Abstract
Purpose
Combining olaparib with carboplatin was recently shown to be active in both BRCA and non-BRCA mutant cancers in a recent phase I/Ib combination trial. The optimal drug sequence recommended was carboplatin 1-day before olaparib. However, carboplatin pre-treatment induced a ∼50% faster olaparib clearance.
Methods
To further explore this drug interaction, a population pharmacokinetic (PK) model was designed that included a lag time parameter, a second absorption compartment from tablet formulation, a single distribution/elimination compartment, and covariance among the clearance and volume parameters.
Results
Clearance (6.8 L/h) and volume (33 L) estimates were comparable with literature. The only significant covariate was the presence of carboplatin on olaparib clearance, consistent with published noncompartmental PK and in vitro data.
Conclusions
Simulations predicted lower steady-state peak/trough olaparib exposure through 24–36 h post carboplatin pre-treatment, but this effect was lost by day 2 and thus no dose adjustment is recommended.
Keywords: Olaparib, Population pharmacokinetics, Carboplatin, Drug interaction
Introduction
DNA damage is occurring constantly and cells have evolved numerous repair mechanisms based on the type of damage. Platinum therapies have been a mainstay of chemotherapy regimens for decades and have largely been efficacious via adducting DNA and disrupting replication. Unlike double-stranded DNA breaks (DSB) that involve non-homologous end joining and/or homologous recombination for repair, platinum–DNA adducts halt replication until nucleotide-excision repair (NER) removes the single-strand adducts. Nucleotide excision and base-excision repair (BER) mechanisms involve single-strand breaks (SSB) that utilize poly (ADP-ribose) polymerases (PARP) to build poly (ADP-ribose) chains (PAR) for recruitment of DNA repair enzymes. Homologous recombination mechanisms for DSB require BRCA proteins [1], thus deleterious BRCA −/− mutations force cells to rely on BER mechanisms that utilize PARPs.
Inhibitors of PARP, such as olaparib, veliparib, rucaparib, and niraparib, are therefore a promising anti-cancer therapeutic avenue to pursue and recent studies of single-agent therapies have extended progression-free survival (PFS) in patients with a variety of cancers, especially carriers of BRCA −/− [2, 3]. The combination of PARP inhibitors with various anti-cancer agents that induce DNA damage requiring SSB repair mechanisms, such as platinums, have potential for additive efficacy. Olaparib (capsule formulation) was the first PARP inhibitor to receive FDA approval (2014), but labeled usage is limited to a BRCA mutated patient population with advanced ovarian cancer that received at least three prior chemotherapies [4]. Olaparib is also approved for second line maintenance therapy by the European Medicine Agencies. Several clinical trials have demonstrated that olaparib monotherapy affords a PFS benefit, especially in a germline BRCA mutated patient population, while having an overall mild adverse event (AE) profile consisting mostly of grade 1–2 nausea (∼32%), fatigue (∼30%), and vomiting (∼20%) [5–13]. Further, numerous trials have combined olaparib with various other agents, including VEGF inhibitors cediranib [14–16] and bevacizumab [17], the anthracycline doxorubicin [18], the topoisomerase inhibitor topotecan [19], paclitaxel [20], and platinum compounds cisplatin [21–23] and carboplatin [24–26].
The anti-cancer mechanisms of olaparib and platinum compounds are proposed to be additive, but it was unknown whether one mechanism was required to occur before the other for maximum benefit. Preliminary in vitro experiments suggested greater cell death when carboplatin was given with or before olaparib, but this effect was lost when olaparib was given before carboplatin (unpublished). Olaparib capsules were combined with carboplatin in treatment of women with BRCA1 or BRCA2 mutation-associated breast or ovarian cancer and demonstrated a maximum tolerated dose (MTD) of 400 mg BID plus AUC5 carboplatin [24]. The AE profile was quite different than single agent olaparib, with 82% of patients having hematologic toxicity, likely due to carboplatin.
Recently, olaparib was studied as being administered orally in tablet formulation (BID days 1–7) before or after carboplatin in order to identify the optimal sequence for in vivo efficacy and safety [26]. Other study endpoints sought to determine the MTD, which was 200 mg BID olaparib + AUC4 carboplatin, as well as study the pharmacokinetics (PK) and pharmacodynamics (PD) of this sequenced combination. There are only a few comparable noncompartmental PK studies published, but most used olaparib in capsule formulation [7, 8, 27, 28], and only two published studies with olaparib tablets, one a food-effect study (that showed slowed absorption, i.e. lower CMAX, later Tmax, consistent with capsules with food) [29], and the other includes population PK analyses determined from a phase I study in 23 patients also given cisplatin and gemcitabine in adults with solid tumors [21]. This trial demonstrated a faster olaparib clearance with carboplatin pre-treatment using noncompartmental approaches, yet the sequence of therapy did not alter the quantity of platinum–DNA adducts. It is therefore the intent of this report to further study the PK of olaparib tablets in the absence or presence of 24-h prior carboplatin pretreatment using a population approach to assess the potential impact of carboplatin on olaparib disposition. Ultimately, the question to be answered is whether olaparib steady-state exposures would be significantly altered to a clinically-relevant extent with carboplatin pre-exposure once per cycle, and whether a dose adjustment should be considered.
Methods
Materials
Olaparib and deuterated olaparib ([2H]8-olaparib) were supplied from AstraZeneca. Stock solutions were prepared in HPLC-grade methanol (Sigma-Aldrich, St. Louis, MO) and all assay solvents were of Optima® grade (Fisher Scientific, Pittsburgh, PA). Pooled human plasma was provided by the NIH blood bank (Bethesda, MD). All water used was ultra-filtered using a MilliPore system (EMD MilliPore, Germany).
Study design
Olaparib was administered orally in tablet formulation at a dose of 200 mg taken approximately every 12 h (BID). Patients included in this two-arm, parallel design phase I/Ib run-in trial (NCT01237067, Clinicaltrials.gov) [26] were treated at the National Cancer Institute (Bethesda, MD, USA). Briefly, eligibility criteria for inclusion on this trial consisted of having recurrent/refractory epithelial ovarian, fallopian, primary peritoneal, uterine papillary serous, malignant mixed Mullerian tumors, or any type of breast cancer (confirmed histologically or cytologically at the NCI). A full list of eligibility criteria and study design can be found in the clinical summary [26]. Patients assigned to Arm A were administered olaparib beginning on cycle 1 day 1 (C1D1) for 7 days with carboplatin treatment beginning on C1D8 of a 21-day cycle. On cycle 2, patients on Arm A were given carboplatin on day 1, followed by 7 days of olaparib beginning on C2D2. Patients on Arm B had the reverse scheme: carboplatin on C1D1, olaparib C1D2–C1D8, then olaparib on C2D1–C2D7 with carboplatin administered on C2D8. Olaparib pharmacokinetics were assessed on C1D1 (before carboplatin) and C2D2 (after carboplatin) for patients on Arm A, and on C1D2 (after carboplatin) and C2D1 (before carboplatin) for patients on Arm B.
Pharmacokinetic sampling and sample bioanalysis
To examine the pharmacokinetic profile of olaparib following its oral administration, blood samples were collected in heparinized collection tubes before drug administration and at 0.5, 1, 2, 3, 4, and 8 h (±15 min) after the first dose of olaparib and immediately prior to the second daily dose of olaparib (approx 12 h after the first daily dose). All samples were analyzed using a validated uHPLC-MS/MS assay with a lower limit of quantitation (LLOQ) of 0.5 ng/mL [30].
Population analyses
A population pharmacokinetic (PPK) model utilizing nonlinear mixed effects (NLME) modeling was developed and validated using Pharsight Phoenix® NLME 1.3 (Certara, Cary, NC) to identify potential covariates (if any) that may influence olaparib pharmacokinetics, such as age, weight, creatinine clearance (CLCR; calculated using the Cock-croft-Gault equation), race, carboplatin pre-treatment, etc. A first-order conditional estimation with extended least squares (FOCE-ELS) estimation algorithm was used to provide maximum likelihood estimates for fixed and random effects.
Development of a multi-compartment absorption model was based on improvement in the objective function value (OFV, aka −2*log likelihood) and mechanistic reasoning from tablet disintegration, dissolution, and gastrointestinal transit. The between-subject variability (BSV) was described by an exponential function, where the individual parameter estimate (θi) is the product of the population parameter estimate (θpop) and the exponentiated BSV estimate for that individual (ηi).
| (1) |
A lag time (Tlag) was also tested based on an observed delay in the appearance of measurable olaparib plasma concentrations post dose. Several residual or unexplained variability (RUV) models were explored as well.
The OFV, calculated by Phoenix NLME 1.3 as minus two times the log likelihood (−2*log likelihood), was used for model diagnostics. Using the likelihood ratio test, a significant (α = 0.05) improvement between nested models requires a delta OFV > 3.84, based on a Chi squared distribution. Non-nested models were compared using the Akaike Information Criterion (AIC). Visual inspection of the model included goodness-of-fit plots, such as observed concentrations (dependent variable, DV), population predicted (PRED) and individual predicted (IPRED) concentrations versus time, DV versus PRED, DV versus IPRED, and conditional weighted residuals (CWRES) versus time or IPRED. Quantile–quantile (QQ) plots for η were assessed for all parameters to check the assumption that η follow a normal distribution. The η-shrinkage for each parameter was also assessed and the IIV estimates for parameters with high η-shrinkage (>30%) were used with caution due to insufficient individual observations that shrink the individual estimate towards the population estimate. Covariance between parameters was evaluated using an omega block variance–covariance matrix.
Identification of potential covariate effects on parameters were made by visual and statistical relationships between individual post hoc η estimates for each parameter and covariate values. Inclusion of covariates was initially performed in a stepwise, univariate, forward-inclusion approach based on significant reductions in the OFV for that parameter (p < 0.005). Covariates that significantly improved the base model in a univariate fashion were then added to each other in a multivariate, forward inclusion approach. Once a full model was achieved, a stepwise backward elimination (p < 0.001) approach was used to identify the final model. Continuous covariates were scaled to the dataset median (Eq. 2), where “COVi” is the covariate value for that individual, “COVmed” is the population median value, and θchng is an estimable parameter. Categorical covariates were included per Eq. 3, where θfct refers to the fractional change, “Cat1” is the first level of specified category, “Catn” refers to subsequent levels
| (2) |
| (3) |
The ability of the final model to predict the DV was tested by visual predictive checks (VPC). Parameter estimates and variances were used to simulate data (58 patients replicated 500 times each) and the 5th, 50th and 95th predicted percentiles were calculated in bins defined by time borders then visually compared with observed data and corresponding distributions. Nonparametric 95% confidence intervals and standard errors were obtained through bootstrap resampling (n = 1000 replicates).
Simulations
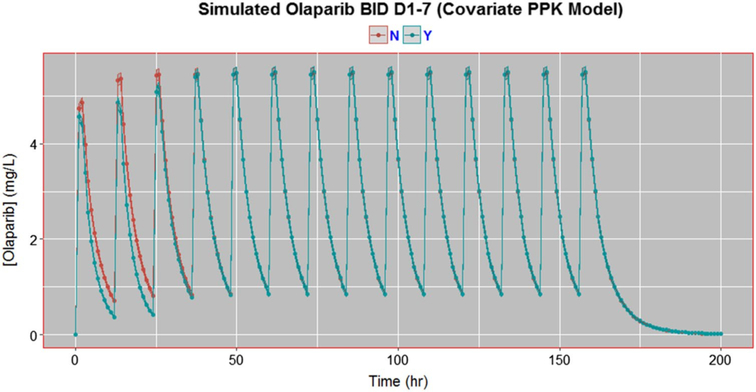
The final covariate model was used to simulate the cumulative effect of carboplatin (given day-1) on olaparib PK over the course of the regimen (BID days 1–7). An assumption of this simulation model is that the effect of carboplatin on olaparib clearance is effective for the first 24 h of olaparib dosing, which is based on literature that detected irreversible carboplatin–DNA mono-adduct formation up to 2 days post bolus dose [31], even with a terminal plasma half-life of 2–3 h [32, 33]. Twenty-Five simulated patients were “pre-exposed” to carboplatin, while 25 simulated patients were not; all patient simulations were replicated 100 times. Mean predicted olaparib concentrations over 8 days was plotted with a 95% confidence interval.
Statistical considerations
Geometric means and associated 95% confidence intervals are reported for PK parameter estimates throughout. Comparison of pharmacokinetic parameters by group was performed using an unpaired t test (GraphPad Prism, v6), where a two-sided p value <0.05 was considered to be statistically significant.
Results
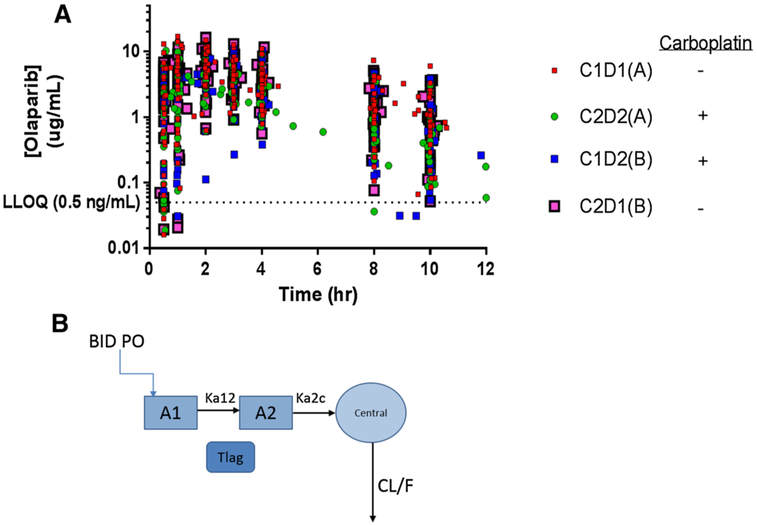
Fifty-eight patients had sufficient evaluable olaparib plasma concentration–time data for pharmacokinetic analysis. Patient descriptive data is available in Table 1. Figure 1a depicts the olaparib concentration–time profiles for all patients (n = 58), encompassing 870 sample points, as well as the near ten-fold inter-patient variability in plasma concentrations. This dataset had noncompartmental PK published along with the clinical trial efficacy and safety [26]. In that report, olaparib apparent oral systemic clearance (CLT/F; calculated as Dose/AUCINF) was approximately 50% faster when carboplatin was administered 24 h prior. This resulted in a lower CMAX and AUC, consistent with unpublished confidential sponsor data for carboplatin plus olaparib.
Table 1.
Patient characteristics (n = 58)
| Parameter | Value |
|---|---|
| Demographic | Median (range) or n (%) |
| Age (year) | 59.3 (25.1–74.3) |
| Body weight (kg) | 73.9 (44.7–124.4) |
| Serum creatinine (mg/dL) | 0.81 (0.45–1.45) |
| Creatinine clearance (mL/min)b | 88.0 (38.5–239.9)a |
| Race (African/Caucasian) | 8 (13.8%)/50 (86.2%) |
Patient with highest CLCr (239 mL/min) also had highest WT (124 kg)
CLCr estimated creatinine clearance per Cockcroft-Gault equation for females: CLCr = [([140—Age] × WT)/(72 × Serum creatinine)] × 0.85
Fig. 1.
Olaparib plasma concentration-time profiles from all patients (n = 58). Plasma concentrations of olaparib during the first dose of either cycle 1 or 2 for patients on both Arms A and B. Cycle 1 day 1 (C1D1) for patients on Arm A represented olaparib before carboplatin, whereas C2D2 for patients on Arm A represented olaparib after carboplatin (given on C2D1). For patients on Arm B, C1D2 represented olaparib after carboplatin (given on C1D1), and C2D1 represented olaparib before carboplatin
Population pharmacokinetics of olaparib
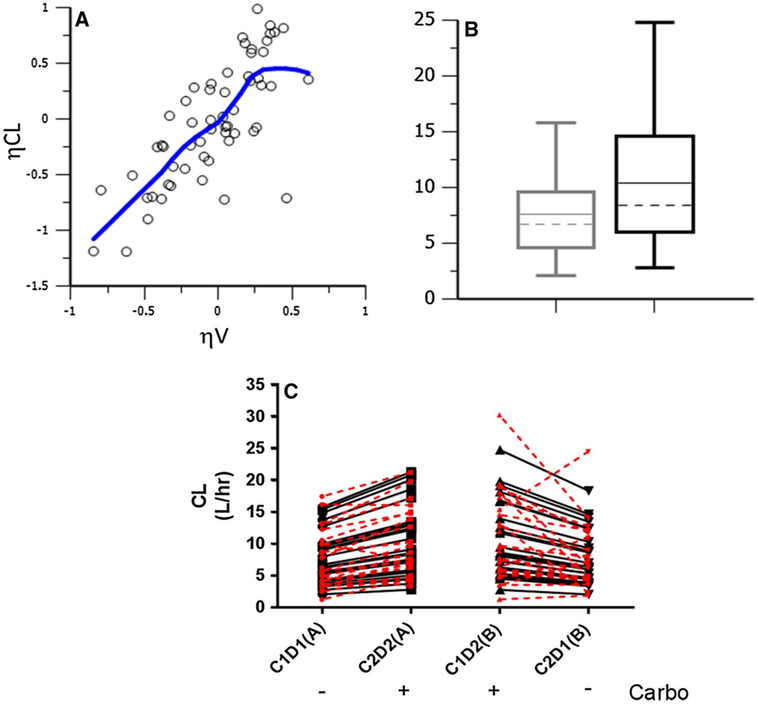
To further investigate these noncompartmental findings, a population PK model based on the patients from this study (NCT01237067) was developed and internally validated, consisting of a 2-transit compartment absorption model with a lag time (Tlag) parameter followed by a single compartment distribution model with first-order elimination (Fig. 1b). The addition of a Tlag parameter greatly improved the model (p = 1.09E–18), as did the second absorption compartment (p = 7.74E–27). A peripheral distribution compartment did not improve the model (p = 0.83) likely due to sampling only to 12 h post dose. The BSV estimates for etaK12 and etaK2c were 216 and 96.3%, respectively, indicating while these structural parameters are mechanistically necessary, there may be insufficient inter-individual data to accurately estimate IIV on these parameters. Therefore, no random effects were estimated for the K12 (absorption rate from 1st to 2nd absorption compartment) and K2c (absorption rate from 2nd absorption compartment to central compartment) parameters, and the typical values (thetas) were fixed and not allowed to vary. There was significant correlation between the CL/F and V/F parameters (ρ > 0.7) that was accounted for through a variance–covariance omega block matrix, which significantly improved the model (p = 3.96E–13) (Fig. 2a). Additionally, a proportional RUV model best described the data and resulted in the greatest statistical improvement in the model based on AIC (−649 points) per Eq. 4, where C0BS and CPRED are the observed and predicted olaparib plasma concentrations and ε is the proportional variability in the data.
Fig. 2.
Justification of covariance and covariate models. There was significant covariance among the random effects for clearance and volume of distribution (ρ > 0.70), that significantly improved the model (p < 4e—13) (a). The presence of carboplatin pretreatment led to significantly faster olaparib clearance, thus carboplatin was factored into the model on the CL parameter as a categorical covariate that led to a significant improvement (p < 2e—13) in the model (b). Model-predicted (black) clearance estimates for each patient with or without carboplatin were very consistent with NCA estimates (red) (c)
| (4) |
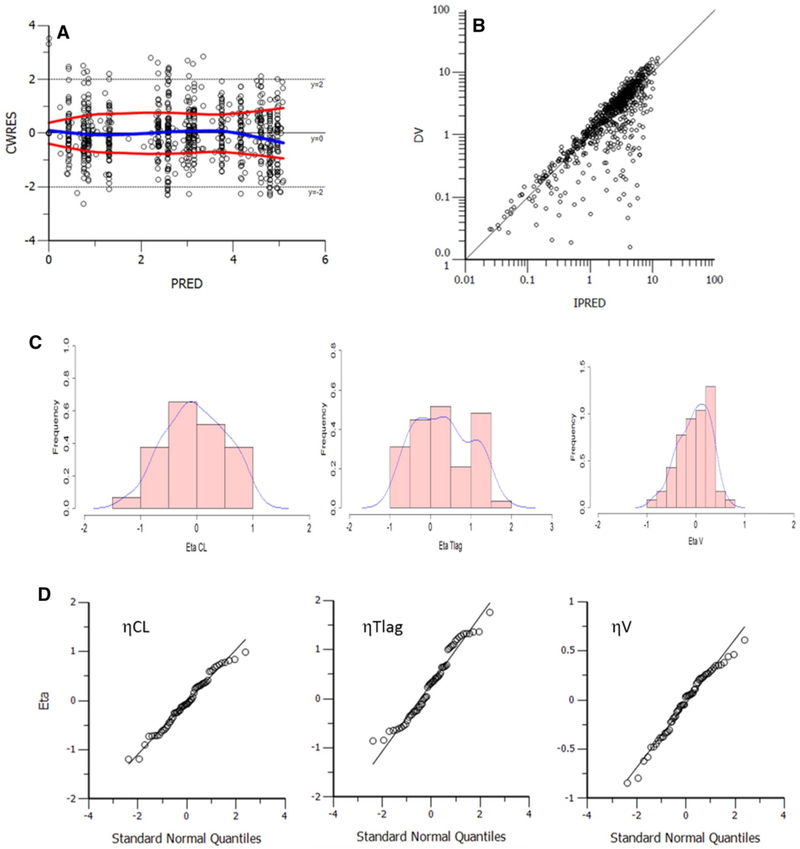
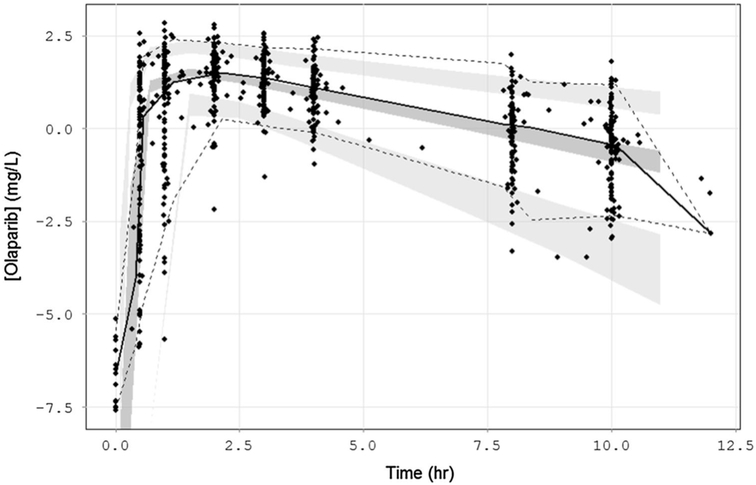
Several covariates were tested on the PPK base model. While age, race, body weight, serum creatinine, and creatinine clearance did not significantly influence the clearance parameter, the presence of carboplatin was identified as a categorical covariate that significantly improved the model (p = 1.89E–13; dOFV = −54.113, df = 1) (Fig. 2b). When Empirical Bayes Estimates (EBEs) for the clearance parameter were outputted for each patient on each cycle (i.e. based on presence/absence of carboplatin), the CL estimates were comparable to the NCA estimates [26] (Fig. 2c). BSV estimates for V/F, CL/F, and Tlag were 37, 55, and 87%, respectively. The addition of carboplatin covariate, while vastly improving the model fit, only explained ∼2% BSV on the CL/F parameter. After inspection of various model diagnostics, the final structural, covariate, and residual and unexplained variability models were deemed adequate (Fig. 3). Point estimates from this final model are listed in Table 2. Eta shrinkage estimates were all <21%, the condition number was 3641.46, and all model diagnostic plots were deemed acceptable. A visual predictive check (VPC) was performed to ensure the model’s ability to adequately describe the data (Fig. 4). All but five patients had data for both cycles available; these five patients all had cycle 1 data only (Arm A or B) and the model was able to predict either a single cycle or both cycles of data.
Fig. 3.
Model diagnostics. There was no trend in the CWRES vs PRED (a), confirming adequacy of structural model; nor any trend in DV vs IPRED (b), suggesting the residual or unexplained variability (RUV) model was adequate. Random effects calculated were normally distributed (c, d)
Table 2.
Final population PK model parameter estimates
| Parameter | Point estimate (%CV) | Bootstrap estimatea (%CV) | Bootstrap 95% CI |
|---|---|---|---|
| CL/F (L/h) | 6.83 (4.21%) | 7.02 (6.80%) | 5.96–7.84 |
| IIV CL/Fb | 55.0% (7.98%) | 57.0% (7.06%) | - |
| V/F (L) | 32.4 (4.30%) | 32.4 (5.39%) | 29.2–36.7 |
| IIV V/F | 37.3% (9.23%) | 38.4% (9.78%) | - |
| Kal2 (1/h)c | 14.5 (0%) | 14.5 (0%) | - |
| IIV Kal2 | N/E | N/E | - |
| Ka2c (1/h)c | 3.22 (0%) | 3.22 (0%) | - |
| IIV Ka2c | N/E | N/E | - |
| Tlag (h) | 0.251 (0.23%) | 0.299(14.1%) | 0.236–0.396 |
| IIV Tlag | 87.1% (13.5%) | 70.7% (19.6%) | - |
| dCLdCarbo (% change) | 1.35 (5.67%) | 1.34 (5.96%) | 1.16–1.51 |
| Proportional RUV (%CV) | 43.6% (5.22%) | 46.36% (5.29%) | 41.75–51.58% |
CI confidence interval; IIV inter-individual variability, expressed as %CV; RSE relative standard error; RUV residual or unexplained variability; N/E not estimated; dCLdCarbo fractional change in clearance parameter based on binary carboplatin covariate status
Boostrap performed with 1000 replicates
All “IIV” values given as point estimates (in %CV) with a percent relative standard error (%RSE)
Parameter estimates fixed
Fig. 4.
Visual predictive check. A visual predictive check (n = 500 reps) generated 95% confidence intervals (shaded areas) around the predicted 5th, 50th, and 95th quantiles (lines) that adequately described the observed data (dots)
Simulations
Simulations were performed using the current olaparib dosing scheme (200 mg BID days 1–7) either with or without carboplatin pre-treatment in order to assess the cumulative effect with 14 doses based on 2 days of carboplatin effect (lasting until end of first day of olaparib dosing). Equation 5 describes how an individual’s clearance (CLi) is a function of the population estimate (CLpop), the IIV parameter for that individual that determines how far off the population estimate that individual is (ηCLi), the fraction change in clearance with carboplatin (dCLdCarbo) that is only in effect when carboplatin was present (Carbo = 1) in the first 24 h (t < 24).
| (5) |
Simulated patients pre-exposed to carboplatin prior to olaparib dosing had lower predicted olaparib concentrations compared to simulated patients without carboplatin for the 24 h until steadily normalizing to “no carboplatin” simulated patients (Fig. 5). While there was a difference in olaparib exposure during the first 24–48 h of simulated PK profiles, this drug effect was not deemed to be clinically relevant or warrant a dose adjustment.
Fig. 5.
Simulations of 200 mg olaparib given BID on Days 1–7 either with carboplatin (blue) or without carboplatin (red). Twenty-five simulated patients in each group were given this dosing regimen; each replicated 100 times
Discussion
This report assessed the PK of olaparib tablets in the presence or absence of carboplatin pre-treatment in advanced women’s cancers. There are numerous studies demonstrating the efficacy of combination of olaparib-platinum therapy [6, 21–26], yet toxicities limit therapeutic efficacy and optimal doses still need to be defined, and none (besides the clinical trial that generated the current PK data [26]) published PK with olaparib tablets and carboplatin. Drug–drug interactions can alter drug exposure to subtherapeutic or supratherapeutic/toxic levels. In this study, carboplatin was shown to increase olaparib clearance ∼50%, resulting in lower olaparib exposure. While there is no comparable literature available for olaparib tablets with carboplatin, this result is consistent with unpublished findings from the drug sponsor that demonstrated a 14% lower olaparib AUC and 17% lower CMAX with carboplatin pre-exposure.
The noncompartmental pharmacokinetics of olaparib tablets from this study [26] were compared to relevant literature on olaparib tablet and capsule formulations [7, 8, 21, 27–29]. Following a 400 mg capsule dose, olaparib CMax and AUC values typically averaged 1–6.4 μg/mL and 10–70 h*μg/mL, respectively, while clearance and volume of distribution had estimated ranges of 7.0–10.8 L/h and 107–210 L, respectively [7, 8, 17, 21, 23, 27, 28]. Tablet doses of 200 mg gave mean values for CMAX, AUC, CL/F, and V/F, of 7 μg/mL, 43 h*μg/mL, 7.95 L/h, and 146 L, respectively, that suggested 200 mg tablets provided comparable exposure to 400 mg capsules [28, 29, 34]. Additionally, the AE profile of olaparib plus carboplatin was very similar for 200 mg BID tablets (with AUC4 carboplatin) and 400 mg BID capsules (with AUC5 carboplatin) [24, 26].
A more relevant comparison to the study presented here is a phase I study, conducted in 2012, where 23 advanced cancer patients received 100 mg olaparib tablets BID along with cisplatin and gemcitabine; population PK analyses were performed [21]. The point estimates for olaparib CL/F and V/F from that population PK model were 6.48 L/h and 38.7 L, respectively, along with a half-life of first dose olaparib of 4.07 h [21]. These values were comparable to this study’s first 200 mg dose of olaparib alone (6.9 L/h and 31.1 L, respectively; Group A C1D1), as well as other literature values (4.6 L/h and 40.3 L) [8]. Only one other study performed similar analyses (PK/PD) on patients given olaparib, but this was not a formal population analyses and no clearance or volume of distribution, nor BSV parameters were described [35].
The model described here had comparable population estimates for CL/F (6.83 L/h) to the NCA estimate (6.08 L/h) and a previous population analysis (6.5 L/h; *from the Supplement) [21]. Likewise, the population estimate for V/F from this analysis (32.4 L) was comparable to the NCA estimate (31 L) [26] and a previous report (39 L) [21]. The population half-life was estimated from this PPK model to be 2.9 h, while the previous PPK model estimated half-life at 4 h [21], which is likely due to the slightly slower CL/F and larger Vd from that report. Several major differences exist between this population PK model and the previous PPK model [21], including a variance–covariance block matrix, lag time, and an additional absorption compartment, whereas the previous PPK model used a simplified (single compartment) absorption model. Further, the absorption rates from the model described here were much faster (14.5 and 3.22/h for K12, K2c, respectively) than the previously estimated absorption rate (Ka) of 2/h [21].
The prescribing information for olaparib, approved in 2014 at 400 mg BID (capsules), reported several PK parameter estimates, including apparent plasma CL/F of 8.6 (±7.1) L/h, apparent volume of distribution at steady-state (Vss/F) of 167 (±196) L, and a half-life of 11.9 (±4.8) h [4]. While the clearance estimate is comparable (6.08 L/h), this study reports a much lower apparent oral volume of distribution (∼30 L) and shorter half-life (∼3 h). Reasons for this discrepancy are unclear, but could be related to capsules vs tablets or different patient population, where not all patients in this study had confirmed deleterious germline BRCA mutations and insufficient terminal PK sampling. The longer half-life of 12 h was based on an elimination rate calculated over a 72-h period [29]. The shorter half-life estimate in this study was likely due to an elimination rate calculation in the first 12 h post dose of a BID regimen, where the true terminal elimination had not yet occurred. The prescribing information also reported that olaparib CMAX and AUC increased 1.5- and 1.2-fold, respectively, in patients with mild renal impairment (CLcr = 50–80 mL/min) [4]. This suggested that renal clearance has a minimal, but still relevant, role in total systemic olaparib clearance.
Carboplatin is largely eliminated renally; patients with normal renal function (CLcr > 60 mL/min) excrete 65% of the dose within 12 h [36]. In this population model, creatinine clearance was not well correlated with olaparib clearance (r < 0.005). Further, it is logical to assume that co-administered carboplatin may slightly decrease olaparib clearance possibly due to competition for renal elimination (carboplatin is not plasma protein bound) and that any olaparib-carboplatin drug interaction may manifest as increased olaparib exposure, possibly in the same range (1.2–1.5 fold) as that observed in renally impaired patients [4]. However, in this study, the presence of carboplatin decreased CMAX and AUCLAST 1.2-fold and 1.3-fold, respectively, due to increased olaparib clearance; this is contrary to expected results and the mechanism for this moderate change is currently unclear. While there is no comparable literature available for olaparib tablets with carboplatin, this result is consistent with unpublished findings from the drug sponsor (NCT00516724) that demonstrated an 11.0% lower steady state olaparib AUC and a 14.8% lower CMAX with carboplatin pre-exposure (n = 4). The magnitude of decreased olaparib exposure in the presence of carboplatin is also comparable to the present study.
It is possible that pretreatment of carboplatin could be inducing elevation of PARP and as an intracellular binding target of olaparib, would lower the plasma concentrations of olaparib relative to monotherapy. The faster disappearance of olaparib from the plasma into the cells to bind PARPs during carboplatin pretreatment could account for the faster clearance estimates. No change in volume of distribution was observed relative to carboplatin pretreatment, consistent with prior data showing no change in olaparib plasma protein binding by carboplatin [26]. In order to elucidate the mechanism of altered olaparib clearance with carboplatin, in vitro experiments were needed and demonstrated greater intracellular olaparib when blood/PMBCs were pretreated with carboplatin [26], which is consistent with clinical results.
Simulations using this population PK model suggested that while the early olaparib doses would incur lower plasma concentrations with carboplatin pre-exposure (carboplatin effect assumed to last 2 days), eventually olaparib plasma exposure normalized. Therefore, on the current carboplatin regimen of a single dose once per 21-day cycle, there is no need for dose adjustment. This is also the consensus from other clinical data with carboplatin and olaparib, that while an effect on PK was observed, it was not deemed clinically relevant, which these simulations support.
In conclusion, this report assessed the pharmacokinetics of olaparib in the presence and absence of carboplatin pretreatment in women with advanced women’s cancers. There is clinical evidence of the additive effect of platinum treatment with PARP inhibitors in this patient population [21, 24, 25], and while a tolerable dose combination was previously determined with carboplatin/olaparib capsules [24], the use of olaparib tablets required a separate study. Pharmacokinetic data was not available for the aforementioned capsule study; therefore, a direct comparison of the carboplatin effect on olaparib clearance, whether in capsule or tablet, could not be assessed. It was evident through both noncompartmental and population analyses that carboplatin induced a faster olaparib clearance in this patient population. The long-term effects on steady-state olaparib are currently not known, as steady-state levels have not been assessed following carboplatin treatment given once per cycle. Thus, it is not known whether the carboplatin effect on olaparib clearance dissipates towards the end of each cycle. Elucidation of the mechanism is ongoing.
Acknowledgements
The authors would like to thank the patients who participated in this trial, all clinical support staff and AstraZeneca for providing the study drug in this trial.
Financial support This project has been funded in whole or in part with federal funds from the National Cancer Institute (National Institutes of Health). This work was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Conflict of interest All authors declared no conflicts of interest.
Disclaimer The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Tutt A, Ashworth A (2002) The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med 8:571–576 [DOI] [PubMed] [Google Scholar]
- 2.Bao Z, Cao C, Geng X, Tian B, Wu Y, Zhang C, Chen Z, Li W, Shen H, Ying S (2015) Effectiveness and safety of poly (ADP-ribose) polymerase inhibitors in cancer therapy: a systematic review and meta-analysis. Oncotarget 7:7629–7639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott CL, Swisher EM, Kaufmann SH (2015) Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol 33:1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration (2014) LYNPARZA prescribing information
- 5.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CF, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U (2014) Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15:852–861 [DOI] [PubMed] [Google Scholar]
- 6.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382–1392 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto N, Nokihara H, Yamada Y, Goto Y, Tanioka M, Shibata T, Yamada K, Asahina H, Kawata T, Shi X, Tamura T (2012) A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci 103:504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Fau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134 [DOI] [PubMed] [Google Scholar]
- 9.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Fubinski J, Shanley S, Messiou C, A’Hem R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB (2010) Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28:2512–2519 [DOI] [PubMed] [Google Scholar]
- 10.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, Carmichael J, Oza A (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12:852–861 [DOI] [PubMed] [Google Scholar]
- 11.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Foman N, Fu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251 [DOI] [PubMed] [Google Scholar]
- 12.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Foman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244 [DOI] [PubMed] [Google Scholar]
- 13.Choy E, Butrynski JE, Harmon DC, Morgan JA, George S, Wagner AJ, D’Adamo D, Cote GM, Flamand Y, Benes CH, Haber DA, Baselga JM, Demetri GD (2014) Phase 11 study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer 14:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiu JF, Tolaney SM, Birrer M, Fleming GF, Buss MK, Dahlberg SE, Fee H, Whalen C, Tyburski K, Winer E, Ivy P, Matulonis UA (2013) A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer 49:2972–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiu JF, Barry WT, Birrer M, Fee JM, Buckanovich RJ, Fleming GF, Rimel B, Buss MK, Nattam S, Hurteau J, Luo W, Quy P, Whalen C, Obermayer L, Lee H, Winer EP, Kohn EC, Ivy SP, Matulonis UA (2014) Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 15:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fee JM, Trepel JB, Choyke P, Cao F, Sissung T, Houston N, Yu M, Figg WD, Turkbey IB, Steinberg SM, Fee MJ, Ivy SP, Fiu JF, Matulonis UA, Kohn EC (2015) CECs and IF-8 Have prognostic and predictive utility in patients with recurrent platinum-sensitive ovarian cancer: biomarker correlates from the randomized phase-2 trial of olaparib and cediranib compared with olaparib in recurrent platinum-sensitive ovarian cancer. Front Oncol 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, Ranson M (2012) Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer 106:468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye SB, Fubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, Amnon A, Bell-McGuinn KM, Chen LM, Friedlander M, Safra T, Vergote I, Wickens M, Fowe ES, Carmichael J, Kaufman B (2012) Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 30:372–379 [DOI] [PubMed] [Google Scholar]
- 19.Samol J, Ranson M, Scott E, Macpherson E, Carmichael J, Thomas A, Cassidy J (2012) Safety and tolerability of the poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Investig New Drugs 30:1493–1500 [DOI] [PubMed] [Google Scholar]
- 20.Dent RA, Findeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, Singer CF, Fowe ES, Watkins CF, Carmichael J (2013) Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res 15:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajan A, Carter CA, Kelly RJ, Gutierrez M, Kummar S, Szabo E, Yancey MA, Ji J, Mannargudi B, Woo S, Spencer S, Figg WD, Giaccone G (2012) A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res 18:2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami D, Takigawa N, Takeda H, Takata M, Ochi N, Ichihara E, Hisamoto A, Hotta K, Tanimoto M, Kiura K (2013) Synergistic effect of olaparib with combination of cisplatin on PTEN-deficient lung cancer cells. Mol Cancer Res 11:140–148 [DOI] [PubMed] [Google Scholar]
- 23.Balmana J, Tung NM, Isakoff SJ, Grana B, Ryan PD, Saura C, Fowe ES, Frewer P, Winer E, Baselga J, Garber JE (2014) Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol 25:1656–1663 [DOI] [PubMed] [Google Scholar]
- 24.Fee JM, Hays JF, Annunziata CM, Noonan AM, Minasian F, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM, Figg WD, Azad N, Wood BJ, Doroshow J, Kohn EC (2014) Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst 106:089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Noll R, Marchetti S, Steeghs N, Beijnen JH, Mergui-Roelvink MW, Harms E, Rehorst H, Sonke GS, Schellens JH (2015) Fong-term safety and anti-tumour activity of olaparib monotherapy after combination with carboplatin and paclitaxel in patients with advanced breast, ovarian or fallopian tube cancer. Br J Cancer 113:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fee JM, Peer CJ, Yu M, Amable F, Gordon N, Annunziata CM, Houston N, Goey AK, Sissung TM, Parker B, Minasian F, Chiou V, Murphy RF, Widemann BC, Figg WD, Kohn EC (2016) Sequence-specific pharmacokinetic and pharmacodynamic phase I/Ib study of olaparib tablets and carboplatin in women’s cancer. Clin Cancer Res 23:1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Conte G, Sessa C, von Moos R, Vigano L, Digena T, Locatelli A, Gallerani E, Fasolo A, Tessari A, Cathomas R, Gianni L (2014) Phase 1 study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer 111:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolfo C, Swaisland H, Leunen K, Rutten A, Soetekouw P, Slater S, Verheul HM, Fielding A, So K, Bannister W, Dean E (2015) Effect of food on the pharmacokinetics of olaparib after oral dosing of the capsule formulation in patients with advanced solid tumors. Adv Ther 32:510–522 [DOI] [PubMed] [Google Scholar]
- 29.Plummer R, Swaisland H, Leunen K, van Herpen CM, Jerusalem G, De Greve J, Lolkema MP, Soetekouw P, Mau-Sorensen M, Nielsen D, Spicer J, Fielding A, So K, Bannister W, Molife LR (2015) Olaparib tablet formulation: effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother Pharmacol 76:723–729 [DOI] [PubMed] [Google Scholar]
- 30.Roth JEP, Peer CJ, Mannargudi B, Swaisland H, Lee JM, Khon EC, Figg WD (2014) A sensitive and robust ultra HPLC assay with tandem mass spectrometric detection for the quantitation of the PARP inhibitor olaparib (AZD2281) in human plasma for pharmacokinetic application. Chromatography 1:82–95 [Google Scholar]
- 31.Hah SS, Stivers KM, de Vere White RW, Henderson PT (2006) Kinetics of carboplatin-DNA binding in genomic DNA and bladder cancer cells as determined by accelerator mass spectrometry. Chem Res Toxicol 19:622–626 [DOI] [PubMed] [Google Scholar]
- 32.Gaver RC, Colombo N, Green MD, George AM, Deeb G, Morris AD, Canetta RM, Speyer JL, Farmen RH, Muggia FM (1988) The disposition of carboplatin in ovarian cancer patients. Cancer Chemother Pharmacol 22:263–270 [DOI] [PubMed] [Google Scholar]
- 33.Oguri S, Sakakibara T, Mase H, Shimizu T, Ishikawa K, Kimura K, Smyth RD (1988) Clinical pharmacokinetics of carboplatin. J Clin Pharmacol 28:208–215 [DOI] [PubMed] [Google Scholar]
- 34.Molife LR, Forster MD, Krebs M, Pwint T, Middleton MR, Kaye SB, McCormack P, Swaisland H, Carmichael J, Ranson M (2010) A phase I study to determine the comparative bioavailability of two different oral formulations of the PARP inhibitor, olaparib (AZD2281), in patients with advanced solid tumors. J Clin Oncol 28:2599–2600 [Google Scholar]
- 35.Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, McCormack P, Swaisland H, Cavallin M, Parry T, Carmichael J, Dixon JM (2013) Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Investig New Drugs 31:949–958 [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration PARAPLATIN prescribing information