Abstract
OBJECTIVE
• To examine the interaction of body mass index (BMI) and race in predicting biochemical failure (BCF) after radical prostatectomy (RP). The relative contribution of BMI and race to BCF after RP has not been well characterized.
PATIENTS AND METHODS
• From 1988 to 2008, 969 white and black men underwent RP and BMI data were available. In all, 168 (17.3%) were black and 801 (82.7%) were white men.
• BCF was defined as a post-surgery PSA level ≥0.2 ng/mL on ≥2 measurements. Cox regression methods were used to model the relationship between race, BMI and BCF.
RESULTS
• The 969 men had a mean age of 59.8 ± 7.2 years. There was no significant difference in BMI between black and white men (P = 0.32).
• The 5-year disease-free survival for black obese men was the lowest at 48%, compared with non-obese black (73%), obese white (82%) and non-obese white men (83%, P < 0.001).
• BMI did not have a significant impact on BCF. In a multivariate analysis, black race remained an independent predictor of BCF [hazard ratio (HR) = 1.76, P = 0.01].
• BMI does not affect the risk of BCF in black men differently than white men (P value for interaction 0.93).
CONCLUSIONS
• Black race is an independent predictor of biochemical failure after adjusting for pathological factors. The impact of BMI on BCF did not vary among different races.
• These findings suggest that elevated BMI does not affect the BCF rates of black men more than in other races, and that other factors may influence the racial variability in disease-free survival and BCF risk.
Keywords: prostate cancer, prostatectomy, obesity, African-American, race, biochemical recurrence
INTRODUCTION
Prostate cancer is the most commonly diagnosed non-cutaneous cancer in men, with approximately 192 280 new cases and 27 360 deaths in 2009 [1]. Black men face a disproportionately high burden of prostate cancer; they have approximately 60% higher incidence rates and almost two-fold higher mortality rates than American-born whites [2]. Black men are more likely to present with a higher stage of disease and tumour grade after adjusting for socioeconomic and clinical factors, and are 50% more likely to present with distant metastases compared with white men [3–5].
Several factors have been suggested to account for the disparity in prostate cancer incidence and mortality, such as differences in tumour biology [6], stage of diagnosis [5] and socioeconomic factors [4]. The characterization of risk factors that are adjustable may help decrease the incidence and mortality associated with prostate cancer with appropriate interventions.
Obesity may be one important modifiable risk factor. Multiple studies have found that increased body mass index (BMI) is associated with an increased risk of death from prostate cancer [7,8]. Although obesity rates do not differ significantly nationwide between white and black adult men [9], obesity may affect the risk profile of racial groups differently, as increased consumption of fat from animal sources has been found to be a more significant risk factor for developing prostate cancer in black men than white [10].
Few studies have investigated the relative contributions of BMI and race to biochemical failure (BCF) after radical prostatectomy (RP). Amling etal. [11] found that while black men had higher obesity rates than white men, only black race was an independent predictor of BCF. Spangler et al. [12] found that obesity affects the biochemical failure rate in black men differently than European American men. It remains unclear if black race [13–18] or obesity [11,18–22] independently predicts BCF, and how BMI and race may interact with each other in predicting BCF after RP. In the present study, the relative contributions of race and BMI in predicting BCF were investigated, and the association of race and BMI with tumour grade, stage and preoperative PSA levels were explored.
PATIENTS AND METHODS
The Institutional Review Board approved Columbia University Urologic Oncology Database was retrospectively reviewed. Between 1988 and 2008, 3736 consecutive men underwent radical prostatectomy for the treatment of prostate cancer. Of those, 824 were excluded due to missing race data, 594 were excluded due to a follow-up time <12 months and were lost to follow-up, and 1160 men were excluded because of unavailable BMI data, leaving 1158 men for the analysis. The cohort included open, laparoscopic and robotic-assisted prostatectomy techniques, performed by multiple attending surgeons. The database was blinded to the surgeon. Sixteen per cent (189) of the men were from Hispanic or Asian heritage and were not included in this analysis. Of the men in the analysis, 801 (82.7%) of the men were white and 168 (17.3%) were black. The data excluded from the study were compared with the data in the study and were not significantly different.
BMI was calculated by dividing the weight (kg) by height squared (m2). Patient weight and height at time of surgery were obtained from the anaesthesia records. BMI was examined as a categorical variable using the National Institute of Health classification: normal (BMI ≤ 25 kg/m2), overweight (BMI 25 to 30 kg/m2) and obese (BMI ≥ 30 kg/m2).
Clinical and pathological data were retrieved from the database, including information on patient age, race, preoperative PSA level, Gleason score, pathological tumour-nodemetastasis system stage and surgical margin. The pathologic tumour stage was classified using the 1997 American Joint Committee on Cancer staging guidelines [23]. After RP, patients were followed until death or disease recurrence. Biochemical failure (BCF) was defined as a serum PSA level of ≥0.2 ng/mL on ≥2 consecutive occasions.
Age, PSA level and year of surgery were examined as continuous variables, whereas race (white vs black), Gleason score (2 to 6 vs 7 vs 8 to 10) and pathological stage (pT2 vs pT3 vs pT4) were examined as categorical variables. PSA was examined using the logarithmic transformation of PSA.
Differences in the distribution of clinical and pathological characteristics were explored between the BMI groups (normal weight vs overweight vs obese), and between the different races (white vs black) using one-way ANOVA F-test or Student's t-test for continuous variables and the chi-square test for categorical variables.
Time to biochemical progression was compared among BMI and ethnic categories using Kaplan–Meier plots and the log-rank test. Biochemical relapse-free survival was defined as the time from prostatectomy until occurrence of a post-treatment PSA level ≥0.2 ng/mL. Multivariate Cox proportional hazards models were used to model the relationship between race, obesity and time to biochemical failure as the primary outcome variable. Interaction terms in these models were tested using Wald's test.
RESULTS
For the 969 men in our analysis who underwent RP, the mean age was 59.8 ± 7.2 years, with a mean BMI of 27.0 ± 3.9 kg/m2 and a median preoperative PSA level of 5.4 ng/mL. Overall, the median follow-up time was 37 months (mean 46 months, range 12 to 168). Table 1 shows the clinical and pathological characteristics of the patients categorized according to race. Black men were significantly younger than white men (P = 0.03), but had higher-risk disease characteristics, with higher pre-operative PSA levels (P < 0.01), greater pT4 disease (P < 0.01) and greater Gleason 7 and 8–10 disease (P < 0.01). Black men also had higher positive surgical margin rates overall (P < 0.01) and in pT2 disease (P < 0.01) compared with white men. There was no significant difference in average BMI levels or representations in the obesity categories (P > 0.05) between racial groups. The follow-up time did not differ significantly according to race.
TABLE 1.
Clinical and pathological characteristics according to race
| Characteristic | White | Black | P value |
|---|---|---|---|
| No. of patients | 801 | 168 | |
| Age, mean ± SD | 60.0 ± 7.3 | 58.6 ± 6.8 | 0.03* |
| Year of surgery, median | 2002 | 2004 | <0.01* |
| Preoperative PSA, median | 5.3 | 6.2 | <0.01* |
| BMI, kg/m2 mean ± SD | 27.0 ± 3.9 | 27.3 ± 3.9 | 0.32* |
| BMI category | 0.50† | ||
| Normal | 240 (30.0%) | 46 (27.4%) | |
| Overweight | 419 (52.3%) | 86 (51.2%) | |
| Obese | 142 (17.7%) | 36 (21.4%) | |
| Pathological stage | <0.01 † | ||
| pT2 | 566 (70.7%) | 111 (66.1%) | |
| pT3 | 218 (27.2%) | 41 (24.4%) | |
| pT4 | 17 (2.1%) | 16 (9.5%) | |
| Pathological Gleason sum | <0.01† | ||
| 2–6 | 252 (31.4%) | 31 (18.5%) | |
| 7 | 433 (54.1%) | 100 (59.5%) | |
| 8–10 | 116 (14.5%) | 37 (22.0%) | |
| Positive surgical margin (PSM) | 169/801 (21.1%) | 67/168 (39.9%) | <0.01† |
| PSM in pT2 patients | 66/566 (11.7%) | 31/111 (27.9%) | <0.01 † |
| Median follow-up time, months (range) | 37 (12–168) | 31 (12–163) | 0.28* |
Student's t-test
Chi-square test.
Compared with normal weight and overweight individuals, obese men were younger at the time of surgery (P < 0.01) and had significantly higher grade disease (P = 0.02, see Table 2). There was no significant association between BMI and year of RP, preoperative PSA levels or length of follow-up time. Obese men tended to have higher stage disease, as 38.7% (68) of obese men had pT3 or pT4 disease compared with 26.9% (77) of normal weight and 28.9% (146) of overweight men, but the difference was not significant (P = 0.08). Obese men tended to have higher positive surgical margin rates in pT2 disease, but the difference was not significant (P = 0.39).
TABLE 2.
Clinical and pathological characteristics according to BMI
| Characteristic | Non-obese (BMI < 25 kg/m2) | Overweight (BMI ≥ 25 and <30 kg/m2) | Obese (BMI ≥ 30 kg/m2) | P value |
|---|---|---|---|---|
| No. of patients | 286 | 505 | 178 | |
| Age, mean ± SD | 60.6 ± 7.4 | 59.7 ± 6.8 | 58.5 ± 7.6 | <0.01* |
| Year of surgery, median | 2003 | 2003 | 2003 | 0.11* |
| Preoperative PSA, median | 5.3 | 5.3 | 5.9 | 0.91* |
| Pathological stage | 0.08† | |||
| pT2 | 209 (73.1%) | 359 (71.1%) | 109 (61.3%) | |
| pT3 | 67 (23.4%) | 130 (25.7%) | 62 (34.8%) | |
| pT4 | 10 (3.5%) | 16 (3.2%) | 7 (3.9%) | |
| Pathological Gleason sum | 0.02† | |||
| 2–6 | 90 (31.5%) | 158 (31.3%) | 35 (19.7%) | |
| 7 | 149 (52.1%) | 277 (54.8%) | 107 (60.1%) | |
| 8–10 | 47 (16.4%) | 70 (13.9%) | 36 (20.2%) | |
| Positive surgical margin (PSM) | 69/286 (24.1%) | 118/505 (23.4%) | 49/178 (27.5%) | 0.54† |
| PSM in pT2 patients | 30/209 (14.4%) | 47/359 (13.1%) | 20/109 (18.4%) | 0.39† |
| Median follow-up time, months | 39 | 37 | 33 | 0.19* |
One-wayANOVA F-test
Chi-square test.
Table 3 describes the characteristics according to race and BMI. White non-obese men were significantly older than white obese men (P= 0.02). Obese black men had significantly higher grade and tumour stage disease than non-obese black men, and obese and non-obese white mne (P < 0.01). Obese black men had significantly higher rates of positive surgical margins overall (P < 0.01), but did not differ significantly in stage pT2 disease (P = 0.39).
TABLE 3.
Clinical and pathological characteristics according to race/ethnicity and BMI
| Characteristic | White and non-obese (BMI < 30 kg/m2) |
White and obese (BMI ≥ 30 kg/m2) |
Black and non-obese (BMI < 30 kg/m2) |
Black and obese (BMI ≥ 30 kg/m2) |
P value |
|---|---|---|---|---|---|
| No. of patients | 658 | 143 | 128 | 40 | |
| Age, mean ± SD | 60.3 ± 7.2 | 58.7 ± 7.4 | 58.9 ± 6.3 | 58.0 ± 8.2 | 0.01* |
| Year of surgery, median | 2002 | 2003 | 2004 | 2004 | 0.02* |
| Preoperative PSA, median | 5.2 | 5.5 | 5.8 | 8.2 | <0.01* |
| Pathological stage | <0.01† | ||||
| pT2 | 476 (72.3%) | 90 (62.9%) | 88 (67.7%) | 23 (57.5%) | |
| pT3 | 167 (25.4%) | 51 (35.7%) | 29 (22.7%) | 12 (30.0%) | |
| pT4 | 15 (2.3%) | 2 (1.4%) | 11 (8.6%) | 5 (12.5%) | |
| Pathological Gleasonsum | <0.01† | ||||
| 2–6 | 220 (33.4%) | 32 (22.4%) | 36 (20.3%) | 5 (12.5%) | |
| 7 | 348 (52.9%) | 85 (59.4%) | 77 (60.2%) | 23 (57.5%) | |
| 8–10 | 90 (13.7%) | 26 (18.2%) | 25 (19.5%) | 12 (30.0%) | |
| Positive surgical margin (PSM) | 137/658 (20.8%) | 32/143 (22.4%) | 48/128 (37.5%) | 19/40 (47.5%) | <0.01† |
| PSM in pT2 patients | 51/476 (10.7%) | 15/90 (16.7%) | 24/88 (27.3%) | 7/23 (30.4%) | 0.39† |
| Median follow-up time, months | 40 | 32 | 27 | 41 | <0.01* |
One-way ANOVA F-test
Chi-square test.
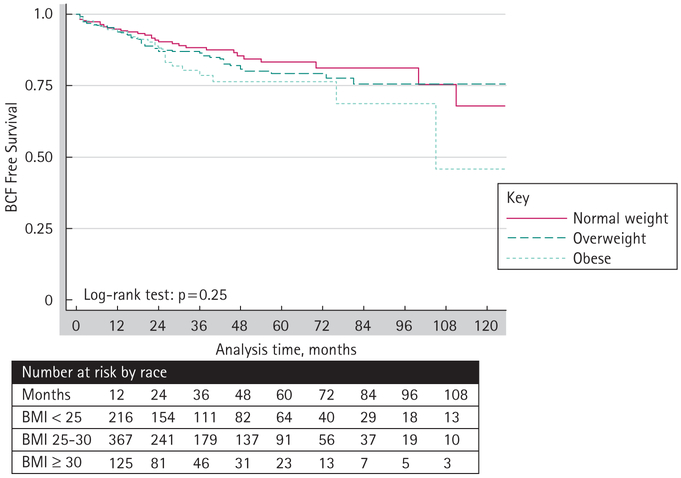
A log-rank survivorship analysis was performed to evaluate disease-free survival among different racial groups and BMI categories. Overall, the median time to BCF was 21 months. The 5-year disease-free survival rates were significantly lower for black men (66%) than for white men (83%, log-rank P < 0.01, Fig. 1). Obese (76%) and overweight (79%) men had lower 5-year disease-free survival rates than normal weight men (83%), but the differences were not significant (log-rank P = 0.25, Fig. 2). Obese black men had significantly worse 5-year disease-free survival than non-obese black men (48% vs 73%, respectively, log-rank P = 0.03), whereas obese white (82%) and non-obese white men (83%) had similar rates (log-rank P = 0.64, Fig. 3).
FIG. 1.
Kaplan–Meier estimates of biochemical failure (BCF)-free survival of patients treated with radical prostatectomy (RP) by race.
FIG. 2.
Kaplan-Meier estimates of biochemical failure-free survival of patients treated with radical prostatectomy by BMI categorization.
FIG. 3.
Kaplan-Meier estimates of biochemical failure-free survival of patients treated with radical prostatectomy by race and obesity.
Table 4 shows factors that were predictive of BCF in both univariate and multivariate Cox regression analysis. In univariate analysis, race, positive surgical margins, higher preoperative PSA levels, higher grade and higher stage disease were associated with a greater risk of having disease progression. After adjusting for the clinical and pathological factors, black race remained an independent predictor of biochemical failure [hazard ratio (HR) = 1.8, P value 0.01], whereas overweight or obesity did not reach statistical significance. Black men who were normal, overweight and obese were 2.0, 3.0 and 2.5 times, respectively, more likely than white men to have BCF in the respective weight categories. An analysis assessing the effect of an interaction between race and BMI in the multivariate Cox regression model demonstrated that HRs for increased BMI did not differ significantly by race (P value for interaction 0.93).
TABLE 4.
Cox regression model predicting biochemical recurrence
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | P value | 95% CI | HR | P value | 95% CI |
| Age | 1.01 | 0.35 | 0.99–1.04 | 0.99 | 0.56 | 0.96–1.02 |
| PSA (log) | 2.43 | <0.01 | 1.86–3.17 | 1.30 | 0.07 | 0.98–1.74 |
| BMI category (relative to non-obese) | ||||||
| Overweight | 1.22 | 0.26 | 0.79–1.88 | 1.31 | 0.48 | 0.84–2.06 |
| Obese | 1.57 | 0.92–2.68 | 1.13 | 0.64–1.99 | ||
| Race (relative to white) | ||||||
| Black | 3.06 | <0.01 | 2.06–4.57 | 1.76 | 0.01 | 1.14–2.72 |
| Tumour stage (relative to pT2) | ||||||
| pT3 | 3.70 | <0.01 | 2.47–5.52 | 1.72 | <0.01 | 1.08–2.76 |
| pT4 | 13.7 | 7.51–24.9 | 4.81 | 2.30–10.0 | ||
| Gleason sum (relative to Gl 6) | ||||||
| 7 | 4.49 | <0.01 | 2.22–9.11 | 3.37 | <0.01 | 1.63–6.95 |
| 8–10 | 14.2 | 7.01–28.9 | 7.64 | 3.51–16.7 | ||
| Surgical margin (relative to negative margin) | 3.31 | <0.01 | 2.30–4.78 | 1.53 | 0.05 | 1.00–2.33 |
DISCUSSION
Previous studies have consistently demonstrated that black men have a greater incidence of prostate cancer and poorer prostate cancer-specific survival compared with other racial groups [2,24], but there is no consensus as to whether race is an independent predictor of BCF [13–18]. In the present study, black men had significantly greater serum PSA levels and had more advanced disease characteristics than white men, which are observations that are consistent with previous studies [11,15]. After adjusting for the higher risk disease, black men were 1.8 times as likely as white men to experience biochemical failure. In a recent meta-analysis of racial differences in prostate cancer prognosis, Evans et al. [25] found a persistent elevated risk of biochemical recurrence of about 10–25% for black men. This finding is consistent with multiple other studies [11,15–17], and suggests that race needs to be reevaluated as a potential risk factor for biochemical recurrence in clinical decision making.
Numerous studies suggest that obesity, or dietary factors leading to obesity, is a risk factor in the development of more aggressive cancer [7,8,26]. Metabolic syndrome was found to be more prevalent in highly aggressive triple-negative breast cancer [26]. In a study of 135 000 men from Sweden, Andersson et al. [27] found that obese men were 40% more likely to die from prostate cancer than non-obese men. Obesity may also be an important modifiable risk factor for prostate cancer, as Rodriguez et al. [28] found that weight loss was associated with a decreased risk of developing prostate cancer. As obesity remains a growing public health concern in the United States, affecting almost a third of the nation [9], clarification of the association between prostate cancer and obesity is essential for patient counselling, treatment planning and to improve outcomes.
In the present study, obese patients had significantly higher pathological Gleason sums and positive surgical margin rates in pT2 disease than the normal weight or overweight men, which are findings generally consistent with previously published series [11,18,22]. The higher positive margin rate reflects the technical difficulty during surgical dissection among obese men. Obese men may have worse outcomes with prostate cancer because they are more likely to have a capsular incision [29] or because they have an artificially low PSA level from haemodilution and present with later stage disease [30]. The obese men in this cohort tended to be younger and have worse disease than normal weight individuals, which is consistent with previous studies [28,31] but did not have significantly different PSA levels. The univariate Kaplan–Meier analysis showed that although there was a trend for overweight and obese men to have lower disease-free survival rates than normal-weight men, the differences were not significant. Using a Cox proportional hazards regression model, neither obesity nor being overweight was predictive of BCF, which was consistent with previous studies [21,22,32].
We also sought to characterize the relative contributions of BMI and race, and how they may influence each other in the prediction of BCF. We found that the rates of obesity did not differ according to race, reflecting the distribution of the general population [9]. Obesity seemed to have a different effect on disease-free survival in black men compared with white men. Obese black men had more aggressive disease and significantly lower disease-free survival rates than non-obese black men in the Kaplan–Meier analysis, while obese and non-obese white men had similar disease-free survival rates. We found that the true interaction between race and BMI was no longer significant after adjusting for pathological tumour stage, grade and age in a multivariate analysis. HRs for increased BMI did not differ significantly by race, indicating that BMI does not have a significant different impact on the risk of BCF among different races.
Our results differ from Spangler et al. [12], who found a significant interaction of BMI on BCF in black men compared with European American men. They reported significant differences in obesity rates between black and white men in their cohort, which may represent differences in the patient population. These discrepancies need to be clarified by further large, multi-centre studies in order to improve the disparities in mortality between the different racial subpopulations.
There are several limitations to the present study that deserve consideration. Our median follow-up time for the cohort was 37 months, and the follow-up time for the non-obese black men was significantly shorter than for obese black men and white men, so subtle associations between groups that need time to mature might have been missed, given the slow progression of prostate cancer. This analysis included only patients who underwent RP as their primary treatment. Therefore, the patient cohort might have been influenced by the selection bias of the physician, who would consider the patient's BMI and comorbidities in selecting the treatment option. Data on occult lymph node positive disease and PSA dynamics were not available in all of these patients, which may influence the differences in biochemical failure in these patients. The black men in the present study also had significantly higher rates of positive surgical margins in patients with pT2 disease than white men. Before exportation from the secure Institutional Review Board (IRB)-approved database, the data is blinded to the surgeon. As there were 13 surgeons in the data set, it is possible that a higher positive margin rate surgeon, or a surgeon with less surgical experience, was operating on a higher number of black patients. In addition, Handa et al. [33] found that the dimensions of the pelvis differ significantly between white and black women, which suggests that the differences in the positive surgical margins in this cohort may be influenced by varying pelvic structures between the races. However, data on the structure of the pelvis have not been prospectively collected at this time, and the rate of positive surgical margins was not an objective in the present study; however they will be a consideration for future studies to address the apparent disparity in surgical outcomes. Finally, the disease-free survival in black men in our sample was based on 76 non-obese and 25 obese men. Even although the data suggests that obesity might be associated with poorer outcomes, the results should be interpreted carefully due to the relatively small numbers.
CONCLUSIONS
This study confirms that black race is an independent predictor of biochemical failure after adjusting for pathological factors. Obese men were younger and had worse disease, but when using an interaction term analysis, no significant differential impact of BMI on BCF could be detected across races. These findings suggest that elevated BMI does not affect the BCF rates of black men more than in other races, and that other factors may influence the racial variability in disease-free survival and BCF risk.
Abbreviations:
- BMI
body mass index
- BCF
biochemical failure
- RP
radical prostatectomy
- IRB
Institutional Review Board
- HR
hazard ratio
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–49 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96 [DOI] [PubMed] [Google Scholar]
- 3.Ghafoor A, Jemal A, Cokkinides V et al. Cancer statistics for African Americans. CA Cancer J Clin 2002; 52: 326–41 [DOI] [PubMed] [Google Scholar]
- 4.Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA 1995; 274: 1599–605 [PubMed] [Google Scholar]
- 5.Hoffman RM, Gilliland FD, Eley JW et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2001; 93: 388–95 [DOI] [PubMed] [Google Scholar]
- 6.Morton RA Jr. Racial differences in adenocarcinoma of the prostate in North American men. Urology 1994; 44: 637–45 [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38 [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev 2001; 10: 345–53 [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549–55 [DOI] [PubMed] [Google Scholar]
- 10.Hayes RB, Ziegler RG, Gridley G et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol Biomarkers Prev 1999; 8: 25–34 [PubMed] [Google Scholar]
- 11.Amling CL, Riffenburgh RH, Sun L et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 2004; 22: 439–45 [DOI] [PubMed] [Google Scholar]
- 12.Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol 2007; 178: 1939–44; discussion 45 [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Amling CL, Dorey F et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology 2002; 60: 670–4 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen ME, Han M, Mangold L et al. Black race does not independently predict adverse outcome following radical retropubic prostatectomy at a tertiary referral center. J Urol 2006; 176: 515–9 [DOI] [PubMed] [Google Scholar]
- 15.Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol 2002; 168: 2510–5 [DOI] [PubMed] [Google Scholar]
- 16.Powell IJ, Dey J, Dudley A et al. Disease-free survival difference between African Americans and whites after radical prostatectomy for local prostate cancer: a multivariable analysis. Urology 2002; 59: 907–12 [DOI] [PubMed] [Google Scholar]
- 17.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol 1996; 155: 1667–73 [PubMed] [Google Scholar]
- 18.Freedland SJ, Aronson WJ, Kane CJ et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol 2004; 22: 446–53 [DOI] [PubMed] [Google Scholar]
- 19.Bassett WW, Cooperberg MR, Sadetsky N et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology 2005; 66: 1060–5 [DOI] [PubMed] [Google Scholar]
- 20.Magheli A, Rais-Bahrami S, Trock BJ et al. Impact of body mass index on biochemical recurrence rates after radical prostatectomy: an analysis utilizing propensity score matching. Urology 2008; 72: 1246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motamedinia P, Korets R, Spencer BA, Benson MC, McKiernan JM. Body mass index trends and role of obesity in predicting outcome after radical prostatectomy. Urology 2008; 72: 1106–10 [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui SA, Inman BA, Sengupta S et al. Obesity and survival after radical prostatectomy: a 10-year prospective cohort study. Cancer 2006; 107: 521–9 [DOI] [PubMed] [Google Scholar]
- 23.American Joint Committee on Cancer. AJCC Cancer Staging Manual, 5th edn. Philadelphia: Lippincott-Raven Publishers, 1997 [Google Scholar]
- 24.Ries LAG, Krapcho M, Mariotto A et al. SEER Cancer Statistics Review, 1975–2004. Bethesda: National Cancer Institute, 2007. Available at: http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007 [Google Scholar]
- 25.Evans S, Metcalfe C, Ibrahim F, Persad R, Ben-Shlomo Y. Investigating Black-White differences in prostate cancer prognosis: a systematic review and meta-analysis. Int J Cancer 2008; 123: 430–5 [DOI] [PubMed] [Google Scholar]
- 26.Maiti B, Kundranda MN, Jin T, Spiro TP, Daw HW. The association of metabolic syndrome with triple-negative breast cancer. J Clin Oncol 2009; 27: (suppl; abstr 1038) [DOI] [PubMed] [Google Scholar]
- 27.Andersson SO, Wolk A, Bergstrom R et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst 1997; 89: 385–9 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez C, Freedland SJ, Deka A et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2007; 16: 63–9 [DOI] [PubMed] [Google Scholar]
- 29.Freedland SJ, Grubb KA, Yiu SK et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol 2005; 174: 1798–801; discussion 801 [DOI] [PubMed] [Google Scholar]
- 30.Bañez LL, Hamilton RJ, Partin AW et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 2007; 298: 2275–80 [DOI] [PubMed] [Google Scholar]
- 31.Wright ME, Chang SC, Schatzkin A et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 2007; 109: 675–84 [DOI] [PubMed] [Google Scholar]
- 32.Chun FK, Briganti A, Graefen M et al. Body mass index does not improve the ability to predict biochemical recurrence after radical prostatectomy. Eur J Cancer 2007; 43: 375–82 [DOI] [PubMed] [Google Scholar]
- 33.Handa VL, Lockhart ME, Fielding JR et al. Pelvic Floor Disorders Network. Racial differences in pelvic anatomy by magnetic resonance imaging. Obstet Gynecol 2008; 111: 914–20 [DOI] [PMC free article] [PubMed] [Google Scholar]