Scholar]
- Wehrhahn C. (1981). Fast and slow flight torque responses in flies and their possible role in visual orientation behaviour. Biol. Cybern. 40, 213-221. 10.1007/BF00453371 [DOI] [Google Scholar]
- Wehrhahn C. and Hausen K. (1980). How is tracking and fixation accomplished in the nervous system of the fly - a behavioral-analysis based on short-time stimulation. Biol. Cybern. 38, 179-186. 10.1007/BF00337407 [DOI] [Google Scholar]
- Weir P. T. and Dickinson M. H. (2015). Functional divisions for visual processing in the central brain of flying Drosophila. Proc. Natl. Acad. Sci. USA 112, E5523-E5532. 10.1073/pnas.1514415112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Nern A., Williamson W. R., Morimoto M. M., Reiser M. B., Card G. M. and Rubin G. M. (2016). Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife 5, e21022 10.7554/eLife.21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala F., Polidoro P., Robie A., Branson K., Perona P. and Dickinson M. H. (2012). A simple strategy for detecting moving objects during locomotion revealed by animal-robot interactions. Curr. Biol. 22, 1344-1350. 10.1016/j.cub.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]