Abstract
Romidepsin is a histone deacetylase inhibitor (HDI), approved by the US FDA for the treatment of cutaneous T-cell lymphoma (CTCL). Although various mechanisms have been proposed for the activity of HDls, including induction of genes controlling cell cycle, acetylation of cytoplasmic proteins and direct induction of apoptosis, the mechanism underlying activity of romidepsin and other HDIs in CTCL is not known. Romidepsin induces long-lasting responses. The side-effect profile is similar to that of other HDIs, causing fatigue, nausea and thrombocytopenia. Management of the CTCL population requires vigilence to prevent infection with skin contaminants, and monitoring of potassium and magnesium, electrolytes found to be low ina large proportion of patients. Electrocardiographic (ECG) changes are common but are not associated with myocardial damage. When molecular end points were evaluated in 61 patients enrolled on a Phase II trial with romidepsin, response was associated with persistence of acetylated histone H3, suggesting that drug exposure is important in effective therapy with romidepsin. Future studies will endeavor to identify combination strategies to increase the efficacy both in resistant CTCL and in solid tumors and to identify biomarkers of response that will allow selection of patients most likely to benefit from the therapy.
Keywords: cutaneous T-cell lymphoma, histone deacetylase inhibitor, peripheral T-cell lymphoma, romidepsin
Romidepsin (Istodax®, formerly depsipeptide, NSC 630176, FR 901228, FK228), a histone deactylase inhibitor (HDI), is among a novel class of anticancer drugs being evaluated for the treatment of cancer. Romidepsin and vorinostat (Zolinza®) were the first agents in this class to receive US FDA approval for treatment of cutaneous T-cell lymphoma (CTCL).
The histone deacetylase (HDAC) enzymes are believed to be the major target of the HDIs and are grouped into four different classes based on their similarity to known yeast HDACSs [1,2]. The current HDIs target class I (HDACs 1, 2, 3 and 8 – isoenzymes homologous to the yeast RPD3 protein), class II (HDACs 4, 5, 6, 7, 9 and 10 – isoenzymes homologous to the yeast HDA1) and HDAC 11 (generally considered class IV) enzymes to varying degrees. Class II HDACs are orthologs of yeast Sir2 [1,2]. Class I and II enzymes are zinc dependent and can be inhibited by zinc chelators [3]. Class I HDACs have ubiquitous tissue expression and localize to the nucleus, while class II HDACs are found in both the nucleus and cytoplasm of various tissues [1].
Histone deacetylase enzymes may play a role in transformation by keeping genes involved in differentiation, apoptosis and cell cycle arrest, in a transcriptionally silent state [4]. Histone acetyl transferase (HAT) enzymes function in opposition to the HDACs, increasing transcriptional activity. Molecular alterations that diminish or dampen HAT activity or shift the balance towards HDAC activity have been noted in a number of different cancers. In vitro, HDIs were found to be effective cytotoxic agents by causing growth arrest, cellular differentiation and apoptosis. Whereas their traditional anti-tumour effect was purported to be due to the increased acetylation of lysine residues on histone proteins comprising the core nucleosomic histones, further investigation has identified a number of intriguing potential mechanisms, which suggest acetylation of nonhistone proteins may be more important.
Pharmacology
Chemical structure
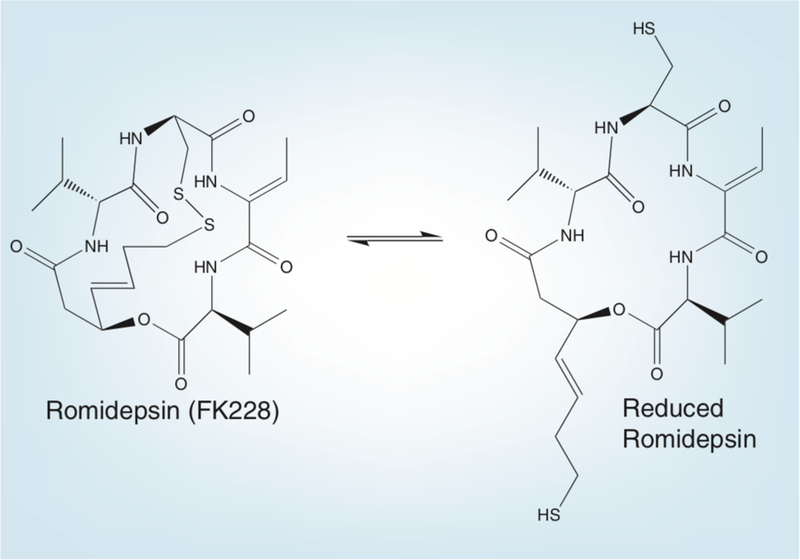
Romidepsin, (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis (1-methyletheyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6] tricos-16-ene-3,6,9,19,22-pentone is a bicyclic peptide (Figure 1). Isolated from the fermentation broth of Chromobacterium violaceum [5,6], romidepsin was identified as a molecule of interest based on its ability to revert H-ras-transformed NIH 3T3 cells; it was later shown to be a potent HDI [5–8]. Laboratory studies revealed it to be a substrate of P-glycoprotein [9]. As an HDI, romidepsin is relatively unique in that it is a prodrug. The disulfide bond of romidepsin is reduced inside the cell to yield the drug’s active form (Figure 1). This active form is then capable of preferentially interacting with the zinc in the active site of the class I and I HDAC enzymes. Romidepsin is a potent inhibitor of class I HDACs, and its ability to inhibit class II enzymes at higher concentrations suggests that, in certain contexts, it could act as a broad-spectrum HDI [10,11].
Figure 1. Romidepsin.
Romidepsin is a bicyclic depsipeptide with a disulfide bond that is converted by cellular reducing activity to yield a free sulfhydryl moiety that is thought to bind to the zinc in the HDAC active site pocket.
Pharmacokinetics
Based on preclinical studies, a 4-h intravenous infusion was selected for development. Phase I studies testing two different schedules determined maximum tolerated doses of 14 mg/m2 on days 1, 8 and 15; and 17.8 mg/m2 on days 1 and 5, of a 21-day schedule [12,13]. Most subsequent Phase II trials adopted the former schedule, and it is this schedule that was approved by the FDA. Dose-limiting toxicities consisted of fatigue, along with nausea, vomiting and thrombocytopenia. Romidepsin pharmacokinetic parameters over each of the eight doses levels explored (1–24 mg/m2), from either noncompartmental or two-compartmental analysis, were comparable. [13]. Furthermore, romidepsin pharmacokinetics were not significantly different when comparing the first cycle of a dose level to a subsequent cycle of the same dose [13]. A separate Phase I study in pediatric patients showed similar pharmacokinetic parameters as adults when adjusting the dose to body surface area, with a comparable recommended Phase II dose of 17 mg/m2 [14].
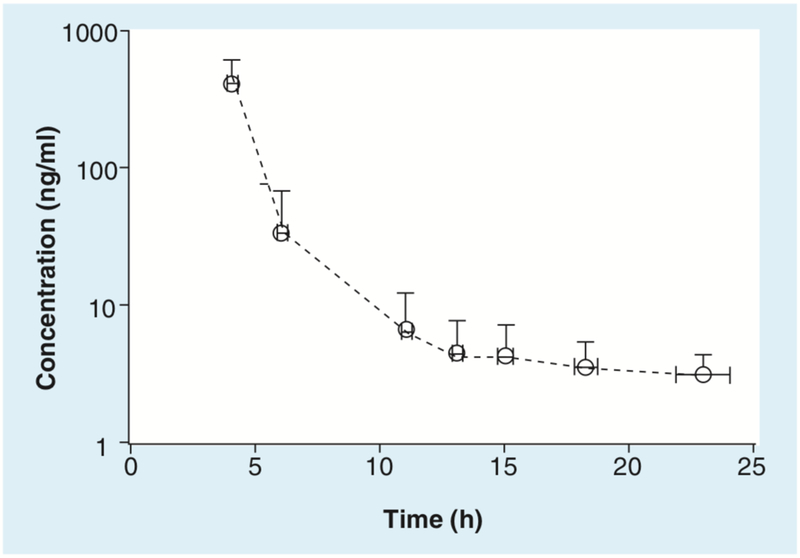
The pharmacokinetics of romidepsin in patients with CTCL, enrolled on the NCI Phase II study, have been described. Using a noncompartmental analysis for the first dose, the half-life (mean value: 2.95 h) and observed clearance (9.6 l/h/m2) at dose levels 14 or 18 mg/m2 were comparable with previously reported parameters in the Phase I studies [13,15,16]. A mean concentration–time profile for 60 patients with CTCL receiving 14 mg/m2 as a 4-h intravenous infusion is shown in Figure 2. It should be noted that terminal elimination rates (kel) could not be calculated for many patients, owing to rapid distribution and/or clearance resulting in plasma concentrations below the limit of quantitation. As expected, increased dose (14 and 18 mg/m2) led to increased Cmax (mean values: 360 and 722 ng/ml), AUClast, (mean value: 1214 and 2571 h ng/ml), AUCinf (mean value: 1456 and 2583 h ng/ml), with decreased pheerved terminal volume of distribution (mean value: 41 and 25 l/m2) [16]. Woo et al. used a two-compartment population modeling of the previous data to evaluate differences in first-cycle romidepsin pharmacokinetics based on genetic polymorphisms involved in romidepsin metabolism and transport (CYP3A4, CYP3A5 and ABCB1). No statistical differences in romidepsin pharmacokinetic parameters were found between patients with wild-type genes and those with polymorphisms [17].
Figure 2. Mean concentration–time profile for 60 patients after receiving romidepsin 14 mg/m2 as a 4-h infusion.
Each circle represents the mean concentration of romidepsin, at the mean collection time. X and Y error bars represent the standard deviation for concentration and sample collection time, respectively.
Romidepsin is administered in its oxidized (disulfide bond intact) prodrug form, and is thought to be activated by reduction of the disulfide bond [10]. The inactive prodrug is thought to be reduced intraellularly by glutathione, where it can then bind HDAC enzymes (Figure 1) [10]. It is metabolized to at least ten metabolites by cytochrome P450 (CYP) enzymes, predominantly CYP3A4, with contributions from CYP3A5, 1A1, 2B6 and 2C19. CYP3A5 accounts for 16.8% of the metabolism, and the rest combine for 8% [18]. Drug–drug interactions to be considered when administering romidepsin include any strong CYP3A4 or CYP3A5 inhibitors, such as ketoconazole, which has been shown to significantly lower romidepsin metabolism in vitro [18]. Other prescription drugs that are CYP3A4 substrates that patients might be taking for noncancer therapy might interact with romidepsin; these include alprazolam, atorvastatin, carbamazepine, dexamethasone, erythromycin, sildenafil and simvastatin [19]. While there are no data to support this, there is potential for an interaction with grapefruit juice, which is known to inhibit CYP3A4 [19].
Pharmacodynamic data
The most direct assay of HDAC inhibition is the extent of histone acetylation. Previously, studies have shown that increased histone acetylation is observed in patients’ peripheral blood mononuclear cells (PBMCs) following treatment with HDIs [20–24]. Increased histone acetylation has also been reported in post-treatment biopsies. Increased acetylation has been observed in PBMCs from patients treated with romidepsin [25].
As part of the Phase II trial with romidepsin in T-cell lymphoma, a comprehensive biomarker study was performed [25]. Four types of molecular end points were assessed in 61 patients enrolled on the trial: histone acetylation in PBMCs, ABCB1 gene expression in PBMCs, ABCB1 gene expression in biopsy samples obtained from patients and blood fetal hemoglobin levels (HbF). Increased global histone acetylation was noted in 73% of PBMC samples within 4 h of treatment and, in approximately 40% of patients, lasted from 24 to 48 h after a single dose [25]. A somewhat lower proportion of patients demonstrated increased ABCB1 expression in PBMCs, with 56% of patients having ABCB1 levels twofold or higher than the baseline at 4 h, but only 30% with levels twofold or higher at 48 h [25]. When each surrogate was evaluated for association with either PK parameters or disease response, it was noted that the fold increase in histone acetylation measured in PBMCs at 24 h correlated with Cmax and area under the curve (AUC) and inversely correlated with clearance. In addition, histone acetylation in PBMCs at the 24-h time point appeared to correlate with response, despite the lack of a statistically significant correlation between PK parameters and response [25]. There was no correlation between the levels of ABCB1 induction and pharmacokinetic parameters, nor was there an association between induction of ABCB1 in biopsy specimens and disease response. In total, 60% of patients also had a greater than fourfold increase in circulating HbF detected in blood [25]. Although this was correlated with response, it was also associated with increased time on study. Taken together, these data suggest that for romidepsin, drug exposure -which is based on drug clearance and in the case of romidepsin, drug activation ‒ is an important determinant of response.
Gene-expression profiling using patient-derived samples has been incorporated in some studies. Genes involved in apoptosis, cell proliferation, immune regulation and angiogenesis are altered as early as 4h after drug administration [24,26,27].
Mechanism of action
The HDIs, including romidepsin, act directly by inhibiting to various extents, members of the family of HDACs, thus allowing unrestrained HAT activity. While this much is clear, the downstream effects of this loss of balance of acetylation and deacetylation vary across model systems. This is, in part, owing to different cellular contexts, levels of expression of the HDAC isoenzymes, and affinities of the HDIs for the HDAC isoenzymes. As discussed earlier, the traditional mechanism of action considered to underlie the antineoplastic activity of romidepsin and other HDIs, has been thought to be increased acetylation of lysine residues of histones forming the octomeric chromatin core. However, more recent studies have recognized that HDIs have multiple potential mechanisms of action [28]. These include:
Histone acetylation with alterations in gene expression that effect cell cycle arrest and limit cell growth, including the upregulation of genes, such as p21, p27 and other genetic markers of differentiation, and downregulation of genes involved in growth, such as cyclin D [29–31];
Acetylation of nonhistone proteins, such as p53, HIF-lα, pRb, STAT-3, Rel A/p65 or estrogen receptor that may impair their function and, thereby, influence cell growth or survival [32–34];
Acetylation of Hsp90, with its attendant loss of ability to chaperone client proteins resulting in their ubiquitinylation and proteasomal degradation [35,36];
Cell cycle arrest in prometaphase, which results from reduced premitotic phosphorylation of pericentromeric histone H3 and disruption of kinetochore assembly [37];
Antiangiogenic effects potentially mediated by impairing hypoxia-inducible factor (HIF)-1 stability [38];
Direct activation of apoptotic pathways through reduction of antiapoptotic proteins, such as Bcl-2, and increased expression of proapoptotic proteins, such as BAX and BAK [39,40];
Enhanced production of reactive oxygen species [41,42] along with increased thioredoxin levels [43];
Disruption of aggresome formation through acetylation of tubulin [44];
Enhanced antitumor immunity through enhancement of TRAIL, or upregulation of antigen expression, which could facilitate cancer cell recognition [45–48];
Disruption of DNA repair through acetylation, or downregulation of proteins, such as Ku70, Ku86, BRCA1 and RAD51 [49–51];
Altered cellular glycolysis by inhibiting the glucose transporter GLUT1, as well as hexokinase activity [52];
Other cellular activities of HDAC inhibitors may also contribute to antitumor activity, such as altered expression of genes promoting or suppressing metastasis [53].
While an array of potential mechanisms of action are described, in fact, the number cited only highlights what we do not know. Two possibilities can be considered. The first possibility is that there is a unifying mechanism of action – perhaps a hierarchy of events that leads to cell death depending upon the proclivity to apoptosis. The poor activity of romidepsin as single-agent therapy in solid tumors would support this model. The cell to cell type variability in gene-expression profiles that results from romidepsin exposure argues somewhat against this, although the possibility exists that it is the totality of dysregulated gene expression that the cell finds intolerable. The second possibility is that the mechanism depends upon cellular context. A T-cell lymphoma cell may find gene induction of death receptors intolerable and undergo rapid apoptosis. A cell with amplification of ErbB2, or mutation and activation of the EGF pathway, will be more sensitive to the loss of Hsp90 chaperone function and client protein degradation than a cell without it [54,55]. A myeloma cell filled with paraproteins may be more sensitive to the loss of HDAC6-mediated transport to the aggresome [56]. The impact of varying mechanism of action could offer almost limitless possibilities for the use of romidepsin and the HDIs in the clinic.
Clinical activity
Based on dramatic responses observed in patients with T-cell lymphoma during Phase I testing reported by Piekarz et al. in 2001 [57], romidepsin entered Phase II testing in that disease. In 2009, the FDA approved romidepsin for the treatment of patients with CTCL who have received at least one prior systemic treatment. Vorinostat was approved for this indication in 2006; the activity in CTCL appears to bea class effect with several other HDIs also demonstrating activity [24,58,59]. Romidepsin has also demonstrated significant activity in patients with peripheral T-cell lymphoma (PTCL), a class of T-cell lymphoma with a poor overall prognosis [60].
While preclinical evidence exists for activity in both solid malignancies and hematological disorders, romidepsin has demonstrated clinical efficacy mainly in lymphomas and hematological malignancies [61]. Success in solid malignancies has remained elusive, with observed responses uncommon in monotherapy trials conducted to date [62–64].
The FDA approved romidepsin in patients with CTCL who have received at least one prior systemic therapy. The approved dose is 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Results from 71 patients with CTCL, 62 with stage IIB or higher disease presentations, were recently reported [16]. Within this patient cohort, four patients achieved a complete response, and an additional 20 patients a partial response, for an overall response rate of 34% with complete responses noted in patients with Sézary syndrome [16]. The median duration of response was 13.7 months. In an independent international trial, of the 96 patients enrolled, six patients achieved a complete response and 27 patients a partial response for an overall response rate of 34% and a median duration of response of 15 months [65]. Responses have also been noted in patients with PTCL, with seven patients achieving a complete response, and an additional 11 patients achieving partial response, for an overall response rate of 38% [60]. Responses were noted in patients previously treated with a stem cell transplant. The median duration of response was 10.3 months, including a patient with ongoing complete response extending over 72 months [60]. In a trial of romidepsin in acute myeloid leukemia (AML), although no responses were noted, antileukemia effects were demonstrated in five patients, including clearance of blasts from the bone marrow in two patients and a greater than 50% decrease in bone marrow blasts in three patients [66]. There were some responses observed in patients in an additional trial in AML [67]. A limited trial in chronic lymphocytic leukemia did not observe any responses [68]. A Phase II trial was initiated for patients with renal cell carcinoma based on an observed partial response in a patient during Phase I testing [13]. Of the 29 evaluable patients, one complete response and one partial response were noted [64]. While intriguing, the single-agent activity in renal cell cancer was not sufficient to pursue romidepsin as a monotherapy. In a Phase II trial of 35 patients with metastatic castration-resistant prostate cancer, two patients had partial responses by Response Evaluation Criteria In Solid Tumors (RECIST) criteria [69]. Little or no response was noted in several other Phase II trials for patients with solid tumors. These include trials for patients with colorectal [70] and lung cancer [26].
Toxicity
Toxicities described for romidepsin have been mainly gastrointestinal and constitutional, and are different than those typically seen for traditional chemotherapeutic agents.
Common romidepsin side effects
Gastrointestinal
Anorexia, dysgeusia, nausea and vomiting are commonly seen in patients receiving romidepsin therapy [13,20,22,71,72]. As a majority of patients receiving these agents will experience nausea when treated at the maximum tolerated dose, antiemetic prophylaxis is recommended. Mild elevations in liver function enzymes have also been noted [13,22,71,73,74]. The National Cancer Institute (NCI) antiemetic routine has been to administer 1 mg granisetron intravenously prior to romidepsin, followed by 1 mg orally every 12 h for 3 days. With this schedule, only 7% of patients have nausea greater than or equal to grade 3 at some point in therapy with romidepsin. Some patients required a change in the antiemetic regimen to include lorazepam, metoclopramide or prochlorperazine; with lorazepam notably effective in this regard. Aprepitant, a CYP3A4 inhibitor, was avoided. Palonosetron (Aloxi®) an agent with a long halflife that can prolong the QT interval, was also avoided. No patient discontinued therapy because of nausea. A complaint of dysgeusia, or taste change, is also associated with nausea, and was noted in 38% of patients on the NCI trial.
Constitutional
Fatigue and occasional fever have been observed with romidepsin (13,20,22,72]. A cytokine mechanism has been postulated for the fever and fatigue associated with HDI therapy [72]. Anecdotally, intravenous hydration has been of some benefit in patients with recurrent fever. Although no fever of grade 3 or higher was reported in the NCI romidepsin Phase II study, 25% of patients were noted to have grade 1 or 2 pyrexia.
Hematologic
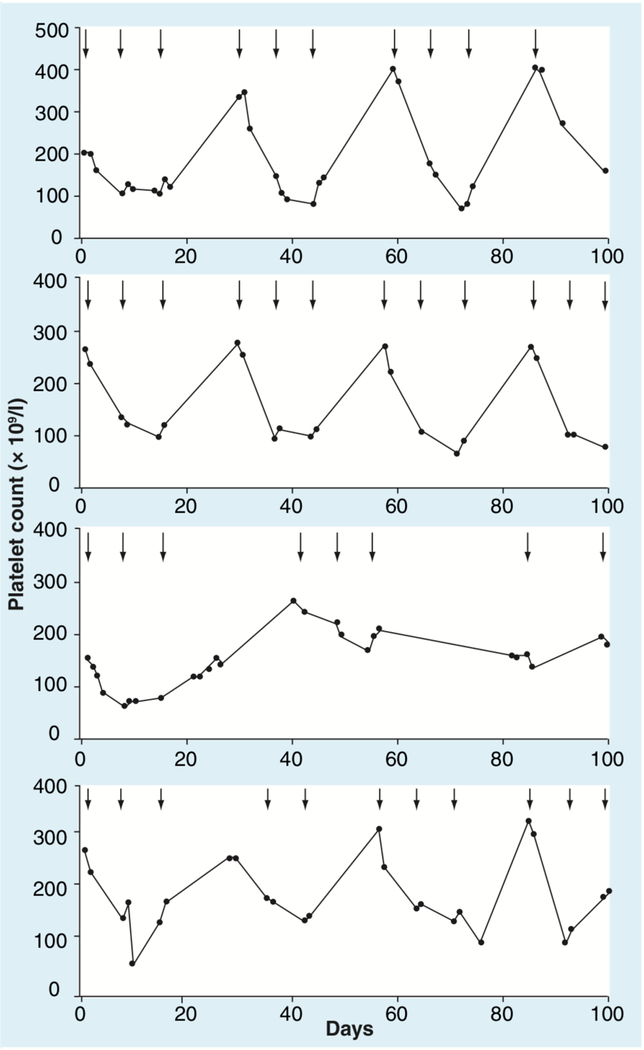
Significant bone marrow suppression at 10–21 days, as occurs following cytotoxic therapy, has not been observed. The leukopenia, granulocytopenia and thrombocytopenia observed after treatment are transient and rapidly reversible. Furthermore, treatment with romidepsin appears to not affect the colony-forming units from bone marrow cells and bone marrow biopsies performed in patients treated with romidepsin do not demonstrate significant myelosuppression. Figure 3 demonstrates the platelet profiles of four patients on treatment with romidepsin whose counts were documented to fall into the grade 3 range. No evidence of cumulative thrombocytopenia was observed and no major bleeding disorders have been reported.
Figure 3. Graphs showing platelet counts (K/μl) over time in four patients who developed grade 3 thrombocytopenia.
Arrows indicate days when treatment was administered.
Cardiac
Electrocardiography changes have been reported in clinical trials with romidepsin and a number of the other HDIs and may be considered a class effect [75–80]. The ECG changes, primarily ST-and T-wave flattening, and ST segment depression, prompted evaluation following HDI administration to exclude myocardial damage or dysfunction. A thorough cardiac assessment of 42 patients with T-cell lymphomas receiving romidepsin demonstrated no impact on myocardial wall motion, by echocardiography, and no impact on left ventricular ejection fraction [81]. Furthermore, there were no clinically significant rises in serum troponin [82].
A related question is the effect on the QT interval; increases have been reported in association with HDI therapy [22,72,74,81,83,84]. There was increased concern with HDIs after a report of torsades de pointes in a patient treated with dacinostat [75] and after several sudden deaths were reported following romidepsin [81,85]. QT interval prolongation is regarded as a signal for depolarization delay that could lead to ventricular tachyarrhythmias [86]. However, it should be noted there are multiple causes of QT prolongation, including electrolyte disturbances often found in patient populations with complicated medical problems [86]. Furthermore, there are inter-individual differences such that some patients without congenital long QT syndrome begin therapy with an elevated QT interval of unknown significance. Importantly, the QT interval can be difficult to measure, particularly in patients with T-wave flattening, and it is well established that standard QT interval correction formulae perform poorly, as the heart rate moves away from 60 beats per min.
The ICH E14 regulatory guidance recommends specific assessment of the potential for QT prolongation as part of the FDA approval process [87,88]. For romidepsin, an independent cardiology review of 4910 ECGs in 135 patients determined a 5.0 ± 13.9 ms QT prolongation (including the effect of antiemetics) following infusion. As the small effect on QT appears to be a class effect, the mechanism may be an effect of HDIs on hERG channel trafficking, not a direct blockade of the channel. Mechanism notwithstanding, it appears the QT prolonging effect of the HDIs is in the same range as that of other drugs known to have slight and occasional effects on the QT interval. Vigilance to avoid concomitant use of agents that significantly prolong the QT (e.g., antiarrhythmics, such as amiodarone) is, therefore, prudent.
While careful studies have not definitively shown a clinically significant QT prolongation, indirect effects of HDIs could increase myocardial electrical instability in patients with underlying heart disease. Clinically insignificant arrhythmias, including supraventricular tachycardias, such as atrial fibrillation, and nonsustained ventricular tachycardias, have been seen following the HDIs [74,81,85]; atrial fibrillation has been reported as a dose-limiting toxicity in Phase I studies of romidepsin, dacinostat and belinostat [13,22,64,72,89]. Some of this may be the patient population; prestudy ambulatory monitoring demonstrated significant ectopy in patients with T-cell lymphoma [81]. In addition, observed events were generally noted in patients with uncorrected low potassium or magnesium. Except for atrial fibrillation, these arrhythmias have been asymptomatic, have not required treatment and have not been recurrent.
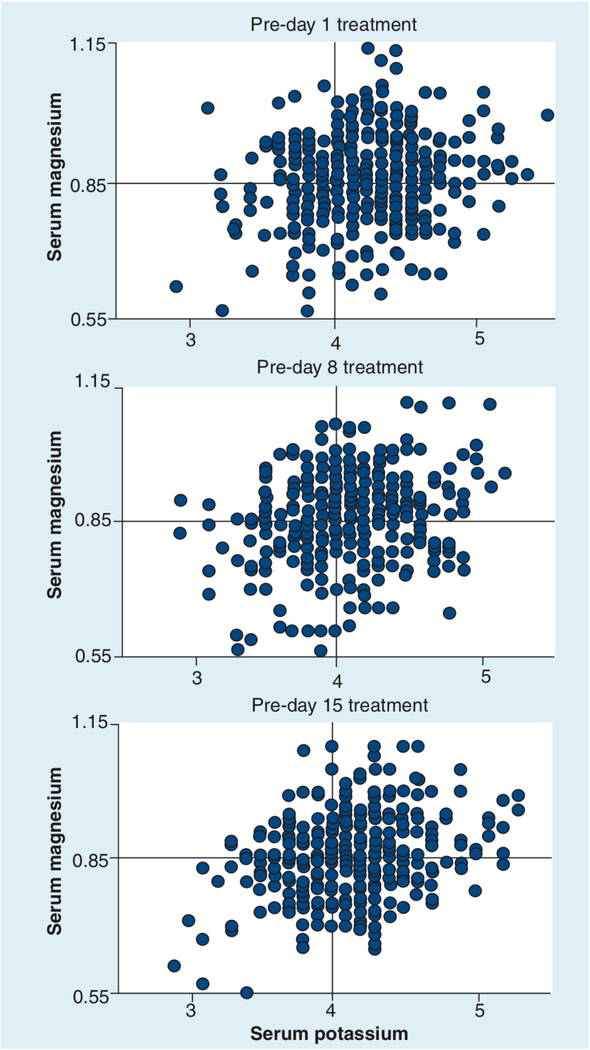
Sudden death occurred in six patients across several Phase II trials of romidepsin. When these deaths were reviewed, it was recognized that all patients had comorbidities that were pre-existing risk factors for sudden death (16,64,81,85]. Entry criteria for romidepsin clinical trials were altered to exclude patients with known heart disease or pre-existing risk factors for sudden death (refer to the sample exclusion criteria in Box 1). In addition, potassium and magnesium were monitored prior to drug administration to ensure levels remained in the high normal range for patients receiving romidepsin. With these safeguards, romidepsin development continued through to its approval by the FDA. Of note, sudden death has also been reported in patients receiving other HDIs [73,83,90,91]. Although infrequent, cardiac arrhythmias including both atrial fibrillation and ventricular tachycardia, have been regularly reported in association with anticancer chemotherapy [92]. Magnesium and potassium replacement is routine in critical care settings [93], and monitoring of these electrolytes should be part of good medical management in the oncologic setting as well. Both the vorinostat and romidepsin package inserts recommend monitoring of potassium and magnesium; the romidepsin insert includes the precaution that physicians should ensure that potassium and magnesium are within the normal range before administration. Particularly in a patient population with active skin disease, or following multiple systemic therapies, low magnesium and potassium levels are common [74,94]. Plots of potassium and magnesium levels in patients presenting for romidepsin dosing are shown in Figure 4. While there was some improvement in the need for electrolyte replacement over time, when the data were analyzed cumulatively, 50% of patients required some form of electrolyte replacement when presenting for day 1 therapy – 13% required both potassium and magnesium, 12% potassium alone and 25% magnesium alone. This analysis suggests electrolytes should be monitored in patients on romidepsin therapy. Electrolyte-replacement guidelines incorporated in the NCI 1312 romidepsin clinical trial are included in Box 2.
Box 1. Typical cardiac exclusion list from an histone deactylase inhibitor clinical trial.
Uncontrolled hypertension
Impaired cardiac function, including any one of the following:
QTc >450 ms on screening ECG
Congenital long QT syndrome
History or presence of sustained ventricular tachycardia
History of ventricular fibrillation or torsades de pointes
Bradycardia, defined as <50 beats per min (patients with a pacemaker and heart rate 250 beats per min are eligible)
New York Heart Association class III–IV congestive heart failure
Right bundle-branch block and left anterior hemiblock (bifascicular block)
Myocardial infarction or unstable angina within the past 6 months
Figure 4. Serum magnesium and potassium (mmol/l) recorded at the time patients presented for romidepsin dosing on days 1, 8 and 15 of treatment.
Per protocol, thresholds for electrolyte replacement were 0.85 mmol/l for magnesium and 4.0 mmol/l for potassium (denoted by quadrants).
Box 2. Guidelines for electrolyte administration in the NCI 1312 romidepsin protocol.
Serum K+ <3.5 mmol/l: 80 mEq of potassium divided 40 mEq intravenously and 40 mEq orally
Serum K+ <4.0 mmol/l: 40 mEq potassium administered by oral and/or intravenous routes
Serum Mg2+ <0.85 mmol/l: 1 g MgSO4, intravenously (8.12 mEq) for every 0.05 below 0.85 mmol/l, maximum of 4 g (32.48 mEq)
Electrolytes were rechecked within 8 h of administration of romidepsin and confirmed to be above the guidelines before administration
Data from [16].
Infections
Infections are a common problem in CTCL and are likely to be caused by both impaired immunity and loss of skin integrity. Patients are colonized with Staphylococcus aureus and insertion of indwelling catheters increases the risk for line infections and sepsis [95]. On the NCI 1312 trial, where patients with advanced CTCL were enrolled, such infections were common. Overall, 52% of patients experienced an infection at some point during therapy. In 28% of patients, infections were reported as serious adverse events, with 37% of patients having infections that were grade 3 or higher. All reported staphylococcal infections were in patients with CTCL. During initial cycles of therapy, in which skin lesions are most active and most subject to bacterial colonization, administration of romidepsin by peripheral intravenous infusion is recommended. In patients with extensive skin involvement, whirlpool baths with antibacterial solutions may reduce bacterial levels. Indwelling catheters should be avoided in patients with erythroderma or with open skin lesions. If a temporary venous access device must be placed in such patients to allow initiation of therapy with romidepsin, a dose of prophylactic antibiotics should be administered while the line is in place, and the device should be removed after each dose. In patients with notable clearing of the skin, subcutaneous cathethers (Port-a-caths) can be safely placed and used, even in those in partial remission, with careful antiseptic preparation of the skin before catheter access.
Less common HDI side effects
Renal dysfunction in the form of benign elevations in serum creatinine have been reported, along with hypoalbuminemia, hypocalcemia, hypomagnesemia and hypophosphatemia. Increased liver enzymes have been reported, including serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase and bilirubin. Furthermore, laboratory studies have suggested viral activation could occur in response to the chromatin effects of the HDIs, and in the NCI study, viral reactivation may have occurred in three patients. Thus, patients with HIV, hepatitis, or Epstein–Barr virus, related illness should be treated with close monitoring or on an experimental study [96,97].
General comments
With the approval of romidepsin, it is important to think about its use in the general population. Romidepsin should not be administered in pregnancy. For a subset of patients with comorbidities, the safety of romidepsin has not yet been proven. It will be important to complete studies in patients with pre-existing liver or renal dysfunction and romidepsin or any HDI should only be used with caution in such patients until further data are available. With regard to cardiac dysfunction, clinical trials have excluded patients with pre-existing cardiac disorders, long QT syndrome, or other risk factors for sudden death. Until more experience is gained with romidepsin, use in this patient population should continue to be avoided or carefully monitored.
Expert commentary
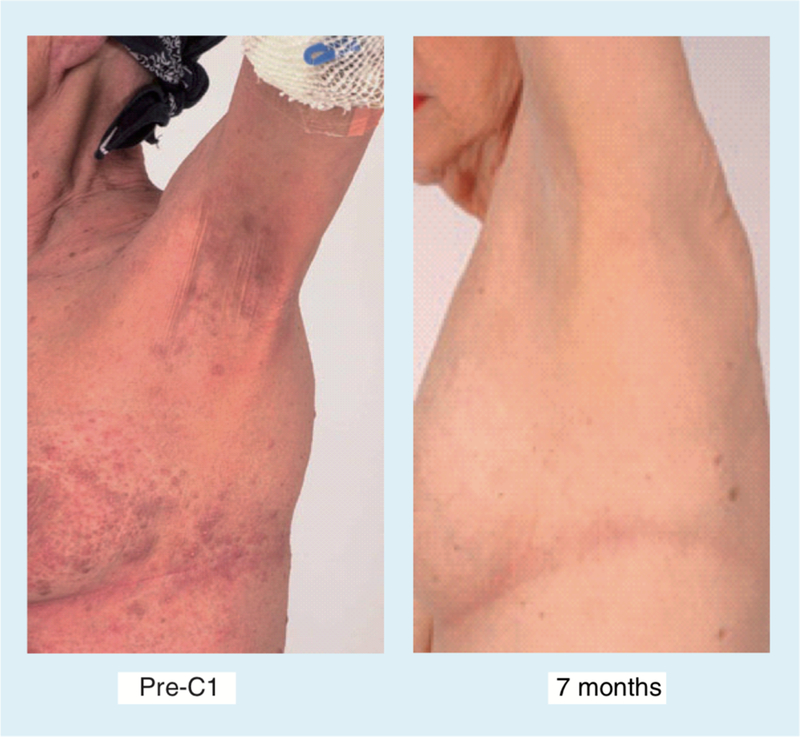
The observation that romidepsin was active in T-cell lymphomas (Figure 5), launched a decade of effort that concluded in its FDA approval for treatment of CTCL in 2009. As highlighted in the previous paragraphs, many questions remain for active investigation. A submission for the PTCL indication is planned for 2010. For these rare lymphomas, this class of drugs offers a new strategy for therapy. As with any new class of agents, the challenge is to use the drugs wisely, to increase their activity, and to develop and extend their indications for use. Increasing the activity of the HDIs will likely require combination with other active therapies. Identifying subsets of tumors in which HDIs have the potential to be effective may allow translation into the difficult to treat solid tumors.
Figure 5. Patient with cutaneous T-cell lymphoma who presented with extensive erythematous skin nodules.
Prior therapy had included Psoralen + UVA, deoxycoformycin, Doxil®, and denileukin diftitox. Complete response noted after 7 months on romidepsin, maintained another 18 months. Developed minimal patch disease, and no therapy was required for an additional 4 years after discontinuation of romidepsin.
The pharmacology of romidepsin has been studied – the Cmax,, is 361 ng/ml (range: 313–416 ng/ml) and the half-life is 2.95 h, with a range of 2.49–3.49 h – but factors influencing interpatient variation remain to be determined. As discussed earlier, the activity in T-cell lymphoma and general toxicities of the HDIs appear to be a class effect, but underlying mechanisms have yet to be elucidated. Major toxicities are fatigue, nausea and thrombocytopenia. ECG changes are common, but not indicative of cardiac damage. At this time, electrolyte replacement must be considered a routine part of the management of patients being treated with this agent, particularly patients with CTCL who are prone to electrolyte imbalance. The infectious complications of CTCL require special attention in patients afflicted with this disease.
Understanding why some lymphomas are so sensitive may help in the design of therapies to overcome resistance. We need to understand what combination strategies would be most effective to increase the overall response rate in T-cell lymphomas, and in what dose and schedule. Furthermore, combinations with cytotoxic therapy, synergy and also some examples of antagonism between agents, have been reported [98–101]. The cell cycle arrest that follows HDI exposure could impair sensitivity to other therapies, allowing time for repair. On the other hand, a marked increase in radiation sensitivity has been noted, despite a pro-found G1 arrest, resulting from HDI pretreatment [102]. Multiple investigators have noted radiosensitization by HDIs, and this is emerging as an important avenue to investigate. The ability of HDIs to reduce levels or impair the function of DNA repair proteins is a likely mechanism for the radiosensitization [102–104].
The second major category of investigation is to identify ways to exploit the unique activities of HDIs for therapy in solid tumors. This could include exploiting genes upregulated or downregulated by the ‘differentiating’ effects of HDIs. Since markers of differentiation occur in a cell-type specific manner, there are potentially unique combinations. For example, induction of the sodium iodide symporter occurs in thyroid cancer cells (105). Patients with thyroid cancer may be treated with HDIs to increase expression of the sodium–iodine symporter in their tumors, with the goal of increasing effectiveness of radioiodine therapy. In T-cell lymphomas, the induction of CD25 as a marker of differentiation offers the potential to combine an HDI with denileukin–diftitox or CD25-targeted antibody [106,107]. Alternatively, thymidylate synthase can be downregulated, which has the potential to increase the activity of 5-fluorouracil or capecitabine [108]. The increased acetylation of Hsp90 that follows HDI exposure promotes loss of mutant EGF receptor or HER2, for example, and should allow synergy in combination with gefitinib or trastuzumab in diseases in which those drugs are active [55].
Several combination strategies have already been translated to the clinic. Synergy based on epigenetic modification has been demonstrated in vitro and needs clinical exploration [109,110]. Trials in combination with DNA methyltransferase inhibitors are already underway [61]. Combinations with other targeted therapies offer nontoxic therapeutic approaches. Both vorinostat and romidepsin have been used in combination with bortezomib, with clinical trials ongoing, providing a potential new strategy for improving outcome in multiple myeloma [111–114].
The addition of the HDIs to the cancer armamentarium brings a multiplicity of new avenues of investigation. The challenge will be to approach these somewhat systematically, because there are so many possibilities. It is critical that combination trials determine whether the molecular effect hypothesized to create synergy between two agents has actually occurred. To this end, laboratory correlates will be an essential component of future clinical trials. Indeed, it can be argued that a clinical trial incorporating a HDI should not be conducted without a translational component that determines whether p21 is induced, a DNA repair protein is acetylated or HER2 is lost. Without this translational component, the science behind trials with these agents cannot truly progress.
Five-year view
Romidepsin represents an exciting addition to the anticancer armamentarium. The second HDI to receive FDA approval for the treatment of CTCL, romidepsin, has a 34% response rate and 11–15 month duration of response. Side effects are manageable and characteristic for this drug class. Concerns regarding cardiac toxicity have largely abated, although treatment of patients with significant heart disease or patients receiving agents that pro-long the QT interval must be viewed with caution. Electrolytes should be monitored and replaced appropriately. Going forward, combination studies are needed to identify strategies to increase efficacy in difficult to treat tumors. Multiple candidate combinations have been proposed; the goal is to select the best combinations to exploit romidepsin’s unique activities. Findings that persistent histone H3 acetylation correlates with response suggests that drug exposure is important, but confirmation is needed to determine potential clinical utility. Biomarkers are needed to identify which patients will benefit from therapy and from which combinations.
Key issues.
Romidepsin is effective as monotherapy for the treatment of cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphoma.
The US FDA approved romidepsin in November 2009, administered intravenously at a dose of 14 mg/m2 on days 1, 8 and 15 of a 28-day cycle in patients who have received at least one prior systemic therapy for CTCL.
Preclinical data have suggested a diverse range of mechanism of action; the precise mechanism of action in T-cell lymphoma is not known.
Toxicities of romidepsin include fatigue, nausea and transient thrombocytopenia. Unique problems in the CTCL patient population include infection and low potassium and magnesium levels. Indwelling lines should be avoided in patients with active skin disease. Particular attention should be paid to maintaining normal to high levels of potassium and magnesium in this patient population.
Electrocardiographic (ECG) changes, including T-wave flattening, are frequently observed. A small increase in QT interval in some patients suggests that concomitant treatment with QT-prolonging agents should be avoided. The mechanism underlying ECG changes has not been determined.
Histone acetylation is observed in peripheral blood mononuclear cells following romidepsin dosing and persists for 24–48 h. Gene induction in biopsy samples is observed, confirming epigenetic effects on chromatin.
A large number of combination therapies are suggested by the diverse biological activities of histone deacetylase inhibitors. Combinations will be needed to effectively translate histone deacetylase inhibitors into successful therapies for solid tumors.
Development of combination therapies should be accompanied by development of biomarkers to confirm the presence of the biological effect underlying the rationale for the combination.
Acknowledgments
TCRADA Partnerships: CRADA 01683. Support for clinical trial effort including monies that were distributed to extramural sites as well as providing intramural funding. Patent applications and Issued patents: PCT/JP2003/003823 US 10/508,958 DHHS E-199–2002/0/US04; PCT/60/811,961; 60/909,780 US 11/759; PCT/US06/31870 DHHS E-238–2005/0-PCT-02; DHHS E-286–2007/0.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Dokmanovic M, Clarke C, Marks P. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res 5(10), 981–989 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Bolden J, Peart M, Johnstone R. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov 5(9), 769–784 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Schrump D Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: mechanisms and potential clinical implications. Clin. Cancer Res 15(12), 3947–3957 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glozak M, Seto E. Histone deacetylases and cancer. Oncogene 26(37), 5420–5432 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res 241(1), 126–133 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968, on Ha-ras transformed NIH3T3 cells. Biosci. Biotech. Biochem 58, 1579–1583 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Nakajima H, Hori Y et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. 1. Taxonomy, fermentation, isolation, physico–chemical and biological properties, and antitumor activity. J. Antibiot. (Tokyo) 47(3), 301–310 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Ueda H, Manda T, Matsumoto S et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. III. Antitumor activities on experimental tumors in mice. J. Antibiot. (Tokyo) 47(3), 315–323 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Paull K, Alvarez M et al. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol 46, 627–638 (1994). [PubMed] [Google Scholar]
- 10.Furumai R, Matsuyama A, Kobashi N et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res 62(17), 4916–4921 (2002). [PubMed] [Google Scholar]
- 11.Bradner J, West N, Grachan M et al. Chemical phylogenetics of histone deacetylases. Nat. Chem. Biol 6(3), 238–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall J, Rizvi N, Kauh J et al. A Phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol 2(6), 325–332 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Sandor V, Bakke S, Robey RW et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res 8(3), 718–728 (2002). [PubMed] [Google Scholar]
- 14.Fouladi M, Furman WL, Chin T et al. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children’s Oncology Group report. J. Clin. Oncol 24(22), 3678–3685 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Marshall JL, Rizvi N, Kauh J et al. A Phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol 2(6), 325–332 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Piekarz RL, Frye R, Turner M et al. Phase I multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol 27(32), 5410–5417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo S, Gardner ER, Chen X et al. Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin. Cancer Res 15(4), 1496–1503 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiraga T, Tozuka Z, Ishimura R, Kawamura A, Kagayama A. Identification of cytochrome P450 enzymes involved in the metabolism of FK228, a potent histone deacetylase inhibitor, in human liver microsomes. Biol. Pharm. Bull 28(1), 124–129 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Kiani J, Imam S. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutr. J 6, 33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly WK, O’Connor OA, Krug LM et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol 23(17), 3923–3931 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robey RW, Zhan Z, Piekarz RL, Kayastha GL, Fojo T, Bates SE. Increased MDR1 expression in normal and malignant peripheral blood mononuclear cells obtained from patients receiving depsipeptide (FR901228, FK228, NSC630176). Clin. Cancer Res 12(5), 1547–1555 (2006). [DOI] [PubMed] [Google Scholar]
- 22.de Bono JS, Kristeleit R, Tolcher A et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin. Cancer Res 14(20), 6663–6673 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Kummar S, Gutierrez M, Gardner ER et al. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin. Cancer Res 13(18 Pt 1), 5411–5417 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Ellis L, Pan Y, Smyth G et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin. Cancer Res 14(14), 4500–4510 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Bates S, Zhan Z, Steadman K et al. Laboratory correlates for a Phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br. J. Haematol 148(2), 256–267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrump DS, Fischette MR, Nguyen DM et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin. Cancer Res 14(1), 188–198 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Manero G, Yang H, Bueso-Ramos C et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 111(3), 1060–1066 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Piekarz R, Bates S. Epigenetic modifiers: basic understanding and clinical development. Clin. Cancer Res 15(12), 3918–3926 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gui C, Ngo L, Xu W, Richon V, Marks P. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl Acad. Sci. USA 101(5), 1241–1246 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl Acad. Sci. USA 97(18), 10014–10019 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandor V, Senderowicz A, Mertins S et al. P21-dependent G(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br.J. Cancer 83 (6), 817–825 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Lu S, Wu L et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Wafl/Cipl). Mol. Cell Biol 26(7), 2782–2790 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai Y, Rahmani M, Dent P, Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-κB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol. Cell Biol 25(13), 5429–5444 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spange S, Wagner T, Heinzel T, Krämer O. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol 41(1), 185–198 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wang S, Zhang X et al. FK228 inhibits Hsp90 chaperone function in K562 cells via hyperacetylation of Hsp70. Biochem. Biophys. Res. Commun 356(4), 998–1003 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Nishioka C, Ikezoe T, Yang J, Takeuchi S, Koeffler H, Yokoyama A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk. Res 32(9), 1382–1392 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Robbins AR, Jablonski SA, Yen TJ et al. Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle 4(5), 717–726 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Jeong J, Park J et al. Regulation of the HIF-1α stability by histone deacetylases. Oncol. Rep 17(3), 647–651 (2007). [PubMed] [Google Scholar]
- 39.Suzuki T, Yokozaki H, Kuniyasu H et al. Effect of trichostatin A on cell growth and expression of cell cycle-and apoptosis-related molecules in human gastric and oral carcinoma cell lines. Int. J. Cancer 88 (6), 992–997 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Dong G, Wang L, Wang C, Yang T, Kumar M, Dong Z. Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J. Pharmacol. Exp. Ther 325 (3), 978–984 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res 63(13), 3637–3645 (2003). [PubMed] [Google Scholar]
- 42.Martirosyan A, Leonard S, Shi X, Griffith B, Gannett P, Strobl J. Actions of a histone deacetylase inhibitor NSC3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells. J. Pharmacol. Exp. Ther 317(2), 546–552 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ungerstedt J, Sowa Y, Xu W et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA 102(3), 673–678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catley L, Weisberg E, Kiziltepe T et al. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 108 (10), 3441–3449 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue H, Shiraki K, Ohmori S et al. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int. J. Mol. Med 9(5), 521–525 (2002). [PubMed] [Google Scholar]
- 46.Guo F, Sigua C, Tao J et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res 64(7), 2580–2589 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Weiser TS, Ohnmacht GA, Guo ZS et al. Induction of MAGE-3 expression in lung and esophageal cancer cells. Ann. Thorac Surg 71(1), 295–301 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Weiser TS, Guo ZS, Ohnmacht GA et al. Sequential 5-Aza-2′-deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J. Immunother 24(2), 151–161 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Munshi A, Kurland J, Nishikawa T et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin. Cancer Res 11(13), 4912–4922 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Rosato RR, Almenara JA, Maggio SC et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol. Cancer Ther 7(10), 3285–3297 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geng L, Cuneo K, Fu A, Tu T, Atadja P, Hallahan D. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AxX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res 66(23), 11298–11304 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Wardell S, Ilkayeva O, Wieman H et al. Glucose metabolism as a target of histone deacetylase inhibitors. Mol. Endocrinol 23(3), 388–401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph J, Mudduluru G, Antony S, Vashistha S, Ajitkumar P, Somasundaram K. Expression profiling of sodium butyrate (NaB)-treated cells: identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene 23 (37), 6304–6315 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Edwards A, Li J, Atadja P, Bhalla K, Haura E. Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor-dependent human lung cancer cells. Mol. Cancer Ther 6(9), 2515–2524 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Yu X, Guo ZS, Marcu MG et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J. Natl Cancer Inst 94(7), 504–513 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Hideshima T, Bradner J, Wong J et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl Acad. Sci. USA 102(24), 8567–8572 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piekarz RL, Robey R, Sandor V et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood 98(9), 2865–2868 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Duvic M, Talpur R, Ni X et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109(1), 31–39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pohlman B, Advani R, Duvic M et al. Final results of a Phase II trial of belinostat (PXD101) in patients with recurrent or refractory peripheral or cutaneous T-cell lymphoma. ASH Ann. Meeting Abstracts 114(22), 920 (2009). [Google Scholar]
- 60.Piekarz R, Wright J, Frye R et al. Final results of a Phase 2 NCI multicenter study of romidepsin in patients with relapsed peripheral T-cell lymphoma (PTCL). ASH Ann. Meeting Abstracts 114(22), 1657 (2009). [Google Scholar]
- 61.Prince H, Bishton M, Harrison S. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res 15(12), 3958–3969 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Molife R, Patterson S, Riggs C et al. Phase II study of FK228 in patients with metastatic hormone refractory prostate cancer. Presented at: 2006 Prostate Cancer Symposium 24–26 February (2006).
- 63.Whitehead RP, McCoy S, Wollner IS et al. Phase II trial of depsipeptide (NSC-630176) in colorectal cancer patients who have received either one or two prior chemotherapy regimens for metastatic or locally advanced, unresectable disease: A Southwest Oncology Group study. J. Clin. Oncol 24(18), 170S–170S (2006). [Google Scholar]
- 64.Stadler WM, Margolin K, Ferber S, McCulloch W, Thompson JA. A Phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin. Genitourinary Cancer 5(1), 57–60 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Whittaker S, Demierre MF, Kim EJ et al. Final results from a multicenter, international pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J. Clin. Oncol (2010) (In Press). [DOI] [PubMed]
- 66.Odenike O, Alkan S, Sher D et al. Histone deacetylase inhibitor romidepsin has differential activity in core binding factor acute myeloid leukemia. Clin. Cancer Res 14(21), 7095–7101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klimek VM, Fircanis S, Maslak P et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin. Cancer Res 14(3), 826–832 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Byrd JC, Marcucci G, Parthun MR et al. A Phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 105(3), 959–967 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Molife L, Attard G, Fong P et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann. Oncol 21(1), 109–113 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Whitehead R, Rankin C, Hoff P et al. Phase II trial of romidepsin (NSC-630176) in previously treated colorectal cancer patients with advanced disease: a Southwest Oncology Group study (S0336). Invest. New Drugs 27(5), 469–475 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan Q, Headlee D, Acharya M et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J. Clin. Oncol 23(17), 3912–3922 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Steele N, Plumb J, Vidal L et al. A Phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin. Cancer Res 14(3), 804–810 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Kelly WK, Richon VM, O’Connor O et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res 9(10 Pt 1), 3578–3588 (2003). [PubMed] [Google Scholar]
- 74.Giles F, Fischer T, Cortes J et al. A Phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin. Cancer Res 12(15), 4628–4635 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Rowinsky EK, de Bono J, Deangelo DJ et al. Cardiac monitoring in Phase I trials of a novel histone deacetylase (HDAC) inhibitor LAQ824 in patients with advanced solid tumors and hematologic malignancies. J. Clin. Oncol. (Meeting Abstracts) 23 (16 Suppl.), 3131 (2005). [Google Scholar]
- 76.Beck J, Fischer T, George D et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of ORAL LBH589B: a novel histone deacetylase (HDAC) inhibitor. J. Clin. Oncol. (Meeting Abstracts) 23(16 Suppl.), 3148 (2005). [Google Scholar]
- 77.Fischer T, Patnaik A, Bhalla K et al. Results of cardiac monitoring during Phase I trials of a novel histone deacetylase (HDAC) inhibitor LBH589 in patients with advanced solid tumors and hematologic malignancies. J. Clin. Oncol. (Meeting Abstracts) 23(16 Suppl.), 3106 (2005). [Google Scholar]
- 78.Kelly WK, DeBono J, Blumenschein G et al. Final results of a Phase I study of oral belinostat (PXD101) in patients with solid tumors. J. Clin. Oncol. (Meeting Abstracts) 27(15 Suppl.), 3531 (2009). [Google Scholar]
- 79.Kristeleit R, Fong P, Aherne G, de Bono J. Histone deacetylase inhibitors: emerging anticancer therapeutic agents? Clin. Lung Cancer 7(Suppl. 1), S19–S30 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J. HDAC inhibitors and cardiac safety. Clin. Cancer Res 13(3), 1068–1069 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Piekarz RL, Frye AR, Wright JJ et al. Cardiac studies in patients treated with depsipeptide, FK228, in a Phase II trial for T-cell lymphoma. Clin. Cancer Res (2006). [DOI] [PubMed]
- 82.O’Mahony D, Peikarz R, Bandettini W, Arai A, Wilson W, Bates S. Cardiac involvement with lymphoma: a review of the literature. Clin. Lymphoma Myeloma 8(4), 249–252 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsen E, Kim Y, Kuzel T et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J. Clin. Oncol 25(21), 3109–3115 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Lebwohl D, Masson E, Laird G, Cooper M, Prince H. Clinically relevant QTe prolongation is not associated with current dose schedules of LBH589 (panobinostat). J. Clin. Oncol 26(2), 332–333 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Shah MH, Binkley P, Chan K et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin. Cancer Res 3997–4003 (2006). [DOI] [PubMed]
- 86.Strevel E, Siu L. Cardiovascular toxicity of molecularly targeted agents. Eur. J. Cancer 45(Suppl. 1), 318–331 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Fingert H, Varterasian M. Cardiac safety, risk management, and oncology drug development. Clin. Cancer Res 12(12), 3646–3647 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Rock E, Finkle J, Fingert H et al. Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am. Heart J 157(5), 827–836, 836.e1 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Ramalingam S, Belani C, Ruel C et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J. Thorac. Oncol 4(1), 97–101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gojo I, Jiemjit A, Trepel JB et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood 109(7), 2781–2790 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galli M, Salmoiraghi S, Golay J et al. A Phase IT multiple dose clinical trial of histone deacetylase inhibitor [TF2357 in patients with relapsed or progressive multiple myeloma. Ann. Hematol 89(2), 185–190 (2010). [DOI] [PubMed] [Google Scholar]
- 92.Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis A. Introducing a new entity: chemotherapy-induced arrhythmia. Europace 11(12), 1579–1586 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Kanji Z, Jung K. Evaluation of an electrolyte replacement protocol in an adult intensive care unit: a retrospective before and after analysis. Intensive Crit. Care Nurs 25(4), 181–189 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Morgan M, Maloney D, Duvic M. Hypomagnesemia and hypocalcemia in mycosis fungoides: a retrospective case series. Leuk. Lymphoma 43(6), 1297–1302 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol 159(1), 105–112 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Ritchie D, Piekarz R, Blombery P et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica 94(11), 1618–1622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edelstein L, Micheva-Viteva S, Phelan B, Dougherty J. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res. Hum. Retroviruses 25(9), 883–887 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bots M, Johnstone R. Rational combinations using HDAC inhibitors. Clin. Cancer Res 15(12), 3970–3977 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Shiozawa K, Nakanishi T, Tan M et al. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin. Cancer Res 15(5), 1698–1707 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Kano Y, Akutsu M, Tsunoda S et al. Cytotoxic effects of histone deacetylase inhibitor FK228 (depsipeptide, formally named FR901228) in combination with conventional anti-leukemia/lymphoma agents against human leukemia/lymphoma cell lines. Invest. New Drugs 25(1), 31–40 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Bishton M, Kenealy M, Johnstone R, Rasheed W, Prince H. Epigenetic targets in hematological malignancies: combination therapies with HDACis and demethylating agents. Expert Rev. Anticancer Ther 7(10), 1439–1449 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Jung M, Dritschilo A. Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat. Res 161(6), 667–674 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Camphausen K, Burgan W, Cerra M et al. Enhanced radiation-induced cell killing and prolongation of γH2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res 64(1), 316–321 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Camphausen K, Tofilon P. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J. Clin. Oncol 25(26), 4051–4056 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Kitazono M, Robey R, Zhan Z et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na+/I-symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J. Clin. Endocrinol. Metab 86(7), 3430–3435 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Chen J, Zhang M, Ju W, Waldmann T. Effective treatment of a murine model of adult T-cell leukemia using depsipeptide and its combination with unmodified daclizumab directed toward CD25. Blood 113(6), 1287–1293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piekarz RL, Robey RW, Zhan Z et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood 103(12), 4636–4643 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Lee J, Park J, Jung Y et al. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol. Cancer Ther 5(12), 3085–3095 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Steiner F, Hong J, Fischette M et al. Sequential 5-Aza 2′-deoxycytidine/depsipeptide FK228 treatment induces tissue factor pathway inhibitor 2 (TFPI-2) expression in cancer cells. Oncogene 24(14), 2386–2397 (2005). [DOI] [PubMed] [Google Scholar]
- 110.Dai Z, Liu S, Marcucci G, Sadee W. 5-Aza-2′-deoxycytidine and depsipeptide synergistically induce expression of BIK (BCL2-interacting killer). Biochem. Biophys. Res. Commun 351(2), 455–461 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Harrison SJ, Quach H, Yuen K et al. High response rates with the combination of bortezomib, dexamethasone and the pan-histone deacetylase inhibitor romidepsin in patients with relapsed or refractory multiple myeloma in a Phase I/II clinical trial. ASH Ann. Meeting Abstracts 112(11), 3698 (2008). [Google Scholar]
- 112.Badros A, Burger A, Philip S et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin. Cancer Res 15(16), 5250–5257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weber DM, Jagannath S, Mazumder A et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in combination with bortezomib in patients with advanced multiple myeloma. ASH Ann. Meeting Abstracts 110(11), 1172 (2007). [Google Scholar]
- 114.Weber D, Badros AZ, Jagannath S et al. Vorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: early clinical experience. ASH Ann. Meeting Abstracts 112(11), 871 (2008). [Google Scholar]