Abstract
Introduction:
Overexpression of antiapoptotic B-cell lymphoma (Bcl-2) proteins confers the dysregulation of apoptosis and results in drug resistance in a variety of cancers, including those of the genitourinary tract. Inhibitors that target prosurvival Bcl-2 proteins are in preclinical and clinical development. The objective of this review is to assess the involvement of Bcl-2 proteins as well as the preclinical and clinical activity of Bcl-2 inhibitors under evaluation for genitourinary neoplasms.
Materials and Methods:
PubMed was used with both medical subject heading terms and free search to identify the relevant literature. Information on clinical trials was obtained using http://Clincaltrials.gov, EU Clinical Trials Register, and meeting abstracts of the American Society of Clinical Oncology.
Results:
To date, 2 Bcl-2 inhibitors have been evaluated in clinical trials for genitourinary tumors (oblimersen and AT-101 (R-(–)-gossypol)). Both agents demonstrated some success in early stages of development, but their clinical activity did not meet expectations. Preclinical studies are under way for other Bcl-2 inhibitors including ABT-737, HA14–1, and Bcl-2 homology 3 inhibitors.
Conclusion:
Antiapoptotic Bcl-2 proteins are potential molecular targets in genitourinary cancers. Bcl-2 inhibitors might be effective as single agents or in combination with conventional therapies. However, the biology of the Bcl-2 family in genitourinary cancers remains poorly understood and robust preclinical studies are needed to inform clinical development. Such studies should aim to identify: (1) pharmacodynamic markers that could help guide patient selection for treatment with Bcl-2 inhibitors, and (2) optimal combinations of Bcl-2 inhibitors with other anticancer agents for future clinical investigation.
Introduction
The cancerous phenotype is characterized by mutations in numerous cellular processes, including those that lead to apoptosis. Apoptosis is dysregulated in numerous malignancies including those of the genitourinary tract.1 Because most chemotherapies and radiation treatments produce their effects by activating various apoptotic pathways, disruption of those pathways can result in profound consequences, including the development of aggressive, drug-resistant tumors.2 With the emergence of drug resistance in genitourinary cancers, apoptosis has become a prime therapeutic target because inhibition of this process might enhance response to standard therapies.
The initiation of apoptosis is mediated through initiator caspases (caspase-8, caspase-9) and effector caspases (caspase-3, caspase-6, and caspase-7). These caspases are activated by cleavage early on in apoptosis. Signals that induce the ‘caspase cascade’ and initiate apoptosis can work through either intrinsic or extrinsic pathways. The extrinsic pathway is mediated independently of the mitochondria and involves activation of death receptors, such as Fas and tumornecrosis factor-related apoptosis-inducing ligand (TRAIL), which activate initiator caspase-8 within a death-inducing signaling complex. The intrinsic pathway is mitochondrial-dependent and is initiated by input from a wide range of signals including radiation, cytotoxic drugs, cellular stress, and growth factor withdrawal. These signals trigger the release of cytochrome c from the mitochondria, which initiates the formation of the apoptosome complex, composed of cytochrome c, apoptosis protease-activating factor-1, and inactive in caspase-9. Caspase-9 is then cleaved to its active form which subsequently initiates the effector caspase cascade.3 The convergence of multiple types of intracellular stimuli to induce cytochrome c release from the mitochondria is mediated by a group of proteins, known as the B-cell lymphoma-2 (Bcl-2) family. Alteration of these proteins is implicated in the tumorigenesis and drug resistance in many cancers.
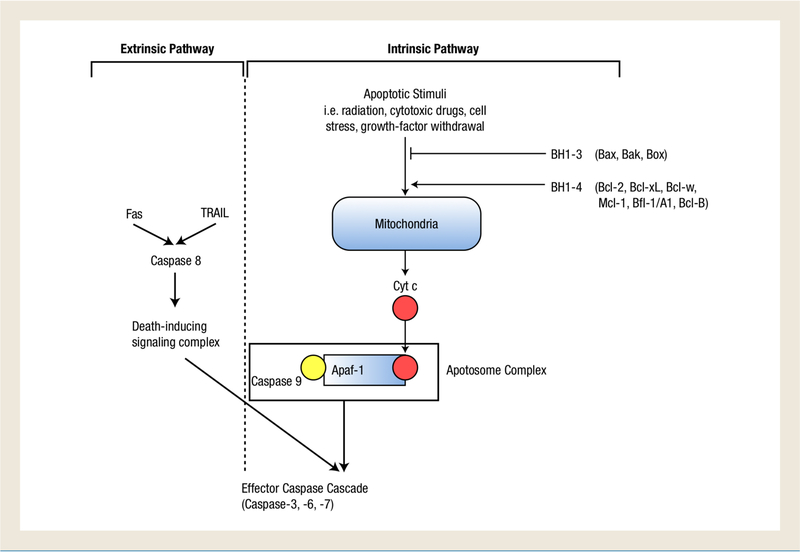
Since the discovery in 1985 of the first Bcl-2 protein, which is associated with the translocation t(l4;18) characteristic of follicular lymphoma,4 more than 25 pro- and antiapoptotic Bcl-2 proteins have been identified and have demonstrated some clinical relevance in a variety of cancers. The defining characteristic of this group of proteins is the presence of up to 4 relatively short sequence specific motifs termed the Bcl-2 homology domains. Bcl-2 proteins are separated into 3 subfamilies based on their structure and function. The antiapoptotic subfamily includes Bcl-2, Bcl-xL, Bcl-w, Mcl-1, Bfl-1/A1, and Bcl-B. They contain all 4 BcL-2 homology domains, and are therefore designated Bcl-2 homology 1–4.3 Another subfamily consisting of the proapoptotic proteins Bax, Bak, and Bok, contains the first 3 homology domains, Bcl-2 homology 1–3 (BH 1–3), and is thus termed proapoptotic multidomain Bcl-2 proteins.3 The other proapoptotic subfamily, named ‘BH3-only proteins,’ consists of proteins that contain just the Bcl-2 homology 3 domain. BH3-only proteins include noxa, Puma, Bad, Bim, Bid, Bik, Bmf, and Hrk. Through an unknown mechanism, BH3-only proteins integrate signals from other parts of the cell with the intrinsic pathway to regulate apoptosis.3,5 Figure 1 illustrates the involvement of Bcl-2 proteins in apoptosis.
Figure 1.
Induction of the Caspase Cascade and Initiation of Apoptosis via the Extrinsic and Intrinsic Apoptotic Pathways. The Extrinsic Pathway of the Caspase Cascade is Mediated Independently of the Mitochondria. It is Activated by Death Receptors, Fas and Tumor-Necrosis Factor-Related Ligand (TRAIL), Which Initiate Caspase-8 in a Death-Signaling Complex. This Complex Subsequently Activates the Effector Caspase Cascade. In the Intrinsic Pathway, Apoptotic Stimuli Trigger the Release of Cytochrome c (cyt c), which Forms a Complex with Caspase 9 and Apoptosis Protease-Activating Factor (Apaf-1). This Apoptotic Complex goes on to Activate the Effector Caspase Cascade. The Signals to Release cyt c from the Mitochondria is Suppressed by the Anti-Apoptotic Bcl-2 Homology 1 to 4 (BH1 −4) Proteins and Incited by the Proapoptotic BH1 −3 Proteins
An imbalance of pro- and antiapoptotic protein, rather than over-expression of antiapoptotic proteins, is believed to be the cause of tumorigenesis. Bcl-2 and related proteins have been implicated in both disease progression and development of treatment resistance in numerous advanced genitourinary cancers. Bcl-2 is overexpressed in 30%–60% of prostate cancers at diagnosis, 100% of castration resistance prostate cancer (CRPC),6,7 50%–70% of renal cell carcinomas (RCC),8,9 and 28%–38% of bladder neoplasms.10,11 Agents targeting antiapoptotic Bcl-2 proteins have demonstrated preclinical activity in restoring sensitivity to chemo- and radioresistant tumors; however, an improved understanding of the biology of Bcl-2 proteins and related pathways is needed to maximize the benefit of these drugs in patients. The objectives of the current review are to evaluate the significance of Bcl-2 proteins in genitourinary malignancies and to analyze the preclinical and clinical activity of Bcl-2 inhibitors in this subset of cancers.
Materials and Methods
Four database resources were searched in March 2012 to acquire evidence: PubMed, http://ClinicalTrials.gov, EU Clinical Trials Register (https://www.clinicaltrialsregister.eu), and American Society of Clinical Oncology (ASCO) abstracts. The terms used for medical subject heading search include genitourinary neoplasms, Bcl-2, Bcl-2 inhibitors, ABT-737, prostate cancer, renal cell carcinoma, and bladder cancer. Only articles in English that published data on humans or human models were considered. Subsequent references were identified from reference lists of retrieved articles. Both http://ClinicalTrials.gov and the EU Clinical Trials Register were surveyed for trials of Bcl-2 inhibitors in genitourinary cancers. ASCO annual meeting abstracts (through 2011) were reviewed to provide the most recent publically available information regarding these trials.
Results
Implications of the Bcl-2 Family
Prostate Cancer.
Prostate cancer is the most common noncutaneous cancer among men in the United States.12 De novo and recurrent metastatic prostate cancer are commonly treated with androgen deprivation therapy (ADT), but in nearly all patients, the disease becomes refractory to androgen ablation.13 Treatment of CRPC has been sought with minimal success. In 2004, docetaxel plus prednisone emerged as the standard treatment for CRPC, but only 50% of men respond to this regimen, and it often causes intolerable toxicides.14 In the past few years, the FDA approved several other agents in the treatment of CRPC including sipuleucel-T, cabazitaxel, and abiraterone, but like docetaxel, they are only effective in a subset of patients.15,16 Improved understanding of the involvement of genes such as Bcl-2 in CRPC is essential for identifying patients most likely to benefit from current therapies and for developing new, more effective and better-tolerated treatment options.
The finding that Bcl-2 expression was observed in prostate intraepithelial neoplasia17 but not in benign prostatic hyperplasia18 led to an interest in apoptosis and Bcl-2 as potential targets in prostate cancer. The progression of CRPC has been correlated with the over-expression of Bcl-2, Bcl-xL, and Mcl-1.19,20 Bcl-2 is overexpressed in 30%–60% of prostate cancers at diagnosis and in nearly 100% of CRPCs.6,7 In addition, Bcl-2 expression has been associated with recurrent localized prostate cancer after treatment with radiation therapy21 and increased levels of Bcl-2 with low Bax levels correlates with high resistance to apoptosis in metastatic prostate carcinoma.22 However, the finding that some bone metastasis in hormonally treated and untreated patients were negative for Bcl-223 suggests that upregulation of Bcl-2 is not an exclusive mechanism for progression. Bcl-xL is expressed in 80%–100% of CRPC and is associated with advanced disease, poor prognosis, decreased survival, recurrence, and metastasis.6 Likewise, Mcl-1 is associated with higher grade and metastatic phenotypes.19 Deciphering the value of each protein with regard to prognosis and therapeutic resistance is still under investigation, but targeting Bcl-2 might constitute a rational treatment approach to CRPC based on the following: (1) the PI3K/NF-κB/Bcl-2 axis seems to be a dominant survival pathway at certain points in the progression of prostate cancer;24–26 (2) expression of Bcl-2 appears to be associated with the development of CRPC;20,27 (3) Bcl-2 has a protective effect against apoptotic stimuli in prostate cancer cells.28,29 This evidence suggests that overexpression of anti-apoptotic Bcl-2 proteins is likely a driver of prostate cancer progression and drug resistance.
Renal Cell Carcinoma.
Though treatment of localized RCC is generally effective, metastatic RCC is unresponsive to existing therapies. The relative rate of 5-year survival after diagnosis in localized, regional, and distant disease is 90.8%, 63.1%, and 11%, respectively.30 Because of a lack of symptoms in early stages, many cases of RCC are already metastatic at the time of diagnosis. When the disease metastasizes, chemotherapies and hormonal agents are minimally effective with no reported response rate more than 15%.31 Targeted therapies including vascular endothelial growth factor and mammalian target of rapamycin inhibitors have demonstrated improved progression free survival (PFS) in metastatic RCC but are not curative and do not improve quality of life.32 Consequently, there is a considerable need for improved therapeutic options for patients with RCC.
The prognostic significance of Bcl-2 expression in advanced renal cancer remains controversial. One study found Bcl-2 overexpression in 70% of RCC compared with infrequent detection in normal renal tissue9 and another showed Bcl-2 and Bcl-xL overexpression in approximately 50% of RCC.8 Cancers overexpressing Bcl-2 and Bcl-xL tended to have low or no apoptotic activity whereas carcinomas with low expression of these proteins had high levels of apoptosis.8 In addition, Bcl-2 was more frequently expressed in metastatic relative to primary tumors.33 Bcl-2 expression might also predict prognosis in RCC.34,35 Similarly, defects in proapoptotic BH3-only proteins, such as Bim, might suppress tumor progression.36,37 Overexpression of the Mcl-1 splicing variant, Mcl-1L, was observed in nearly 60% of paired samples of clear cell renal cell carcinoma, whereas decreased expression of the variant was associated with more aggressive phenotypes.38 Conversely, some studies found no association between Bcl-2 expression and proliferation or apoptosis in RCC.39
Despite these conflicting results on the role of Bcl-2 proteins in tumorigenesis and prognosis of RCC, the data consistently show that modulation of Bcl-2 can alter chemosensitivity. Inhibition of Bcl-2 with a pharmacologic modulator40 or antisense oligonucleotide (ASO)41–45 resulted in increased sensitivity of renal cell carcinoma to apoptotic stimuli in cell lines. Although it remains unclear whether Bcl-2 overexpression is a driver or passenger alteration of RCC, results provide encouraging evidence that modulating anti-apoptotic Bcl-2 proteins might provide a therapeutic benefit in renal cell cancer by improving the efficacy of standard therapies.
Bladder Cancer.
Cancer of the bladder is highly aggressive and treatment of metastatic disease has shown little or no efficacy. Even though 70%–80% of newly diagnosed cases are noninvasive,46 nearly 70% of those cancers will recur despite initial treatment with cystectomy with adjuvant chemotherapy or radiation, and 25% of recurrent bladder cancers will ultimately progress to invasive disease.47 When the disease becomes metastatic, the median survival is 14 months, even with standard chemotherapy 48 Some studies have assessed the efficacy of various combinations of chemotherapies and increased doses, but no significant benefits have been observed.49–51 It is essential to improve the understanding of the disease in order to identify new targets and develop more effective therapies for both early-stage and metastatic bladder cancer.
Bel-2 and its homologs have been implicated in the progression of bladder neoplasms, especially transitional cell carcinoma.52 The results regarding the prognostic value of the Bcl-2 proteins in bladder cancer have been variable,10,11,53 though this might be because of an inadequate number of participants. In 1 study, immunostaining revealed Bcl-2 positivity in 26% of muscle-invasive transitional cell carcinoma of the bladder though it was not correlated with prognosis.10 Another found altered Bcl-2 expression in 32% of bladder cancer recurrent after radical cystectomy and that this altered expression was significantly associated with high probability of disease recurrence and disease-specific mortality.11 They also found that Bcl-2 positivity was associated with poorer outcome in patients treated with radiotherapy for transition cell carcinoma of the bladder. It has also been demonstrated that overexpression of Bcl-2 is associated with resistance to both chemotherapy and radiation treatment in advanced bladder cancer.54,55 The current standard of care for metastatic bladder cancer is a cisplatin-based combination chemotherapy.56 Based on preclinical data suggesting that high levels of Bcl-2 expression might confer resistance to cisplatin,57,58 Bcl-2 inhibitors might be relevant in treating bladder cancer. It remains unclear at this time whether Bcl-2 alterations are drivers of bladder carcinoma, but evidence suggests that it does contribute to drug resistance.
Advances In Targeting the Bcl-2 Family In Genitourinary Malignancies
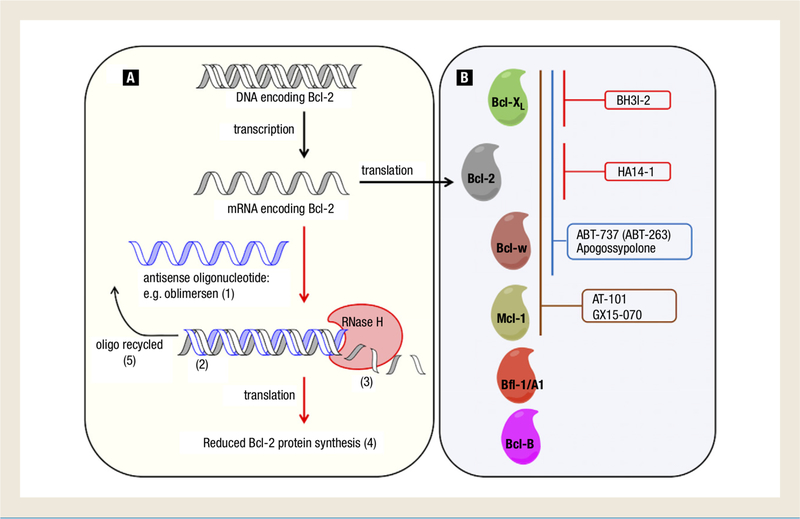
The development of Bcl-2 inhibitors has evolved over the past 2 decades since the antiapoptotic properties of Bcl-2 were first discovered. During that time, it was shown that the dysregulation of Bcl-2 confers chemo- and radio resistance and the 3-dimensional structure of Bcl-xL was discovered.3 The first Bcl-2 inhibitors were the ASOs,59 1 of which, oblimersen sodium, has been used in clinical trials for more than a decade. More recent advances have led to the development of a new class of agents known as Bcl-2 homology 3 (BH3) mimetics. These agents bind to the hydrophobic BH3 binding domain on antiapoptotic proteins to block their interaction with proapoptotic proteins. This allows proapoptotic proteins to dimerize and depolarize the outer mitochondrial membrane, releasing cytochrome c and inducing the caspase cascade. Several agents targeting the antiapoptotic Bcl-2 family have been developed, and some are undergoing preclinical and clinical testing as novel treatments for advanced genitourinary cancer. Figure 2 illustrates the mechanisms of action of Bcl-2 inhibitors in various stages of development for the treatment of genitourinary malignancies.
Figure 2.
B-Cell Lymphoma (Bcl-2) Inhibitors in Clinical and Preclinical Development for Genitourinary Cancers. There are Currently 2 Classes of Bcl-2 Inhibitors in Clinical and Preclinical Development for Genitourinary Cancers: (A) Antisense Oligonucleotides (ASO) and (B) Small Molecule BH3 Mimetics. The ASO, Oblimersen Sodium, (1) is in Phase ll/Phase III Clinical Trials in Prostate Cancer and Renal Cell Carcinoma and Specifically Hybridizes to the First 6 Codons of the Open Reading Frame of bcl-2 mRNA. (2) Oblimersen Promotes the Recruitment of Endogenous RNase H to Mediate bcl-2 mRNA Degradation, (3) Thereby Reducing Bcl-2 Protein Synthesis and Expression (4) and is Able to Recycle and Repeat the Process. (5) BH3 Mimetics Bind to the Hydrophobic BH3 Binding Domain of Specific Anti-Apoptotic Bcl-2 Family Members and Disrupt Their Ability to Oppose Apoptosis. BH3I-2 (Preclinical; Prostate Cancer) Targets Bcl-2. HA14–1 (Preclinical; Prostate Cancer) Targets Bcl-2. ABT-737 (Preclinical; Prostate Cancer, Renal Cell Carcinoma, Transitional Cell Carcinoma of Bladder) and Apogossypolone (Preclinical; Prostate Cancer) Target Bcl-xL, Bcl-2, and Bcl-w. AT-101 (Phase II; Prostate Cancer, Renal Cell Carcinoma, Transitional Cell Carcinoma of Bladder) and GX15–070 (not in Development in Genitourinary Cancers) Target Bcl-xL, Bcl-2, Bcl-w, and Mcl-1. Parenthesis Denotes Furthest Stage of Development for Genitourinary Cancers
Oblimersen Sodium.
Since the conceptualization and development of ASOs in the 1960s and 1970s, preclinical and clinical research has examined their use particularly in human malignancies. Oblimersen (Genasense, G3139) is an 18-base ASO complimentary to the first 6 codons of the open reading frame of human bcl-2 mRNA.60 It specifically hybridizes with targeted mRNA, promoting the recruitment of Bendogenous RNase H to mediate its scission and subsequently reducing Bcl-2 protein expression. It resists cleavage by substituting.sulfur for the nonbridging oxygen in the phosphate backbone, therefore evading intra- and extracellular nucleases and resulting in greater in vivo stability.60 The cell death mechanisms of Bcl-2 antisense were previously reviewed.3 Oblimersen has been tested for its efficacy in numerous preclinical and clinical studies for various malignancies. For example, a phase III clinical trial demonstrated that the addition of oblimersen to dacarbazine, the standard treatment for advanced melanoma, improved PFS and response rate but did not significantly improve overall survival (OS) and increased the risk of neutropenia and thrombocytopenia. In other clinical trials, addition of oblimersen to the standard chemotherapy failed to demonstrate improved 5-year survival in patients with chronic lymphocytic leukemia (CLL)61 and did not reduce time to progression in multiple myeloma.62,63 In both trials, frequent thrombocytopenia was noted. A new drug application seeking FDA registration of oblimersen based on a randomized phase III trial comparing fludarabine/cyclophosphamide (FC) with or without oblimersen in relapse or refractory CLL62 has gone through multiple appeal and amendment steps. Results of the trial indicated that 17% of patients receiving FC plus oblimersen had partial or complete responses compared with only 7% of those receiving FC alone, and the addition of oblimersen increased the duration of the response. However, there was no significant difference in OS or time to progression and toxicides increased with oblimersen. A recent decision by the FDA Center for Drug Evaluation and Research indicated that the available data were insufficient for the approval of the drug for treatment of padents with relapsed or refractory CLL. A confirmatory clinical trial was recommended by the agency.
Oblimersen has shown profound activity in combination with standard treatment for CRPC in cell lines and animal models.64 Prostate cancer cells positive for Bcl-2 expression were sensitized to the apoptotic effect of docetaxel by increasing the proportion of Bcl-2 existing in a phosphorylated, inactive state.65 A phase I study of oblimersen in combinations with mitoxantrone, the previous standard therapy for CRPC, found limited efficacy and low toxicity.66 Phase I and II clinical trials of docetaxel plus oblimersen were conducted in patients with CRPC. Preliminary evaluation of the phase I study suggested that Bcl-2 levels in diagnostic tumor specimens did not predict response to treatment,67 but results of the phase II study indicated a correlation between response to treatment and plasma concentrations of the drug and showed that oblimersen reduced Bcl-2 expression by a median of 49.9% in peripheral blood mononuclear cells.68 However, in an expanded multicenter phase II trial, docetaxel plus oblimersen failed to show significant benefit versus docetaxel alone using prostate specific antigen (PSA) as the primary end point and significantly increased risk of grade ≥ 3 fatigue, mucositis, and thrombocytopenia.69 Similarly, preclinical data of oblimersen in combination with alpha-interferon for advanced renal neoplasms showed promising results, but a combined phase I/II trial failed to replicate the benefits of the combination seen in preclinical studies.70 One study examining the effect of oblimersen in combination with mitomycin C for the treatment of transition cell carcinoma of the bladder found that oblimersen reduced Bcl-2 expression in 4 bladder cancer cell lines, but this downregulation only enhanced mitomycin C-induced apoptosis in 1 cell line, T24/83.71 Table I66–70,72–75 summarizes clinical trial results of oblimersen.
Table I.
Current and Completed Clinical Trials of Bcl-2 Inhibitors, Oblimersen, and AT-101, in Genitourinary Neoplasms
| Agent | Phase | Regimen | Trial Number | Cancer Type | n | Status |
|---|---|---|---|---|---|---|
| I66 | Genasense, mitoxantrone | NA | CRPC | 26 | Response was limited. Two of 26 patients had PSA decline ≥ 50%, Well tolerated in combination. | |
| I67 | Oblimersen, docetaxel | NCT00003103 | Solid tumors | 20 | Dose reduced from 7 to 5 mg/kg per day to reduce toxicity, PSA responses were observed in 7 of 12 taxane-naive patients, but no responses were observed in taxane-refractory patients. | |
| II68 | Oblimersen,Docetaxel | NCT00003103 | Solid tumors | 28 | PSA response observed in 14 of 27 patients (52%) in whom steady-state concentration was well correlated with drug efficacy, but was variable Median survival was 19.8 months. Regimen was well tolerated. | |
| Oblimersen (Genasense) | II69 | Docetaxel with or without oblimersen | NCT00085288 EORTC 30,021 | CRPC | 111 | Confirmed PSA response was observed in 46% in docetaxel-alone group and 37% in docetaxel with oblimersen group. Partial response using RECIST criteria was achieved in 18% and 24%, respectively. Oblimersen and docetaxel combination was associated with greater grade ≥ 3 toxicity with 40.7% patients vs. 20.8% patients given docetaxel alone. |
| II70 | Oblimersen | NCT00059813 | RC | 23 | One patient had partial response lasting 2.5 months. Grade 3/4 toxicities were fatigue, fever, myelosuppression. hepatic enzyme, and metabolic abnormalities. | |
| I/II72 | AT-101 | NCT00286793 | CRPC | 23 | Two patients had a confirmed ≥ 50% PSA decline High incidence of grade 3 intestinal obstruction led to dose reduction from 30 mg per day for 21 to 28 days to 20 mg per day for 21 to 28 days. | |
| I73 | AT-101, paclitaxel carboplatin | NCT00891072 | CRPC, other solid tumors | 24 | Four of 11 patients (36%) with CRPC (all previously treated with docetaxel) had PSA declines ≥ 50% and 4 patients had stable disease. Two patients with CRPC had PR. Dose limiting toxicities were grade 3 abdominal pain. | |
| Gossypol (AT-101) | I/II74 | AT-101, docetaxel, prednisone | NCT00571675 | Chemonaive CRPC | 220 | Docetaxel plus prednisone (placebo-DP arm) (n = 110) vs. AT-101 plus docetaxel and prednisone (ADP arm) (n = 110). No significant difference in OS (18.1 vs. 17.8 months), PSA reduction, measurable disease control rates, objective response rates, pain response rates. Median overall survival in high risk CRPC (n = 34) numerically favored ADP (19 vs. 14 months). Grade 3/4 AEs, discontinuation of therapy and AE-related dose reduction were more common in ADP arm than placebo with DP arm. |
| II85 | AT-101, ADT | NCT00666666 | Newly diagnosed androgen-dependent PC | 42 | Out of the 23 of 42 patients who completed the 7 months of treatment, 11 met primary end points (PSA ≤ 0.2 ng/mL) and 10 had PSA > 0.2 and ≤ 0.4 ng/mL. Grade 3 toxicities were sensory neuropathy (2 patients), GI obstruction (1 patient) and syncope (1 patient). |
Abbreviations: ADP = AT-101 + docetaxel+prednisone; AE = adverse event; Bcl-2 = B-cell lymphoma; CRPC = castration-resistant prostate cancer; DP = docetaxel + prednisone; GI = gastrointestinal; OS = overall survival; PC = prostate cancer; PR = partial response; PSA = prostate specific antigen; RC = renal cell cancer; RECIST = Response Evaluation Criteria In Solid Tumors.
The discrepancy between preclinical data and clinical results might be because of: (1) issues in the delivery of ASOs, (2) failure of the drug to inhibit other antiapoptotic Bcl-2 family members, leading to chemo- and radioresistance, and (3) the possibility that effects in preclinical models might be related to nonimmunostimulation by the oligonucleotide which does not translate to the clinic. To improve the delivery of AS Os, alternative mechanisms of delivery have been proposed including liposomal delivery systems and preradiation sensitizing of tumor cells to increase uptake of oblimersen.76 Even with the problem of delivery resolved, the specificity of oblimersen (only targets bcl-2) might limit its efficacy in tumors expressing numerous antiapoptotic Bcl-2 proteins, such as Bcl-xL and Mcl-1. Multispecific ASOs might alleviate this problem. Bispecific ASOs are currently being evaluated in preclinical models.77
Gossypol.
Gossypol (AT-101, (−)-gossypol) is a naturally occurring polyphenolic pigment derived from cotton seeds and cotton products (ie, cottonseed oil and cottonseed meal flour).78 Gossypol has been used in ancient Chinese medicine and studied for decades as a male contraceptive agent, but more recently has garnered interest for its antineoplastic effects.79,80 Initial studies found that natural (−)-gossypol promoted antiproliferative activities in a variety of cancers as a BH3 mimetic.81–83 Because it was discovered that (−)-gossypol was the more active enantiomer, recent investigations have focused on (−)-gossypol for the treatment of malignancies.79 Gossypol is known as a ‘pan–bcl-2 inhibitor,5 being unique in its lack of selectivity among antiapoptotic Bcl-2 members. It has moderate affinity (in μM range) for several antiapoptotic Bcl-2 family members, namely Bcl-2, Bcl-xL, Mcl-1, and Bcl-w. However, investigators have unveiled an assortment of other possible mechanisms and molecular targets of gossypol that contribute to its antineoplastic activity. In addition to blocking the association of antiapoptotic Bcl-2 proteins with their corresponding effectors and sensitizing the cells to apoptosis, gossypol also increases expression of proapoptotic proteins noxa and Puma, further enhancing the Bcl-2 pathway of intrinsic apoptosis.13 It is additionally believed to be antiangiogenic, have DNA damaging properties, promote G0/G1 cell cycle arrest, inhibit nuclear enzymes, inhibit protein kinase C, and be toxic to mitochondria.78,84 Furthermore, the ability of gossypol to induce complimentary levels of both intrinsic and extrinsic pathways of apoptosis and autophagy based on expression patterns of Bcl-2 demonstrates its lack of specificity.85 Though this low specificity might improve efficacy, it might also increase the risk of unexpected responses and systemic toxicities.
In both non-CRPC and CRPC in vitro and in vivo models, gossypol has been shown to induce apoptosis as a single agent and to interact synergistically with standard therapies, such as docetaxel, radiation, and ADT.13,84,86,87 In addition, studies indicate that gossypol activates p5388 and therefore p53 might serve as a predictive biomarker for gossypol efficacy. Bladder cancer cell lines that are resistant to chemotherapeutic agents for metastatic bladder cancer in vitro, including gemcitabine and carboplatin, demonstrated significantly increased apoptosis under cotreatment with gossypol.72 Currently, AT-101 is in multiple phase I and II trials in combination with other agents for CRPC. Although phase I/II clinical trials with AT-101 in combination with docetaxel have shown modest effect in CRPC (2 of 23 patients with declined PSA), the initial dose of 30 mg per day was reduced to 20 mg per day for all patients because of gastrointestinal toxicities. 5 Despite this finding, initial evidence from later phase I/II trials that are currently underway have shown gossypol to be well tolerated when combined with paclitaxel plus carboplatin, or ADT while still showing moderate efficacy.73,89 However, a more recent phase II randomized control trial comparing docetaxel plus prednisone treatment with or without AT −101 found that while the combination was well tolerated, AT-101 did not significantly improve overall survival, though a trend was noted in a subset of high-risk CRPC.74 Table I66–70,72–75 summarizes clinical trial data of AT-101.
A gossypol derivative, apogossypolone (ApoGII) was developed in an effort to reduce systemic toxicides from gossypol. ApoGII has better affinity for Bel-2, Bcl-xL, and Mcl-1 (binding affinity (Ki) = 35, 660, and 25 nmol/L, respectively). A preclinical study comparing gossypol with ApoGII suggests that both are equally efficacious in vivo and in vitro, but the toxicity profile for ApoGII is more favorable.90,91 Despite good preclinical activity, the increased number of cells undergoing autophagy poses another problem because autophagy might imply a survival mechanism. It has been suggested that using an agent to inhibit autophagy might enhance the activity of ApoGII.91 In addition, Dash et al92 developed and tested a series of apogossypol derivatives in in vitro and in vivo models of human prostate cancer. One of these derivatives, BI-97C1 (sabutoclax), which has affinity for Mcl-1, sensitized prostate cancer cells to apoptosis mediated by melanoma differentiation-associated gene-7/interleukin (IL)-24 (mda-7/IL-24), and IL-10 family cytokine.91,92
ABT-737.
ABT-737 is a potent (Ki ≤ 1 nmol/L) small molecular inhibitor of Bcl-2, Bcl-xL, and Bcl-w, but has poor affinity for Mcl-1, Bcl-Al, or Bcl-B (Ki = 0.46 ± 0.11 mmol/L, > 1 and > 1 mmol/L, respectively).93 Preclinical activity of ABT-737 has been demonstrated in urinary, renal, and prostate cancer cell lines as a single agent as well as in combination with other chemotherapeutic agents.44,91,94 However, because of the low affinity of ABT-737 for Mcl-1 and Bcl-Al, overexpression of these 2 proteins might confer drug resistance in various genitourinary cancers, especially prostate cancer in which Mcl-1 has been implicated in treatment resistance.92 Combining agents with adjuvant or other chemotherapeutic agents to decrease expression of Mcl-1 has been proposed as a means of overcoming resistance to ABT-737. In renal, bladder, and prostate cancer cell lines, gemcitabine promoted degradation of Mcl-1 by preventing USP9X, a ubiquitin-specific protease, from interacting with and deubiquinating Mcl-1.91 In renal cell carcinoma cell lines, the DNA damaging agent, etoposide, and the microtubule toxins, vinblastine and paclitaxel, demonstrate that traditional chemotherapeutic agents can induce, in part through noxa, the neutralization of Mcl-145
The clinical use of ABT-737 is limited by its lack of oral bioavailability, but an oral analogue, ABT-263 (navitoclax), has demonstrated 20%–50% oral bioavailability in animal models. 95 Preclinical trials have also shown apoptosis as early as within 2 hours of treatment and complete tumor regression in xenografts of acute lymphoblastic leukemia and small-cell lung cancer (SCLC). In a phase I study in patients with SCLC and other nonhematologic solid tumors, 225 mg ABT-263 per oral led to drug plasma concentrations within the therapeutic range and the maximum tolerated dose was 325 mg. Of the 38 patients enrolled in the trial, 1 experienced a partial response and 8 had stable disease. Dose-dependent transient thrombocytopenia was noted as the most concerning systemic toxic ity.96 These effects were attributed to the dependence of mature platelets on Bcl-xL.97,98 Otherwise, ABT-263 was well tolerated.96 It was also found that biomarkers of clinical benefit in SCLC of ABT-263 included baseline levels of cytokeratin 19 fragment antigen 21–1, neuron-specific enolase, progastrin-releasing peptide, and circulating tumor cell number.99 These might be relevant biomarkers in selecting patients for future studies of ABT-263 in genitourinary cancers.
Currently, the use of ABT-737/ABT-263 for the treatment of tumors of the genitourinary tract remains in preclinical development. The combination of docetaxel and ABT-737 reduced PC3 cell viability from 44.2% under docetaxel alone and 93.2% under ABT-737 alone to 19.7%.100 ABT-737 in combination with the Pim kinase inhibitor, SMI-4a, synergistically enhanced apoptosis in LNCaP and PC3 cell and xenografts.101 Similarly, though it failed to induce apoptosis as a single agent, ABT-373 led to considerable cell death when combined with 2-deoxyglucose (2DG), which partially blocks glycolysis.102 It is believed that 2DG disrupts the Bak-Mcl-1 association and thereby promotes Bak-induced apoptosis.102 Combination of ABT-737 with gemcitabine synergistically enhanced cytotoxicity in prostate (PC3), renal carcinoma (SW-13), and bladder carcinoma (5637 and SCABER) cell lines via disruption of the interaction between ubiquitin-specific peptidase 9, X-linked (USP9X) and Mcl-1.91 In PV-10 cells, a renal cell carcinoma cell line, senescence was found to be p5 3-dependent44 indicating that p53 might be an important predictive biomarker.
Other Bcl-2 Inhibitors in Development.
Other Bcl-2 inhibitors in preclinical development include HA 14–1 and BH3 inhibitor-1 and −2. HA14–1 is another BH3-mimetic that has undergone preclinical studies for a number of malignancies including leukemia, lymphoma, breast, colon, and prostate cancer. It was developed using computer-based structural screening of compounds that bind to the predicted BakBH3 peptide binding pocket located on the Bcl-2 protein.103 Similar to gossypol, the mechanism of action of HA14–1 goes beyond the binding properties of antiapoptotic Bcl-2 proteins as it is believed to induce apoptosis via changes in Ca2+ homeostasis, reactive oxygen species generation, Bax translocation, cytochrome c release, and caspase-9/−3 activation.104 HA14–1, in sequential combination with radiation or topotecan, synergistically produced apoptosis in prostate and renal cell carcinoma cell lines, respectively.104,105 When coadministered with TRAIL, HA14–1 was also able to reconstitute the mitochondrial pathway of apoptosis in renal cell carcinoma cell lines.106 However, the high concentration required to produce an effect (half maximal inhibitory concentration [IC50] approximately 9 μmol103) might limit the future development of HA14–1. In addition, because of its rapid degradation in solution, HA14–1 might require chemical modification.107
BH3 inhibitors (BH3I-1 and BH3I-2) are small peptides that disrupt the interactions between proapoptotic and antiapoptotic members of the Bcl-2 family, resulting in increased availability of Bax and/or Bak to induce mitochondrial membrane permeabilization.3,108 The combination of BH3I-2 with the TRAIL ligand synergistically enhanced apoptosis in prostate cancer cell lines.108 However, BH3Is also require high concentrations (approximately 20 mmol/L) which might limit their clinical utility.3 Another BH3 mimetic, GX15–070 (obatoclax), has demonstrated pan-Bcl-2 inhibitory effects and has affinity for Mcl-1, but minimal work has examined its effects in genitourinary neoplasms.109
Discussion
There is a need for new treatment strategies for patients with advanced genitourinary neoplasms. Inhibition of the Bcl-2 family of proteins might be a viable treatment option because this class of proteins is overexpressed in genitourinary neoplasms, especially in advanced stages of prostate and bladder cancer. An assortment of Bcl-2 inhibitors are in preclinical and clinical investigation for advanced genitourinary tumors but further preclinical and biological research is needed to optimize the use of this class of drugs. One limitation with most Bcl-2 inhibitors currently in development is their poor affinity for antiapoptotic Mcl-1. However, focusing on agents that target all the known antiapoptotic Bcl-2 family of proteins might not be the solution because those agents which inhibit Mcl-1 as well as other antiapoptotic Bcl-2 proteins do not appear to be associated with improved antitumor activity.
For future development of Bcl-2 family inhibitors in genitourinary cancers 2 strategies are suggested. First, studies must be conducted to improve the understanding of the target biology including identification of pharmacodynamic markers for predicting potential responders. Second, robust preclinical studies assessing combination therapy with Bcl-2 inhibitors are necessary to enhance the activity of conventional chemotherapeutic agents. Research into the biology of potential molecular targets in the context of specific cancers is essential for developing clinical trials as well as for interpreting data from both successful and failed therapeutic studies.
The second strategy, developing drug combinations in preclinical studies, has been the rationale for most of the current clinical trials of Bcl-2 inhibitors. However, there is a lack of preclinical models for genitourinary cancers in general, and especially of models known to be predictive of clinical activity for investigational drugs. As clinical testing of drug combinations in clinical trials can be costly, it is worthwhile to develop preclinical models to inform such clinical trials. Thus, research programs seeking to develop clinically relevant preclinical models for genitourinary cancers should be a high priority for cancer funding organizations.
Antiapoptotic Bcl-2 proteins have been identified as potential targets in some genitourinary cancers. Currently, clinical trials of Bcl-2 inhibitors in genitourinary neoplasms are being conducted with the ASO, oblimersen, and a small molecule Bcl-2 protein inhibitor, AT-101. For the development of this class of agents to be successful, a better understanding of the biology of the Bcl-2 family in genitourinary cancers is needed. Such insight will lead to the identification of pharmacodynamic markers of the single drug activity of these agents as well as guide the development of drug combinations. Because Bcl-2 inhibitors have been shown to enhance efficacy of conventional chemotherapy agents in some clinical studies, preclinical studies exploring drug combinations in robust preclinical models might inform future clinical development of this class of agents.
Footnotes
Disclosure
All authors have no conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281:1309–12. [DOI] [PubMed] [Google Scholar]
- 3.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 2009; 15:1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985; 228:1440–3. [DOI] [PubMed] [Google Scholar]
- 5.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 2011; 18:1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuya Y, Krajewski S, Epstein JI, et al. Expression of bcl-2 and the progression of human and rodent prostatic cancers. Clin Cancer Res 1996; 2:389–98. [PubMed] [Google Scholar]
- 7.Konopleva M, Zhao S, Hu W, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol 2002; 118:521–34. [DOI] [PubMed] [Google Scholar]
- 8.Pammer J, Exner M, Regele H, et al. Expression of bcl-2, bcl-x, bax and bak in renal parenchyma, oncocytomas and renal cell carcinomas. Pathol Res Pract 1998; 194:837–45. [DOI] [PubMed] [Google Scholar]
- 9.Huang A, Fone PD, Gandour-Edwards R, et al. Immunohistochemical analysis of BCL-2 protein expression in renal cell carcinoma. J Urol 1999; 162:610–3. [PubMed] [Google Scholar]
- 10.Glick SH, Howell LP, White RW. Relationship of p53 and bcl-2 to prognosis in muscle-invasive transitional cell carcinoma of the bladder. J Urol 1996; 155: 1754–7. [PubMed] [Google Scholar]
- 11.Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol 2007; 8:128–36. [DOI] [PubMed] [Google Scholar]
- 12.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin 2012; 2012:10–29. [DOI] [PubMed] [Google Scholar]
- 13.Meng Y, Tang W, Dai Y, et al. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of puma and noxa. Mol Cancer Ther 2008; 7:2192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. [DOI] [PubMed] [Google Scholar]
- 15.Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol 2011; 29:3686–94. [DOI] [PubMed] [Google Scholar]
- 16.Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol 2011; 29:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol 1993; 143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Hockenbery DM, Zutter M, Hickey W, et al. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 1991; 88:6961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and Mcl-1 expression in prostate cancers. Am J Pathol 1996; 148:1567–76. [PMC free article] [PubMed] [Google Scholar]
- 20.McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992; 52:6940–4. [PubMed] [Google Scholar]
- 21.Rosser CJ, Reyes AO, Vakar-Lopez F, et al. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol BiolPhys 2003; 56:1–6. [DOI] [PubMed] [Google Scholar]
- 22.McConkey DJ, Greene G, Pettaway CA. Apoptosis resistance increases with metastatic potential in cells of the human LNCaP prostate carcinoma line. Cancer Res 1996; 56:5594–9. [PubMed] [Google Scholar]
- 23.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996; 28:251–65. [DOI] [PubMed] [Google Scholar]
- 24.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 2001; 20:7342–51. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Adam RM, Santiestevan E, et al. The phosphatidylinositol 3’-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res 1999; 59:2891–7. [PubMed] [Google Scholar]
- 26.Murillo H, Huang H, Schmidt LJ, et al. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 2001; 142:4795–805. [DOI] [PubMed] [Google Scholar]
- 27.Raffo AJ, Perlman H, Chen MW, et al. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res 1995; 55:4438–45– [PubMed] [Google Scholar]
- 28.Huang A, Gandour-Edwards R, Rosenthal SA, et al. p53 and bcl-2 immunohistochemical alterations in prostate cancer treated with radiation therapy. Urology 1998; 51:346–51. [DOI] [PubMed] [Google Scholar]
- 29.Mackey TJ, Borkowski A, Amin P, et al. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 1998; 52:1085–90. [DOI] [PubMed] [Google Scholar]
- 30.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2008 Bethesda, MD: National Cancer Institute, SEER Program; Available at: http://seer.cancer.gov/csr/1975_2008/. Accessed: May 25, 2011. [Google Scholar]
- 31.Yagoda A, Abi-Rached B, Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol 1995; 22:42–60. [PubMed] [Google Scholar]
- 32.Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011; 108:1556–63. [DOI] [PubMed] [Google Scholar]
- 33.Lee CT, Genega EM, Hutchinson B, et al. Conventional (clear cell) renal carcinoma metastases have greater bcl-2 expression than high-risk primary tumors. Urol Oncol 2003; 21:179–84. [DOI] [PubMed] [Google Scholar]
- 34.Itoi T, Yamana K, Bilim V, et al. Impact of frequent Bcl-2 expression on better prognosis in renal cell carcinoma patients. Br J Cancer 2004; 90:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phuoc NB, Ehara H, Gotoh T, et al. Immunohistochemical analysis with multiple antibodies in search of prognostic markers for clear cell renal cell carcinoma. Urology 2007; 69:843–8. [DOI] [PubMed] [Google Scholar]
- 36.Sturm I, Stephan C, Gillissen B, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ 2006; 13:619–27. [DOI] [PubMed] [Google Scholar]
- 37.Zantl N, Weirich G, Zall H, et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene 2007; 26:7038–48. [DOI] [PubMed] [Google Scholar]
- 38.Kempkensteffen C, Hinz S, Johannsen M, et al. Expression of Mcl-1 splicing variants in clear-cell renal cancer and their correlation with histopathological parameters and prognosis. Tumour Biol 2009; 30:73–9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Takenaka I. Cell proliferation and apoptosis with BCL-2 expression in renal cell carcinoma. Urology 2000; 56:510–5. [DOI] [PubMed] [Google Scholar]
- 40.Ahn KS, Bae E, Jeon SS, et al. Microenvironment effects on promoting upregulation of matrix metalloproteinases in Bcl-2-overexpressing renal cell carcinoma as a response to doxorubicin treatment inducing the production of metastasis. Tumour Biol 2007; 28:181–8. [DOI] [PubMed] [Google Scholar]
- 41.Bilim V, Yuuki K, Itoi T, et al. Double inhibition of XIAP and Bcl-2 axis is beneficial for retrieving sensitivity of renal cell cancer to apoptosis. Br J Cancer 2008; 98:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kausch I, Jiang H, Thode B, et al. Inhibition of bcl-2 enhances the efficacy of chemotherapy in renal cell carcinoma. Eur Urol 2005; 47:703–9. [DOI] [PubMed] [Google Scholar]
- 43.Kelly JD, Dai J, Eschwege P, et al. Downregulation of Bcl-2 sensitises interferon-resistant renal cancer cells to Fas. Br J Cancer 2004; 91:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song JH, Kandasamy K, Zemskova M, et al. The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res 2011; 71:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zall H, Weber A, Besch R, et al. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the noxa-dependent inactivation of Mcl-1 or Al. Mol Cancer 2010; 9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005; 66:4–34. [DOI] [PubMed] [Google Scholar]
- 47.Soloway MS, Sofer M, Vaidya A. Contemporary management of stage Tl transitional cell carcinoma of the bladder. J Urol 2002; 167:1573–83. [PubMed] [Google Scholar]
- 48.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23:4602–8. [DOI] [PubMed] [Google Scholar]
- 49.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol 2012; 30:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loehrer PJ, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study, J Clin Oncol 1992; 10:1066–73. [DOI] [PubMed] [Google Scholar]
- 51.Logothetis CJ, Finn LD, Smith T, et al. Escalated MVAC with or without recombinant human granulocyte-macrophage colony-stimulating factor for the initial treatment of advanced malignant urothelial tumors: results of a randomized trial. J Clin Oncol 1995; 13:2272–7. [DOI] [PubMed] [Google Scholar]
- 52.King ED, Matteson J, Jacobs SC, et al. Incidence of apoptosis, cell proliferation and bcl-2 expression in transitional cell carcinoma of the bladder: association with tumor progression. J Urol 1996; 155:316–20. [PubMed] [Google Scholar]
- 53.Giannopoulou I, Nakopoulou L, Zervas A, et al. Immunohistochemical study of pro-apoptotic factors Bax, Fas and CPP32 in urinary bladder cancer: prognostic implications. Urol Res 2002; 30:342–5. [DOI] [PubMed] [Google Scholar]
- 54.Hussain SA, Ganesan R, Hiller L, et al. BCL2 expression predicts survival in patients receiving synchronous chemoradiotherapy in advanced transitional cell carcinoma of the bladder. Oncol Rep 2003; 10:571–6. [PubMed] [Google Scholar]
- 55.Ong F, Moonen LM, Gallee MP, et al. Prognostic factors in transitional cell cancer of the bladder: an emerging role for Bcl-2 and p53. Radiother Oncol 2001; 61: 169–75. [DOI] [PubMed] [Google Scholar]
- 56.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 2011; 59: 1009–18. [DOI] [PubMed] [Google Scholar]
- 57.Cho HJ, Kim JK, Kim KD, et al. Upregulation of Bcl-2 is associated with cisplatin-resistance via inhibition of Bax translocation in human bladder cancer cells. Cancer Lett 2006; 237:56–66. [DOI] [PubMed] [Google Scholar]
- 58.Miyake H, Hanada N, Nakamura H, et al. Overexpression of Bcl-2 in bladder cancer cells inhibits apoptosis induced by cisplatin and adenoviral-mediated p53 gene transfer. Oncogene 1998; 16:933–43. [DOI] [PubMed] [Google Scholar]
- 59.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets 2010; 11:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chi KN. Targeting Bcl-2 with oblimersen for patients with hormone refractory prostate cancer. World J Urol 2005; 23:33–7. [DOI] [PubMed] [Google Scholar]
- 61.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy (+/−) oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. J Clin Oncol 2007; 25:7012. [Google Scholar]
- 62.O’Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2007; 25:11 14–20. [DOI] [PubMed] [Google Scholar]
- 63.Chanan-Khan AA, Niesvizky R, Hohl RJ, et al. Phase III randomised study of dexamethasone with or without oblimersen sodium for patients with advanced multiple myeloma. Leuk Lymphoma 2009; 50:559–65. [DOI] [PubMed] [Google Scholar]
- 64.Gleave M, Tolcher A, Miyake H, et al. Progression to androgen independence is delayed by adjuvant treatment with antisense Bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate tumor model. Clin Cancer Res 1999; 5:2891–8. [PubMed] [Google Scholar]
- 65.Haidar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res 1996; 56:1253–5. [PubMed] [Google Scholar]
- 66.Chi KN, Gleave ME, Klasa R, et al. A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin Cancer Res 2001; 7:3920–7. [PubMed] [Google Scholar]
- 67.Tolcher AW, Kuhn J, Schwartz G, et al. A phase I pharmacokinetic and biological correlative study of oblimersen sodium (genasense, g3139), an antisense oligonucleotide to the bcl-2 mRNA, and of docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 2004; 10:5048–57. [DOI] [PubMed] [Google Scholar]
- 68.Tolcher AW, Chi K, Kuhn J, et al. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 2005; 11:3854–61. [DOI] [PubMed] [Google Scholar]
- 69.Sternberg CN, Dumez H, Van Poppel H, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol 2009; 20: 1264–9. [DOI] [PubMed] [Google Scholar]
- 70.Margolin K, Synold TW, Lara P, et al. Oblimersen and alpha-interferon in metastatic renal cancer: a phase II study of the California Cancer Consortium. J Cancer Res Clin Oncol 2007; 133:705–11. [DOI] [PubMed] [Google Scholar]
- 71.Duggan BJ, Maxwell P, Kelly JD, et al. The effect of antisense Bcl-2 oligonucleotides on Bcl-2 protein expression and apoptosis in human bladder transitional cell carcinoma. J Urol 2001; 166:1098–105. [PubMed] [Google Scholar]
- 72.Macoska JA, Adsule S, Tantivejkul K, et al. -(−)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol Res 2008; 58:323–31. [DOI] [PubMed] [Google Scholar]
- 73.Stein MN, Khan I, Hussain M, et al. Phase II study of AT-101 to abrogate Bcl-2-mediated resistance to androgen-deprivation therapy (ADT) in patients (pts) with newly diagnosed androgen-dependent metastatic prostate cancer (ADM PC). ASCO Meeting Abstracts 2011; 29:137. [Google Scholar]
- 74.Sonpavde G, Matveev V, Burke JM, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol 2012; 23:1803–8. [DOI] [PubMed] [Google Scholar]
- 75.Liu G, Kelly WK, Wilding G, et al. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res 2009; 15:3172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anai S, Brown BD, Nakamura K, et al. Irradiation of human prostate cancer cells increases uptake of antisense oligodeoxynucleotide. Int J Radiat Oncol Biol Phys 2007; 68:1161–8. [DOI] [PubMed] [Google Scholar]
- 77.Rubenstein M, Tsui P, Guinan P. Treatment of prostate and breast tumors employing mono- and bi-specific antisense oligonucleotides targeting apoptosis inhibitory proteins clusterin and bcl-2. Med Oncol 2010; 27:592–9. [DOI] [PubMed] [Google Scholar]
- 78.Huang YW, Wang LS, Chang HL, et al. Molecular mechanisms of (−)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res 2006; 26: 1925–33. [PubMed] [Google Scholar]
- 79.Liu S, Kulp SK, Sugimoto Y, et al. The (−)-enantiomer of gossypol possesses higher anticancer potency than racemic gossypol in human breast cancer. Anticancer Res 2002; 22:33–8. [PubMed] [Google Scholar]
- 80.Tuszynski GP, Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 1984; 44:768–71. [PubMed] [Google Scholar]
- 81.Jaroszewski JW, Kaplan O, Cohen JS. Action of gossypol and rhodamine 123 on wild type and multidrug-resistant MCF-7 human breast cancer cells: 31P nuclear magnetic resonance and toxicity studies. Cancer Res 1990; 50:6936–43. [PubMed] [Google Scholar]
- 82.Rao PN, Wang YC, Lotzova E, et al. Antitumor effects of gossypol on murine tumors. Cancer Chernother Pharmacol 1985; 15:20–5. [DOI] [PubMed] [Google Scholar]
- 83.Tso WW. Gossypol inhibits Ehrlich ascites tumor cell proliferation. Cancer Lett 1984; 24:257–61. [DOI] [PubMed] [Google Scholar]
- 84.Xu L, Yang D. Wang S, et al. (−)-gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther 2005; 4:197–205. [PubMed] [Google Scholar]
- 85.Lian J, Wu X, He F, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin 1 interaction at endoplasmic reticulum. Cell Death Differ 2011; 18:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cengiz E, Karaca B, Kucukzeybek Y, et al. Overcoming drug resistance in hormone- and drug-refractory prostate cancer cell line, PC-3 by docetaxel and gossypol combination. Mol Biol Rep 2010; 37:1269–77. [DOI] [PubMed] [Google Scholar]
- 87.McGregor N, Patel L, Craig M, et al. AT-101 (R-(−)-gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. J Cell Biochem 2010; 110:1187–94. [DOI] [PubMed] [Google Scholar]
- 88.Volate SR, Kawasaki BT, Hurt EM, et al. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol Cancer Ther 2010; 9:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y, Gounder M, Lin H, et al. Phase I study of at-101 (R-(−)-gossypol) in combination with paclitaxel (P) and carboplatin (C) in solid tumors including castrate-resistant prostate cancer (CRPC). ASCO Meeting Abstracts 2011; 29:169. [Google Scholar]
- 90.Zhan Y, Jia G, Wu D, et al. Design and synthesis of a gossypol derivative with improved antitumor activities. Arch Pharm (Weinheim) 2009; 342:223–9. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C, Cai TY, Zhu H, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther 2011; 10:1264–75. [DOI] [PubMed] [Google Scholar]
- 92.Dash R, Azab B, Quinn BA, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A 2011; 108:8785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435:677–81. [DOI] [PubMed] [Google Scholar]
- 94.Pandit B, Gartel AL. New potential anti-cancer agents synergize with bortezomib and ABT-737 against prostate cancer. Prostate 2010; 70:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68:3421–8. [DOI] [PubMed] [Google Scholar]
- 96.Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 2011; 29:909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128:1173–86. [DOI] [PubMed] [Google Scholar]
- 98.Schoenwaelder SM, Jarman KE, Gardiner EE, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011; 118:1663–74. [DOI] [PubMed] [Google Scholar]
- 99.Rudin CM, Elann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012; 18:3163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hao JW, Mao XP, Ding DG, et al. The effect of cell killing by ABT-737 synergized with docetaxel in human prostate cancer PC-3 cells [in Chinese]. Zhonghua Wai Ke Za Zhi 2012; 50:161–5. [PubMed] [Google Scholar]
- 101.Song JH, Kraft AS. Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737. Cancer Res 2012; 72: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamaguchi R, Janssen E, Perkins G, et al. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One 2011; 6:e24l02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 2000; 97:7124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An J, Chervin AS, Nie A, et al. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene 2007; 26:652–61. [DOI] [PubMed] [Google Scholar]
- 105.Heikaus S, Pejin I, Gabbert HE, et al. PIDDosome expression and the role of caspase-2 activation for chemotherapy-induced apoptosis in RCCs. Cell Oncol 2010; 32:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heikaus S, van den Berg L, Kempf T, et al. HA14–1 is able to reconstitute the impaired mitochondrial pathway of apoptosis in renal cell carcinoma cell lines. Cell Oncol 2008; 30:419–33– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian D, Das SG, Doshi JM, et al. sHA 14–1, a stable and ROS-free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer Lett 2008; 259:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis 2005; 10:1411–8. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15–070) antagonizes Mcl-1 and overcomes Mcl-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA 2007; 104:19512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]